Abstract
High pressure electrospray ionization mass spectrometry has been performed by pressurizing a custom made ion source chamber with compressed air to a pressure higher than the atmospheric pressure. The ion source was coupled to a commercial time-of-flight mass spectrometer using a nozzle-skimmer arrangement. The onset voltage for the electrospray of aqueous solution was found to be independent on the operating pressure. The onset voltage for the corona discharge, however, increased with the rise of pressure following the Paschen’s law. Thus, besides having more working gas for the desolvation process, gaseous breakdown could also be avoided by pressurizing the ESI ion source with air to an appropriate level. Stable electrospray ionization has been achieved for the sample solution with high surface tension such as pure water in both positive and negative ion modes. Fragmentation of labile compounds during the ionization process could also be reduced by optimizing the operating pressure of the ion source.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
At present, nearly all electrospray ion sources employed in the commercial mass spectrometer are operated at atmospheric pressure. Carrying out the electrospray ionization under the atmospheric pressure condition is advantageous compared with that in high vacuum because the ambient gas that surrounds the electrosprayed charged droplets acts as a heat bath, and can provide sufficient thermal energy to vaporize the volatile solvent without freezing the aerosol droplets [1, 2]. This allows ESI to work with wide range of solvents including those used in the liquid chromatography, while its vacuum counterpart, electrohydrodynamic (EHD) spray could only handle low-volatility solvents [3, 4].
Although it has been mentioned that it could be advantageous to operate the ion source at pressure >1000 Torr [5], there has been little report on the ESI performed at pressure much higher than the atmospheric pressure. Under a higher pressure condition, more working gas could be used for the desolvation process, and due to the reduction of mean free path for electron and gas molecules, electric potential that induces the gaseous breakdown will become higher according to Paschen’s law [6]. Gaseous breakdown that leads to the corona or arc discharge is troublesome to ESI because it reduces the electric field near the ESI emitter and degrades the performance of electrospray. For liquid with high surface tension such as pure water, it is difficult to achieve stable electrospray because the gaseous breakdown voltage could fall below or about the same with the onset voltage of electrospray. Suppression of electrical discharge can be done to a certain extent by using CO2, or SF6 as the sheath gas [7, 8]. Because the onset voltage for electrospray is nearly independent to the pressure [7], the corona discharge could also be quenched by pressurizing the ESI ion source with air to an appropriate level.
In this work, we carried out high pressure electrospray ionization (HP-ESI) under a pressurized condition of up to 6 bar, and coupled this ion source to a commercial time-of-flight mass spectrometer. Direct coupling of the high pressure ion source to the mass spectrometer was difficult because it would add a tremendous gas load to the vacuum and could pose hazardous failure to the mass analyzer. Furthermore, the geometric arrangement for the skimmer and ion guiding electrodes inside the mass spectrometer are optimized with atmospheric pressure as the upstream pressure [9] and, thus, deviation from the designed parameters could affect the efficiency of the ion transmission to the analyzer. Here, instead of raising the pressure directly around the ion inlet of the mass spectrometer, the high pressure ion source was separated from the first pumping stage with an additional stage at near atmospheric pressure. The arrangement is like a nozzle-skimmer system [10], where the ions exit from the high pressure stage via a nozzle to an open space at ~1 atm and re-enter to the vacuum through the ion sampling skimmer.
2 Experimental
2.1 Mass Spectrometer
The experiments were performed with a commercial orthogonal-acceleration time-of-flight mass spectrometer (AccuTOF; JEOL, Akishima, Japan). The original electrospray ion source was removed, and the interlock was disabled to accommodate the new ion source. The potential difference between the ion inlet and the skimmer was tuned between 50 and 200 V. Mass spectra were acquired using a continuous digital signal averager (Fastflight; Ametek, Oak, Ridge, TN, USA).
2.2 High Pressure ESI Ion Source
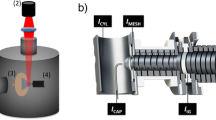
Figure 1 shows the schematic of the high pressure electrospray ion source. Photographs for ion source are depicted in the Supplementary Figure S1 in the supporting information. The chamber of the ion source was made from the housing of a commercial high pressure air filter (AMF150C-02; SMC Corp., Tokyo, Japan), which has a transparent view port attached on it. The electrospray condition could be monitored using a long working distance microscope (VHX; Keyence, Osaka, Japan) via the view port. The ion source chamber was filled with purified air from an air compressor. ESI emitter was a stainless steel capillary with i.d. of 50 μm (New Objective, Woburn, MA, USA). Distance between the ESI emitter tip and the counter electrode was 2.5 mm. Sample solution was pumped through the ESI capillary using a syringe pump with high linear force (PHD 4400; Harvard Apparatus, Holliston, MA, USA). The flow rates for sample solution were 1–5 μL/min.
Schematic of the high pressure electrospray ion source. Ions and charged droplets are transported from the high pressure side (>1 atm) to the atmospheric pressure side (1 atm) via a 1/16 in. stainless steel tube (i.d. = 0.8 mm), and are sampled to first pumping stage (<1 atm) of the mass spectrometry via a 0.4 mm (i. d.) orifice. Distance between the outlet nozzle of the ion source and the ion sampling orifice is 1.5 mm. Distance between the ESI emitter and the counter electrode is 2.5 mm
Charged droplets and ions were transported to the atmospheric pressure side via a 1/16 in. stainless steel tube (i.d. = 0.8 mm, length = 72 mm, obtained from GL Scientific, Tokyo, Japan). Two heaters (Heater 1 and Heater 2) were employed in the ion source. The temperature for Heater 1 was kept at 150 °C for heating the air inside the ion source chamber, and Heater 2 was used to heat the ion transport tube to 250 °C for desolvation and to prevent the condensation of droplets on the inner wall. The distance between the outlet of the ion source and the ion-sampling orifice of the mass spectrometer was 1.5 mm. The flow rate of the compressed air and the pressure in the first vacuum stage of the mass spectrometer depend on the operating pressure of the ion source. The plots of flow rate and the vacuum gauge reading versus the ion source pressure are shown in Supplementary Figure S2a and b, respectively. At typical operating pressure of 4 bar, the flow rate of the working gas is ~17 L/min. Despite high gas flow rate, the spraying of liquid was driven electro-statically like an unassisted electrospray, and is thus different from other pneumatically assisted sprayers like ion spray [11], sonic spray [12], and electro-sonic spray [13]. The excess charge was primarily produced from the electrospray, but when the liquid droplets were transferred to the mass spectrometer, there could also be some additional charging caused by the statistically unbalanced charge distribution similar to that in the sonic spray ionization.
Ions and fine charged droplets that exited from the outlet nozzle of the ion source were guided to the mass spectrometer by the high speed flowing gas, and the ion transmission was weakly influenced by the surrounding electric field. No improvement was observed by applying potential difference between the ion source outlet and the ion sampling orifice. Thus, for the purpose of simplicity, the metallic housing of the ion source, the counter electrode for the electrospray, and the ion outlet nozzle were all held at ground potential.
2.3 Chemicals and Sample Preparation
Myoglobin, cytochrome c, lysozyme, and ubiquitin, were purchased from Sigma. All samples were prepared in pure water without any addition of organic solvent or acid. Pure water was prepared using Milli-Q system (Millipore, Bedford, MA, USA).
3 Results and Discussion
Figure 2a shows the onset voltages for positive and negative corona discharges under different operating pressures of the HP-ESI ion source. The working gas was air. The onset voltages are determined by monitoring the discharge current in the counter electrodes. The gaseous breakdown due to the negative corona discharge took place at lower voltage compared to that in the positive ion mode. Also shown in Figure 2a are the threshold voltage for the initialization of electrospray and the required voltages for the establishment of different electrospray modes for pure water (distilled and deionized, liquid flow rate = 1.6 μL/min).
(a) Plot of the onset voltage for the occurrence of corona discharge versus pressure for positive (solid square) and negative (open circle) ion modes. The y-axis represents the magnitude of the voltage. The polarity of the onset voltage is “+” for positive ion mode and “−” for negative ion mode. Also shown are the onset voltage for the electrospray of pure water, and the voltages required to establish the steady cone-jet, two cone-jet, and multi cone-jet modes. Dashed blue lines represent conditions that can only be met for the positive ion mode, and solid blue lines represent conditions that can be met for both positive and negative ion modes. (b)-(d): Photographs showing the electrospray of pure water in cone-jet mode (b), two cone-jet mode (c), and multi cone-jet mode (d). The electrospray was conducted under an operating pressure of 4 bar (~4 atm) in negative ion mode
When the electrospray was first initiated, the initial size of the sprayed droplets was rather large and the spray was not stable. To obtain stable electrospray such as that in the steady cone-jet mode, one needs to apply few hundreds volts higher than the onset voltage. Spraying mode changed to two cone-jet and multiple cone-jet modes when the applied electric field was further increased. In this paper, we followed the nomenclature used by Cloupeau and Prunet-Foch [14], for describing the spraying mode. The spraying modes were determined by microscopic inspection and measurement on the electrospray current. The onset voltage for electrospray and the voltages required for different cone-jet modes were the same for both positive and negative ion mode and were found to be independent to the operating pressure if there was no occurrence of corona or arc discharge.
Due to its high surface tension (0.073 N/m), water is difficult to electrospray under atmospheric pressure, particularly in the negative ion mode. As shown in Figure 2a, although an operational region exists for the electrospray in the positive ion mode, the negative ion mode could only be operated near the threshold of the onset of electrospray, which is of poor ionization efficiency. Once the corona discharge was initiated, further increase in applied voltage would only increase the discharge current, which could reduce the electric field near the ESI emitter and interfere with the formation of charged droplets [15].
Increasing the ion source pressure to about 4 bar could nearly eliminate the corona discharge and the electric field on the emitter tip could be raised until the formation of the multi cone-jet mode. Photographs showing the electrospray of pure water in different spraying modes conducted under the operating pressure of 4 bar (~4 atm) in negative ion mode are depicted in Figures 2b–d. As shown in Supplementary Figure S3, when the operating pressure of the ion source was raised to 4 bar, both positive and negative mass spectra of various proteins (e.g., cytochrome c, lysozyme, and ubiquitin) could be obtained even from the sample solution prepared with pure deionized water.
Recently, Marginean et al. noticed that the electrospray condition (spray current and stability) changed with the operating pressure in a sub-atmospheric pressure environment, and the stability was limited by the vapor pressure of the liquid [16]. This pressure effect was not observed in our experiments, and the electrospray current remained nearly constant with the applied pressure once the stability of liquid flow rate was maintained.
The influence of ion source pressure on the softness of ionization process with myoglobin as the model analyte is shown in Figure 3. Myoglobin consists of an iron-containing porphyrin (heme), which is bound by noncovalent bond. Myoglobin is thermally labile, and heme can be easily detached due to the excessive energy deposited to the protein during the desolvation process. In Figure 3, peaks originated from the apomyoglobin (without heme) are denoted with solid circle, and those from holomyoglobin (with heme) are denoted with open circle.
High pressure ESI mass Spectra of 10–5 M myoglobin in pure water (pH ~7), obtained at different operating pressures of the ion source. (a) 6 bar (~6 atm), (b) 5 bar, (c) 4 bar, (d) 3 bar, (e) 2.5 bar, (f) 2 bar, (g) 1.5 bar, and (h) 1.2 bar. Peaks originated from the holomyoglobin are denoted with open circle, and those from the apomyoglobin are denoted with solid circle. Asterisk signs * indicate the peaks originated from Heme+. Potential difference between the ion inlet and the skimmer is 100 V. Temperatures of Heater 1 and Heater 2 are 150 °C and 250 °C, respectively. ESI emitter potential = 3.3 kV
The desolvation appeared to be more gentle when the operating pressure of the ion source was equal or greater than 4 bar (Figure 3a–c), and the obtained mass spectra were dominated by the heme bound holomyoglobin. Although heme and apomyoglobin were also detected, their abundance was significantly lower than that of intact holomyoglobin. The distribution of holo- and apo-species changed when the pressure was reduced, and the abundance of the apomyoglobin became higher than that of holomyoglobin when the pressure was reduced to 2 bar (Figure 3f). Corona discharge took place occasionally at 1.5 bar, (Figure 3g), and the total ion signal became weaker and less stable. The discharge became more severe when the pressure was further reduced to 1.2 bar.
The result in Figure 3 shows that when other experimental parameters (i.e., temperatures of Heater 1 and Heater 2, and the potential between ion inlet and skimmer) are kept constant, the ionization process tends to be milder in the cases of higher ion source pressure. It is conceivable because, (1) the rise in ion source pressure increases the flow rate of the working gas and the temperature of the flowing gas that surrounds the electrospray plume and charged droplets is not in equilibrium with the heaters’ temperature, and is lower in the case of higher pressure; (2) when ion source pressure is increased, it also increases the pressure in the first pumping stage of the mass spectrometer (see Supplementary Figure S2b), and the pressure in this vacuum stage has been known to influence the softness of the desolvation [17–19]. When the ions/charged droplets enter the vacuum, they are accelerated by the nozzle-skimmer potential. If the pressure in the first pumping stage is increased while keeping the nozzle-skimmer potential unchanged, the velocity that an ion can reach before colliding with a residual gas molecule will be reduced because the acceleration distance becomes shorter due to the decrease in mean free path. As a result, the number of collisions between ions and residual gas molecules increases but each collision becomes less energetic because the center-of-mass energy which depends on the relative velocity of ion and gas is reduced [19].
4 Conclusion
High pressure electrospray ionization mass spectrometry with operating pressure higher than 1 atm has been demonstrated on a commercial mass spectrometer without additional modification on the pumping system. Corona discharge or arc discharge could be quenched using pressurized air as the working gas, and thus allowed the high pressure ion source to deal with aqueous sample solution. Future works include the improvement of ion transmission using additional pumping and the study of ion–molecule reactions under the high pressure conditions.
References
Dole, M.: Molecular beams of macroions. J. Chem. Phys. 49, 2240–2249 (1968)
Yamashita, M., Fenn, J.B.: Electrospray ion source. Another variation on the free-jet theme. J. Phys. Chem. 88, 4451–4459 (1984)
Evans, C.A.: An electrohydrodynamic ion source for the mass spectrometry of liquids. Rev. Sci. Instrum. 43, 1527–1530 (1972)
Simons, D.S., Colby, B.N., Evans, C.A.: Electrohydrodynamic ionization mass spectrometry—the ionization of liquid glycerol and nonvolatile organic solutes. Int. J. Mass Spectrom. Ion Phys. 15, 291–302 (1974)
Fenn, J.B.: Mass spectrometric implications of high-pressure ion sources. Int. J. Mass Spectrom. 200, 459–478 (2000)
Paschen, F.: Ueber die zum Funkenübergang in Luft, Wasserstoff und Kohlensäure bei verschiedenen Drucken erforderliche Potentialdifferenz. Ann. Phys. 273, 69–96 (1889)
Smith, D.P.H.: The electrohydrodynamic atomization of liquids. IEEE Trans. Ind. Appl IA-22, 527–535 (1986)
Ikonomou, M. G.; Blades, A. T.; Kebarle, P. Electrospray Mass Spectrometry of Methanol and Water Solutions Suppression of Electric Discharge with SF6 Gas. J. Am. Soc. Mass Spectrom. 2, 497–505 (1991)
Covey, T.R., Thomson, B.A., Schneider, B.B.: Atmospheric pressure ion sources. Mass Spectrom. Rev. 28, 870–897 (2009)
Kantrowitz, A., Grey, J.: A high intensity source for the molecular beam. Part I. Theoretical. Rev. Sci. Instrum. 22, 328–332 (1951)
Bruins, A.P., Covey, T.R., Henion, J.D.: Ion spray interface for combined liquid chromatography/atmospheric pressure ionization mass spectrometry. Anal. Chem. 59, 2642–2646 (1987)
Hirabayashi, A., Sakairi, M., Koizumi, H.: Sonic spray ionization method for atmospheric pressure ionization mass spectrometry. Anal. Chem. 66, 4557–4559 (1994)
Takáts, Z., Wiseman, J.M., Gologan, B., Cooks, R.G.: Electrosonic spray ionization. A gentle technique for generating folded proteins and protein complexes in the gas phase and for studying ion − molecule reactions at atmospheric pressure. Anal. Chem. 76, 4050–4058 (2004)
Cloupeau, M., Prunet-Foch, B.: Electrohydrodynamic spraying functioning modes: a critical review. J. Aerosol Sci. 25, 1021–1036 (1994)
Cloupeau, M.: Recipes for use of EHD spraying in cone-jet mode and notes on corona discharge effects. J. Aerosol Sci. 25, 1143–1157 (1994)
Marginean, I., Page, J.S., Kelly, R.T., Tang, K., Smith, R.D.: Effect of pressure on electrospray characteristics. Appl. Phys. Lett. 95, 184103 (2009)
Van Berkel, W.J.H., Van Den Heuvel, R.H.H., Versluis, C., Heck, A.J.R.: Detection of intact megadalton protein assemblies of vanillyl-alcohol oxidase by mass spectrometry. Protein Sci. 9, 435–439 (2008)
Tahallah, N., Pinkse, M., Maier, C.S., Heck, A.J.R.: The effect of the source pressure on the abundance of ions of noncovalent protein assemblies in an electrospray ionization orthogonal time-of-flight instrument. Rapid Commun. Mass Spectrom. 15, 596–601 (2001)
Schmidt, A., Bahr, U., Karas, M.: Influence of pressure in the first pumping stage on analyte desolvation and fragmentation in Nano-ESI MS. Anal. Chem. 73, 6040–6046 (2001)
Acknowledgments
This work was supported by the Special Coordination Funds for Promoting Science and Technology from the Ministry of Education, Culture, Sports, Science and Technology of the Japanese government.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESSM 1
Photographs showing the prototype of the high pressure electrospray ion source (a) and (b) and the coupling of the ion source to a commercial time-of-flight mass spectrometer (c). (PDF 2.44 MB)
Rights and permissions
About this article
Cite this article
Chen, L.C., Mandal, M.K. & Hiraoka, K. High Pressure (>1 atm) Electrospray Ionization Mass Spectrometry. J. Am. Soc. Mass Spectrom. 22, 539–544 (2011). https://doi.org/10.1007/s13361-010-0058-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13361-010-0058-8