Abstract
Lung cancer (LC), particularly nonsmall cell lung cancer (NSCLC), is one of the most prevalent types of neoplasia worldwide, regardless of gender, with the highest mortality rates in oncology. Over the years, treatment for NSCLC has evolved from conventional surgery, chemotherapy, and radiotherapy to more tailored and minimally invasive approaches. The use of personalised therapies has increased the expected efficacy of treatment while simultaneously reducing the frequency of severe adverse effects (AEs). In this review, we discuss established modern approaches, including immunotherapy and targeted therapy, as well as experimental molecular methods like clustered regularly interspaced short palindromic repeat (CRISPR) and nanoparticles. These emerging methods offer promising outcomes and shorten the recovery time for various patients. Recent advances in the diagnostic field, including imaging and genetic profiling, have enabled the implementation of these methods. The versatility of these modern therapies allows for multiple treatment options, such as single-agent use, combination with existing conventional treatments, or incorporation into new regimens. As a result, patients can survive even in the advanced stages of NSCLC, leading to increased survival indicators such as overall survival (OS) and progression-free survival (PFS).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to the latest data from GLOBOCAN, in 2020, more than 2.2 million people were diagnosed with lung cancer (LC), and almost 1.8 million deaths were noted worldwide. It made LC the most common cause of cancer-related death globally (Sung et al. 2021). Even with the increased smoking cessation and subsequent decrease in cases among men in developed countries, the overall statistics have grown since the last analysis in 2018 (Bray et al. 2018). National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program indicates that the 5-year survival rate in the USA (2010–2016) was 20.5%, which is a significant improvement compared to the rate from 1975, which was 11.5% (Thandra et al. 2021). This phenomenon can be attributed to the rapid development of diagnosis and treatment methods such as computed tomography (CT) and the introduction of immunotherapy and targeted therapies for LC. There are several risk factors responsible for LC development, such as age, gender, race/ethnicity, family history, tobacco smoking, cannabis smoking, environmental pollution, infections, inflammation, and chronic obstructive pulmonary disease (Thandra et al. 2021).
Tobacco smoking is the most critical risk factor in LC formation, as it accounts for over two-thirds of deaths and is a traceable cause in up to 80% of cases (Sung et al. 2021; Thandra et al. 2021). Combustion products contain various carcinogenic substances, such as polycyclic aromatic hydrocarbons and tobacco-specific N-nitrosamines. The presence of these carcinogens may lead to the activation of specific oncogenes (e.g. KRAS) and inhibition of tumour suppressor genes (TSGs) (Dela Cruz et al. 2011; Akopyan and Bonavida 2006).
LC is divided into types and subtypes based on the histological features of the tumour cells. Small cell lung carcinoma (SCLC) and nonsmall cell lung carcinoma (NSCLC) are the two main types of LC, with NSCLC being much more common (85% of all LC cases) (Veronesi et al. 2017). NSCLC’s further subtypes include adenocarcinoma (LUAD), squamous cell carcinoma, and large cell carcinoma. In 2015, WHO introduced another subtype, neuroendocrine tumours, which consist of SCLC, large cell neuroendocrine carcinoma, and carcinoid. LUAD and squamous cell carcinoma are the most common types of lung tumours. Current diagnosis is more often based on immunochemistry than morphology, with biomarkers being the determining factor in situations of uncertainty (Veronesi et al. 2017). Genetic screening is becoming more common in LC with the development and cost reduction of next-generation sequencing (NGS). Specific mutations and gene alterations have been found to influence lung tumorigenesis. Those alterations are present in genes which encode proteins such as the epidermal growth factor receptor (EGFR), Kirsten rat sarcoma virus (KRAS), anaplastic lymphoma kinase (ALK), B-RAF, and ROS1.
Recognition of those mutations has facilitated the development of targeted therapy. Most defects affect signalling pathways within the cell due to tyrosine kinase (TK) activation. Monoclonal antibodies (mAbs) and tyrosine kinase inhibitors (TKIs) are used in selected patients to restrain the effects of mentioned alterations (Larsen et al. 2011). Epigenetic modifications have also been found to affect LC development. Hypermethylation of certain TSGs, due to increased levels of DNA methyltransferases, prevents them from being expressed and impacts lung tumorigenesis from its early stages (Langevin et al. 2015). There is also evidence of hypomethylation in the CpG sequences, which may lead to increased recombination during proliferation and chromosome instability. Some chromatin structural changes can be reversed, hence the potential for chromatin modifiers to be used in targeted therapy (Mehta et al. 2015). The main aim of this brief review is to collect and introduce the potential option of available, modern ways of battles with LC.
Conventional therapies
Due to the high mortality rate of NSCLC, there is a great need to develop new, more effective ways of curing patients (Siegel et al. 2014). New therapies begin to play a more significant role in NSCLC treatment. Nonetheless, classic approaches such as surgery, radiotherapy, and chemotherapy are still commonly used worldwide. Ongoing research on traditional therapies allows improving and using them more effectively. In this section, we want to outline the most common conventional treatment approaches briefly.
Surgery
Surgical removal of a tumour has been the golden standard for patients in the early stage of NSCLC, but only if the tumour is resectable and the patient can survive the surgery (Zappa and Mousa 2016). The operation consists of removing the lobe, segment, or entire lung, depending on the tumour size, location, and level of the pulmonary reserve (Tandberg et al. 2018).
The oldest surgical intervention technique is open, posterolateral thoracotomy (conventional), and muscle-sparing thoracotomy (Wang et al. 2019). However, due to technological development, video-assisted thoracic surgery (VATS) has gained popularity. According to numerous studies, VATS appears significantly superior to thoracotomy, with fewer removed lymph nodes, less intraoperative bleeding and trauma, fewer postoperative complications, shorter hospitalisation, faster rehabilitation, and better 3- and 5-year survivals (Wang et al. 2019; Dziedzic et al. 2021). Usability limitations of VATS may arise from low experience in using this technique and higher costs. Nonetheless, available data shows that shorter hospitalisation and reduced postoperative healthcare requirements lower the costs of using VATS (Wang et al. 2019). In the past two decades, three-port VATS has been positively established and has become a conventional treatment for NSCLC. This relatively new variant of VATS involves making one 4-cm-long incision. It is a less invasive form of lung surgery proven to reduce trauma, decrease postoperative pain, and shorten the time needed for rehabilitation. However, it requires the extensive experience of the surgeon (Wang et al. 2017).
The latest and most challenging technique of surgical tumour removal in NSCLC is robot-assisted thoracic surgery (RATS). The biggest problems encountered in using RATS are related to the costs of this procedure and learning how to perform this technique (Lee et al. 2021). Robotic lobectomy may be recommended for operations requiring greater precision because robotic arms can provide more precise movements. The only systems currently available for RATS are da Vinci Systems (Veronesi 2015). According to most studies that compare RATS and VATS, there are no differences in mortality, overall survival (OS), disease-free survival, and intraoperative and postoperative parameters (Ma et al. 2021; Guo et al. 2019). However, a meta-analysis published in December 2020 suggests that RATS may result in better OS (Wu et al. 2021). This research implies that RATS is a safe and practicable technique in NSCLC treatment.
Radiotherapy
Radiotherapy is another important form of NSCLC treatment. It can be applied in every stage of NSCLC as adjuvant or neoadjuvant therapy (Uzel et al. 2019). Technological development and diagnostic imaging have made radiotherapy more precise and safer for patients. Research shows the superiority of three-dimensional conformal radiotherapy over two-dimensional therapy, which is characterised by poor control of tumour location (Chang et al. 2008). One of the latest techniques of this treatment is image-guided radiotherapy (IGRT). It can be used with four-dimensional CT or positron emission tomography, allowing tumour imaging before and during the procedure. IGRT maximised the precision of the radiation beam and reduced damage to healthy near-tumoural tissue (Kilburn 2016). One factor that can limit radiotherapy’s precision is respiratory movement during irradiation. Respiratory-gated conformal radiotherapy (RGRT) can be used to limit the impact of this factor. This technique involves sending the radiation beam toward the tumour during a predefined gate or blocking the patient’s breathing. Studies have shown that RGRT can reduce acute and late toxicity during chest irradiation. Nevertheless, this therapy is associated with higher financial costs (Giraud et al. 2011).
There are several techniques for radiotherapy: conventional with low doses of radiation and prolonged treatment time, stereotactic body radiation therapy (SBRT) with high doses but short treatment time, and intensity-modulated radiation therapy with modulated doses and longer treatment time. SBRT is a more complex technique, but many studies have shown that it is more convenient for patients due to shorter treatment times and lower morbidity, especially when used against peripheral tumours. Cancer foci located centrally are more challenging because of the increased risk of damage to the mediastinal structure (Tandberg et al. 2018; Donovan and Swaminath 2018; Kepka and Socha 2021).
Radiotherapy can be performed alone or with other types of NSCLC treatment, such as surgery, chemotherapy, immunotherapy, therapy with nanoparticles, targeted therapy, and CRISPR-Cas9 therapy.
Chemotherapy
Chemotherapy is commonly used in the IV stadium of NSCLC. Drugs used in chemotherapy, including LC, utilise the neoplastic cells’ capability of more intense mitosis compared to host cells, which makes these medicaments more toxic to the tumour. Platinum-based drugs are frequently used as they preferentially adduct with the N7 atom on guanine and adenosine. This bonding process impedes DNA replication and transcription, ultimately leading to apoptosis. Research has shown that the most effective outcomes are achieved through 4 rounds of chemotherapy that include platinum (Masters et al. 2015; Gridelli et al. 2004; Gerber and Schiller 2013).
The type of drugs administered to patients depends on their performance status (PS, scale 0–5), which describes their ability to function independently. According to The American Society of Clinical Oncology, the best combination of cytotoxic medicaments for patients with PS of 0 or 1 is cisplatin or carboplatin with paclitaxel, gemcitabine, docetaxel, vinorelbine, irinotecan, or pemetrexed (Masters et al. 2015). Patients treated this way can survive 8–10 months (Zappa and Mousa 2016). Nonetheless, a combination of these medicaments should be determined individually. Patients with a PS of 2 may be effectively treated only with nonplatinum drugs (Gridelli et al. 2004). Patients with a PS of 3 usually do not benefit from chemotherapy due to adverse effects (AE) and worse quality of life (Zappa and Mousa 2016).
Four of the most discussed forms of chemotherapy treatment are standard, sequential, alternating, and maintenance chemotherapy. All of them are effective in different groups of patients. However, it should be noted that only standard therapy is a validated and non-experimental method of chemotherapy (Grossi et al. 2007).
Sequential chemotherapy is based on applying non-cross-resistant medicaments in a specific sequence. It allows the application of more drugs with reduced toxicity by optimisation of dose of each medicament (Grossi et al. 2007). According to studies, sequential chemotherapy with a single agent followed by another can be promising for elderly feeble patients who do not qualify for platinum-based chemotherapy. For patients with good PS, an optimistic sequence of medicaments is a platinum-based doublet followed by a single, third-generation agent. The preliminary results of studies on this method have shown lower toxicity in this method with no differences in progression-free survival (PFS) and OS between this method and standard chemotherapy (Castro et al. 2002; Clark et al. 2001; Hosoe et al. 2003; Binder et al. 2007; Grossi et al. 2004; Edelman et al. 2004; Rinaldi et al. 2005; Chiappori et al. 2005).
Alternating chemotherapy is based on applying non-cross-resistant medicaments or their combination for a predetermined number of cycles or until progression. Optimal doses should be used as early as possible. Alternating administration of medicaments can reduce the toxicity of specific drugs (Grossi et al. 2007). Nonetheless, studies have shown that alternating chemotherapy is less effective for patients with good PS than standard therapy (Grossi et al. 2007). Despite this, several phase II trials have shown that alternating single-agent chemotherapy can be promising for elderly patients with bad PS (Mattson et al. 2000; Aguiar et al. 2005; Gridelli et al. 2007).
Maintenance chemotherapy is based on the administration of additional medicaments after planned in-advance chemotherapy. The drug used in this method can be one of those used in the standard cycle or a non-cross-resistant drug. The most effective outcome of maintenance chemotherapy is observed when the drug has been efficient during previous induction chemotherapy (Grossi et al. 2007). Studies have shown that maintenance chemotherapy can increase PFS, but OS is often not improved (Gerber and Schiller 2013). Two of the most promising drugs used in maintenance treatment are pemetrexed and bevacizumab. They present better OS; however, further research is required in this field (Gerber and Schiller 2013).
Chemotherapy can be combined with radiotherapy, immunotherapy, targeted therapy, surgery, and nanoparticle therapy. Currently, the most extensive research on cancer treatment has been on the combination of chemotherapy and surgical methods. The studies demonstrate that neo-adjuvant chemotherapy is primarily administered for the early stages of lung cancer. The procedure enables the reduction of tumour mass, making the operation feasible. Research shows that adjuvant chemotherapy is highly successful in decreasing mortality rates among patients diagnosed with stage IB cancer. There is a possibility that adjuvant chemotherapy, utilising platinum compounds, could become the standard for stage IB–IIIA of NSCL treatment.
Immunotherapy
Immunotherapy is one of the latest forms of cancer treatment and focuses on the patient’s immune system. Neoplastic cells have developed numerous ways to avoid apoptosis and destruction by the immune system, so the immune system needs a specific boost provided by immunological treatment. There are several branches of immunotherapy, and the two focus on CD8 + T cells and factors that modify their function, i.e. cytokines—small proteins responsible for interactions between immune system cells. Interleukin-2 is the most effective cytokine regarding T cell activation, which can eventually lead to a reduction of tumour size (Rosenberg et al. 1984, 1985; Rosenberg 2014; Taniguchi et al. 1983). Another option is using immune checkpoint inhibitors (ICIs). Their mechanism of action includes binding with receptor proteins located on the cytoplasmatic membrane of CD8 + T cells. These receptors enable cancer cells to avoid an immune system response by binding with cancer-produced proteins. Immunotherapeutic mAbs prevent from binding with these particles, increasing the capability of T cells to attack the tumour. The most commonly used antibodies in LC treatment are ipilimumab, pembrolizumab, and nivolumab (Steven et al. 2016). However, the tumour microenvironment may have highly immunosuppressive effects, which is the main obstacle in applying such medicaments. This area of research is being extensively investigated; learning about and eliminating these effects could be a promising possibility in helping the immune system destroy neoplastic cells (Steven et al. 2016).
Another successful immunotherapy method is adoptive cell therapy, based on genetic modification and proliferation of a patient’s T cells so they can effectively attack neoplastic cells. After modification, T cells are moved back to the patient. This approach has proven effective, but several potentially dangerous AEs currently limit the use of adoptive cell therapy (Dobosz and Dzieciątkowski 2019). Other promising immunotherapy options are oncolytic viruses and vaccines. The first one can be used to boost the immune system. Genetically modified viruses, which can penetrate only neoplastic cells, can cause their destruction. Molecules released during the disintegration of cancer cells stimulate the immune system to respond (Oiseth and Aziz 2017). Years ago, much hope arose among the scientific community that vaccines could also be used in preventing and treating cancers. Currently, there are only a few available and approved anticancer vaccines, but many of them, both allogeneic and autologous, are being investigated, and high hopes are placed on them (Dobosz and Dzieciątkowski 2019).
Immunotherapy appears to be extremely promising. Thanks to a large body of research conducted worldwide, it will be increasingly developed and may become a primary form of cancer treatment.
Immunotherapy in lung cancer
Immunotherapy and its incorporation of ICIs is the most promising branch of LC treatment. There have been attempts at developing alternative immunotherapeutic drugs, but none of them reached the efficacy achieved by ICIs (Steven et al. 2016).
Efficacy and safety
Compared to the standard NSCLC chemotherapy treatment approach, immunotherapy has proven to be more effective. Generally, ICIs may contribute to better OS and PFS values in previously treated NSCLC patients and be invaluable as first-line treatment (Reck et al. 2021; Zhou et al. 2021; Wang et al. 2020a). In the clinical study of the metastatic LC, the use of ICIs alone with programmed death ligand-1 (PD-L1) expression ≥ 50% versus chemotherapy resulted in a doubling of the median OS and 5-year OS rate (Reck et al. 2021). Also, when combined with the cycles of standard chemotherapy, results have shown an increased OS and PFS (Reck et al. 2021). Additionally, clinical trials showed the overall relative safety of this approach compared to the conventional approach, even in cases of combining immunotherapies. However, this depends on the individual patient’s characteristics and draws particular attention to potential AEs (hui Jia et al. 2020; Zhao et al. 2021). To summarise the abovementioned results, many approaches combine treatment methods to achieve the best possible efficacy (Huang et al. 2021). Nevertheless, it is significant to consider the sex of a patient, as the analysis of clinical trials in cases of NSCLC and melanoma denotes that the particular ICI efficiency (i.e. pembrolizumab) decreases for women (Conforti et al. 2018).
Observed possible mild AEs include nausea, fatigue, dyspnea, and decreased appetite; however, given the antibody nature of immunotherapeutic drugs, AEs can escalate to severe conditions such as pneumonitis, hepatitis, colitis, psoriasis, transient thyroid malfunction, and diabetes mellitus (Zarogoulidis et al. 2017; Godwin et al. 2017; Iwama and Arima 2017). Furthermore, this implies that careful observation must continue long after termination of the treatment because of the hazard of developing long-term autoimmune conditions (Suresh et al. 2018; Puzanov et al. 2017).
The mechanism
The immune system destroys cancer cells through tumour-specific T cells, utilising the tumour-specific differentiation 8 T (CD8 T) cluster to distinguish hostile cells from the host cells. This response requires the presentation of tumour antigens by the antigen-presenting cells (Steven et al. 2016). In LC, several steps of this system may be disturbed, impairing the immune response, therefore allowing cancer cells to thrive. It may include an insufficient antigen expression on the surface of the tumour cells, a disturbance in the antigen-presenting process or local immunosuppressive conditions. To further suppress the immune response, cancer cells may exploit the ICI system by expressing specific ligands on their surface, such as the PD-L1 or B7 compound family, which bind to corresponding receptors on the T cells and inhibit their anti-tumour activity (Steven et al. 2016; Dobosz and Dzieciątkowski 2019; Oiseth and Aziz 2017).
Additionally, regulatory T cells may favour immunosuppression in the presence of specific cancer molecules. Furthermore, prolonged exposure to increased concentrations of hostile antigens or inflammatory signals (as in chronic infections or cancers) may lead to T cell exhaustion, making T cells lose their effector functions and express inhibitory signals (Wherry and Kurachi 2015). Both ICIs and T cell exhaustion can be reversed using immunotherapeutic drugs, allowing the reinduction of the immune cell response.
PD-1/PD-L1 axis
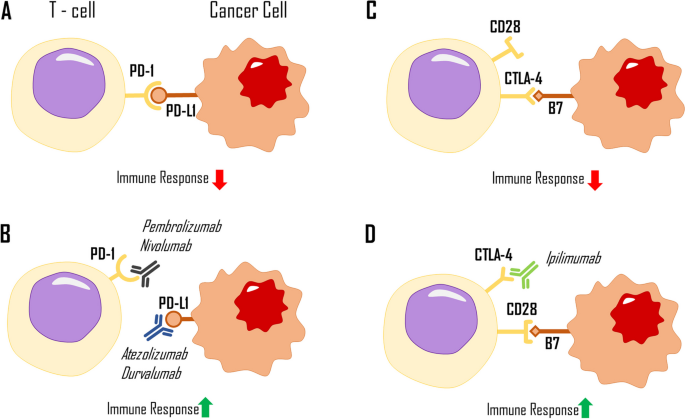
The most crucial immunological checkpoint exploit in LC involves the activation of a T cell programmed death receptor-1 (PD-1). PD-1 can be expressed in many haematopoietic and epithelial cells and the tumour microenvironment. Matching PD-L1 binds to the PD-1 receptor located on the surface of the T cell, downregulating its immune response (Fig. 1A). In NSCLC, a matching PD-L1 may be expressed in approximately half of the cancer cells, making the tumour highly susceptible to immunotherapy (Steven et al. 2016). On the other hand, programmed death ligand-2 (PD-L2) is located on Th2 cells, helping to mediate the inflammation.
Interaction between immune checkpoint inhibitor ligands, their corresponding receptors (A, C), and most commonly used immunotherapeutic drugs (B, D). PD-1, programmed death receptor; PD-L1, programmed death ligand 1; CTLA4, cytotoxic T-lymphocyte associated protein 4; B7, B7 compound family molecules; CD28, cluster differentiation protein 28; immunoterapeutic drugs are portrayed as symbols of antibodies, visualising their immune checkpoint blocking capabilities
Depending on the specific drug, immunotherapeutic drugs in the form of mAbs adhere to either PD-L1 or its receptor. This binding prevents the immunosuppressive T cell signal by rendering them unable to bind together (Xia et al. 2019). The leading pharmaceuticals that use this axis are IgG-type antibodies such as atezolizumab and durvalumab, which target PD-L1, and nivolumab and pembrolizumab, which target the PD-1 receptor (Fig. 1B). These drugs differ in their half-life, accompanying adverse effects, and possible immune-mediated adverse effects.
A crucial criterion of immunotherapy is the degree of PD-L1 expression on the surface of cancer cells, where PD-L1 ≥ 50% is the most favourable (Nasser et al. 2020). Individual predispositions, such as specific biomarkers and genetic landscapes, should be evaluated to ensure the best treatment option for a given patient (Rizvi et al. 2015). In the case of pembrolizumab, diagnostic trials include assessing tumour mutational burden, PD-L1 expression, and T cell response gene expression profile (Cristescu et al. 2018). Analysing these variables allows tailored therapy to a specific patient, advancing it into personalised precision therapy territory. The best-case scenario assumes treatment options with minimal use of chemotherapy or chemotherapy-free. However, knowing what is beneficial to a patient is most relevant, hence the importance of individual testing. Clinical trials, such as KEYNOTE (pembrolizumab), CHECKMATE (nivolumab + ipilimumab), and Impower (atezolizumab), confirm the efficacy of ICIs both as a monotherapy and in combination with standard chemotherapy such as cisplatin/carboplatin or paclitaxel, resulting in an overall increased overall survival median reaching 30 months. This makes it one of the most successful and currently best in use option for first-line treatments in NSCLC (Nasser et al. 2020).
CTLA-4 axis
Another important immune checkpoint involves the cytotoxic T-lymphocyte-associated protein-4 (CTLA-4) axis, which is one of the earliest and most studied ICIs. This receptor is located on the surface of T regulatory cells alongside CD28. These two compete for adherence to B7 compound family molecules, such as CD80 and CD86 (Fig. 1C). The CTLA-4 receptor has a higher affinity for these ligands than CD28 and favours the downregulation of the immune response. Ipilimumab, a fully human kappa mAb used in treating melanoma, renal, and hepatic carcinoma, binds to the CTLA-4 receptor, making it unable to attach to its ligand, resulting in a more robust immune response due to the lack of prior-established inhibition (Fig. 1D) (Nasser et al. 2020). This drug can be used in combination with PD-1/PD-L1 checkpoint inhibitors and standard chemotherapy while still producing beneficial results. In a clinical trial of CheckMate 9LA, after a 2-year update, treatment with nivolumab (PD-1), ipilimumab (CTLA-4), and chemotherapy resulted in higher overall survival (OS) and double the progression-free survival (PFS) compared to standard chemotherapy (Reck et al. 2021). Case studies have also observed a correlation between radiotherapy and CTLA-4 blockade immunotherapy, showing increased efficacy when both are combined in treatment (Formenti et al. 2018).
Other immunotherapies
There have been attempts at stimulating the immune system through Toll-like receptors located on the surface of B cells and plasmacytoid dendritic cells (Steven et al. 2016).
VEGF-targeted therapy
In NSCLC, cancer cells produce vascular endothelial growth factor (VEGF), particularly two forms of this molecule, VEGF-A and VEGF-B, which leads to intense angiogenesis and a better blood supply for tumour cells, enabling their growth and proliferation (Bonnesen et al. 2009; Holm et al. 1996). Targeting VEGF and its receptors can suppress angiogenesis and slow tumour progression by reducing the oxygen and nutrient supply to the tumour (Yamazaki and Morita 2006). VEGF is a glycoprotein produced by various cells, including macrophages, keratinocytes, endothelial cells, mesangial cells, platelets, and tumour cells (Sunderkotter et al. 1994; Rosen 2002; Duffy et al. 2013; Zhang et al. 2022). There are several forms of VEGF, including VEGF-A, VEGF-B, VEGF-C, VEGF-D, VEGF-E, and VEGF-F (Lohela et al. 2009). Hypoxia-induced factor (HIF) is a crucial factor for VEGF secretion, which is produced in response to oxygen deprivation. VEGF binds to the vascular endothelial growth factor receptor (VEGFR) to initiate the pathway. VEGFR is a tyrosine kinase receptor with three domains: intracellular (with TK activity), transmembrane, and extracellular (Ferrara et al. 2003). The binding of VEGF to the extracellular domain activates the intracellular domain, leading to the phosphorylation of signalling proteins essential for cell migration, proliferation, and survival (Kowanetz and Ferrara 2006; Neufeld et al. 1999).
VEGFR1 and VEGFR2 are the two most important receptors in the VEGFR group, expressed on the surface of various cells, including endothelial cells (Yamazaki and Morita 2006; Neufeld et al. 1999). Both receptors bind VEGF, but VEGFR1 shows a ten times higher affinity for VEGF than VEGFR2. However, VEGFR2 has stronger TK activity, and some studies suggest that VEGFR1 regulates VEGFR2 function (Ferrara 2004; Cébe-Suarez et al. 2006). There is also VEGFR3, which plays a role in lymphangiogenesis and is located on the surface of endothelial cells in lymphatic vessels (Hamrah et al. 2004).
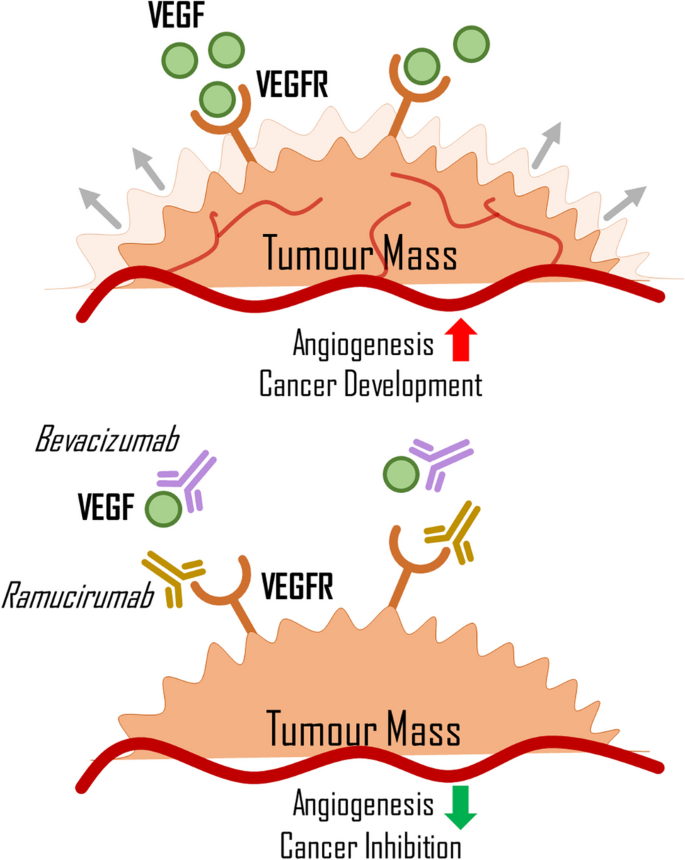
During angiogenesis, VEGF initially induces vasodilation and increased vessel permeability. Next, endothelial cells proliferate and migrate to a site where a new capillary will be formed (Carmeliet 2000). Blood then flows through the preexisting vessel to the new vessel. Angiogenesis is a crucial process, not only important in wound healing or embryogenesis but also in carcinogenesis. Tumour cells require oxygen and nutrients to grow and proliferate, and new capillaries deliver these compounds. However, angiogenesis in tumours differs from that in healthy tissues. New vessels grow incoherently, often spiralling, and their permeability increases (Dudley 2012; Carmeliet 2005). This can lead to a weaker oxygen supply to the tumour cells, resulting in hypoxia. As a result, cells will produce more HIF, intensifying VEGF secretion and angiogenesis (Fig. 2) (Carmeliet 2005). Some monoclonal antibodies (mAbs), such as bevacizumab and ramucirumab, which can be used in LC treatment, operate by blocking angiogenesis.
Interaction of VEGF and VEGFR with immunotherapeutic drugs. VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor; immunoterapeutic drugs are portrayed as symbols of antibodies, visualising their blocking capabilities. Red lines represent blood vessels; grey arrows visualize expanding tumour mass
Bevacizumab is a humanised monoclonal antibody that targets the VEGF-A molecule, preventing its attachment to its receptor and potentially inhibiting angiogenesis and tumour growth (Li et al. 2014; Garcia 2020). It is often used in combination with drugs like erlotinib or another monoclonal antibody, such as atezolizumab, in the treatment of NSCLC (Garcia 2020; Socinski et al. 2018; Cohen et al. 2005). While research shows that combination therapy with bevacizumab is generally safe, it can negatively affect the body, such as hypertension or pulmonary haemorrhage (Cohen et al. 2005; Piperdi et al. 2014). This is because bevacizumab reduces the level of VEGF, which impairs the remedial ability of the endothelium and weakens blood vessels, increasing the risk of rupture (Kamba and McDonald 2007; Johnson et al. 2004). Ramucirumab is another monoclonal antibody that has antiangiogenic properties. It binds to the extracellular domain of VEGFR2, preventing VEGF from binding to its receptor and blocking the formation of new blood vessels (Fig. 2) (Garon et al. 2014; Skobe et al. 1997). Recently approved in May 2020 for LC therapy, ramucirumab can be used with erlotinib when an activating mutation of EGFR is present in cancer cells (Nakagawa et al. 2019). As a newer drug in LC therapy, there is still some risk associated with its use.
Pathway targeted therapy
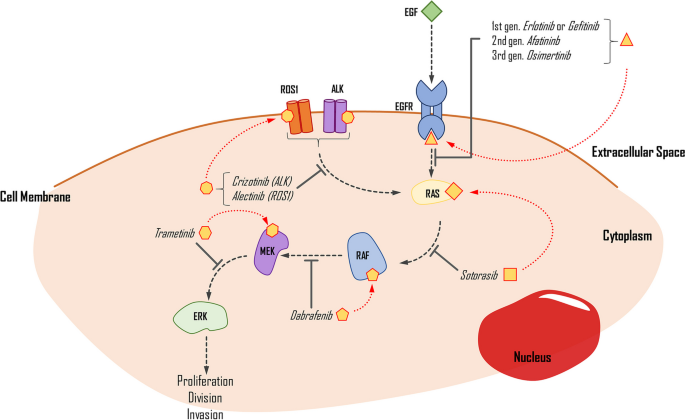
Targeted therapy for NSCLC focuses on identifying mutations in the EGFR and MAPK/ERK pathways, along with ALK and ROS1 gene fusions (Fig. 3). These mutations cause excessive stimulation of cell proliferation, differentiation, and growth, ultimately leading to carcinogenesis. Multiple studies demonstrate that these mutations can exist separately or concurrently. Molecular testing has confirmed that most forms of NSCLC, particularly LUADs, are positively correlated with mutations or alterations in EGFR, KRAS, ALK, ROS1, and B-RAF, in decreasing frequency. Polymerase chain reaction and NGS, coupled with fluorescence in situ hybridisation (FISH), are widely employed for diagnosing these mutations in NSCLC patients (Dugay et al. 2017; Dogan et al. 2012).
The simplified impact of selected drugs on the inhibition of the EGFR pathway. Medication is applied when proper mutations are detected in the genes that encode the pathway proteins. EGF, epidermal growth factor; EGFR, epidermal growth factor receptor; ROS1, proto-oncogene C-Ros 1; ALK, anaplastic lymphoma kinase; RAS, KRAS proto-oncogene; RAF, B-Raf proto-oncogene; MEK, mitogen-activated protein kinase kinase 1; ERK, mitogen-activated protein kinase kinase 3
EGFR
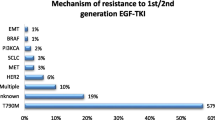
EGFR is one of the most frequently overexpressed proteins in NSCLC, found in both wild-type and mutated variants, with prevalence rates of over 11% in Caucasian and about 50% in Asian populations. It is also more common in non-smokers than smokers (Dogan et al. 2012; Rude Voldborg et al. 1997). About 66% of patients with EGFR mutation have the in-frame deletion in exon 19, while less than 30% have a point mutation in L858R exon 21. Rare mutations make up the remainder (Castellanos et al. 2017).
Currently, there are several options for first-line treatment in advanced NSCLC, but different variants of mutations respond differently to the administered drugs, which are TKIs. These drugs work by preventing the phosphorylation process of compounds in the EGFR pathway, thus limiting cell division, causing cell cycle arrest, and initiating apoptosis (Abdelgalil et al. 2020). The first- and second-generation drugs work as reversible inhibitors of ATP binding to the TK domain, while third-generation drugs bind irreversibly (Gelatti et al. 2019). The most common variant, exon 19 deletion, responds better to first-line treatment (such as erlotinib or gefitinib) than L858R. However, most EGFR-mutated cancers can develop resistance caused by a T790M mutation, which can be pre-existent or appear after implementing the TKIs (Castellanos et al. 2017). To address this resistance, a new approach has been taken that introduces an irreversibly binding drug called osimertinib. Initially, it was used as a second-line treatment in T790M-mutated NSCLC and significantly extended OS in patients. The genuine success of its implementation also transferred to first-line treatment. The FLAURA phase 3 trial compared osimertinib with first-generation TKIs, showing that the PFS was 18.9 and 10.2 months in osimertinib and standard TKIs (Soria et al. 2018). The findings imply that osimertinib treatment, irrespective of the presence of T790M mutation, improves overall survival (OS) and causes fewer adverse events (AEs) compared to standard treatment. Additionally, both approaches outperform platinum-based chemotherapy. However, the sequential approach gives more consistent results, with the osimertinib treatment implemented only after the gain of T790M mutation in EGFR-mutated NSCLC (Leonetti et al. 2019).
KRAS
KRAS mutation occurs in 27.5% of NSCLC, mostly in LUAD (Judd et al. 2021). The most common variants are G12C (40%), G12V (19%), and G12D (15%). It is more often detected in women than men (Judd et al. 2021). Another study shows a correlation between smoking and the presence of KRAS mutations in 34% of patients diagnosed with NSCLC (Dogan et al. 2012). The protein product of this mutation, the consistently active form of KRAS, influences several pathways, with the most significant impact on the MAPK/ERK pathway, which causes overstimulation of cell proliferation and growth. KRAS mutated protein is hard to target, as its binding is not as tight as expected (Salgia et al. 2021). A recent trial of a new drug, sotorasib, a G12C KRAS protein irreversible inhibitor, shows promising effects in treatment. With a 37.1% response rate and 80.6% disease control, the PFS of patients who used this drug is 6.8 months, and the OS is 12.5 months, with 19.8% of grade 3 AES and 0.8% of grade 4 events. This drug binds to GDP-KRAS, the inactive form of the KRAS protein, and prevents it from changing into the GTP-KRAS form, which causes oncogenesis when the G12C mutation is present. The drug use is limited and is usually implemented as second-line treatment in advanced malignant NSCLC. Although superior to platinum-based chemotherapy, the outcome of the treatment varies depending on other mutations and the tolerability of the drug itself (Skoulidis et al. 2021).
B-RAF
The B-RAF proto-oncogene is commonly mutated in 2–4% of NSCLC cases, with most of these mutations occurring in LUADs (> 85%) and in current smokers. Different variants of B-RAF mutations determine the differentiation, with two main types identified—the V600E variant accounting for approximately 50% of all B-RAF mutations and non-V600E variants comprising the remainder. It has been reported that wild-type B-RAF patients have more prolonged OS than B-RAF mutated patients in those with EGFR mutations (O’Leary et al. 2019; Baik et al. 2017). B-RAF encodes a serine/threonine kinase, part of the MAPK/ERK pathway. Currently, two targets for inhibiting this pathway are available—B-RAF inhibitors (B-RAFi) or MEK inhibitors (MEKi). These drugs were previously used in metastatic melanoma and are now being used in NSCLC. Monotherapy with B-RAF inhibitors is not sufficient. Thus, combination therapy using both types of drugs is necessary to enhance cell cycle arrest and activate apoptosis. In clinical trials, treatment with dabrafenib and trametinib resulted in a 63% response rate and approximately 9.7 months of progression-free survival. The most common AEs reported in various trials were pyrexia, ALT increase, and vomiting. These findings suggest that BRAFi and MEKi therapy is effective and provides a promising treatment option for patients with B-RAF mutation (Baik et al. 2017; Planchard et al. 2017).
ALK/ROS1 gene rearrangement
The most common mutation of the ALK gene in NSCLC is the ALK-R gene rearrangement, which can be detected in up to 6% of patients. The ALK gene commonly fuses with EML4, KIF5B, KLC1, and TRP in cancers, with EMLA4 being the most frequent partner gene in NSCLC. These fusions increase the expression of an ALK protein—TK (Holla 2017; Du et al. 2018; Camidge et al. 2019). The ROS1 rearrangement is another common targetable mutation in NSCLC, present in 1–2% of patients. CD74-ROS1 and SLC34A2-ROS1 gene fusions are the most frequent variants, resulting in constant TK activity. Both fusions cause phosphorylation, which activates components in several pathways, such as MAPK/ERK, PI3K, and PLC-γ, leading to uncontrolled cell proliferation and inhibiting cell termination (Holla 2017; Mao and Wu 2017a, b). The proteins resulting from the EML4-ALK and both ROS1 chromosomal rearrangements are target molecules for treating NSCLC ALK/ROS1.
The first drug approved for ALK or ROS1 pathway signalling inhibition was crizotinib, which has an objective response rate (ORR) of 72% that is fast and durable. The median PFS was 19.3 months, and the median OS was 52.4 months when such mutations were present (Shaw et al. 2014; Shaw et al. 2019; Moro-Sibilot et al. 2019; Recondo et al. 2018). However, crizotinib lacks a durable response due to resistance gained toward it, resulting from the L1196M mutation. Alectinib is a drug introduced into clinical trials to respond to this mutation, with a 48–55% response rate in ALK-positive crizotinib-resistant NSCLCs. It has also been used in patients with no previous treatment, with a 93.5% ORR (Holla 2017). Another study showed that alectinib had significantly higher PFS (68.4% vs 48.7%) and ORR (82% vs 75.5%) over 12 months, in combination with lower toxicity (41% vs 50%) than crizotinib. These findings suggest that alectinib is solid choice to be used in first-line treatment for ALK-positive NSCLC patients (Camidge et al. 2019; McKeage 2015).
Brigatinib is a next-generation ALK inhibitor (since 2020 is approved by FDA as first-line treatment in NSCLC), and comparison with crizotinib favours the newer drug. The comparison shows a rate of PFS, 67% vs 43% (12-month period), a response rate of 71% vs 60%, and an intracranial response of 78% vs 29% (Camidge et al. 2018). Another study described a 24.0-month vs 11-month PFS in 24.9 months, with additional information about a delayed median time to worsening compared to crizotinib (Camidge et al. 2020).
CRISPR and nanotechnology
In this section, clustered regularly interspaced short palindromic repeat (CRISPR) and nanoparticles as the latest approaches in NSCLC treatment will be discussed separately and cooperatively. CRISPR is a genome-editing tool helpful in developing personalised therapies, whereas nanotechnology is mainly used to improve drug delivery systems. The latest research shows promising results of combining nanomedicine and CRISPR to improve delivery and activation for in vivo genome editing therapy in NSCLC (Taha et al. 2022).
CRISPR-CAS9/12A
CRISPR was initially discovered as part of an adaptive defence system in the Escherichia coli genome and was later adapted into an in vivo editing and targeting tool (Wiedenheft et al. 2012). What creates CRISPR is a small guide RNA that forms a complex with the inactivated DNA-binding protein Cas9 and together they attach to a precise region of the genome. With the production of RNA-guided nucleases (RGNs) like Cas9, allowing to obtain tailored specificities, many targets in the genomes of cells can be reached. The first step in genome editing involves creating a double-stranded DNA break at specific loci using the CRISPR-Cas system. The resulting break can be repaired by different pathways, including nonhomologous end-joining (NHEJ) and homology-directed repair (HDR), with HDR being a more precise mechanism (Sander and Joung 2014). The CRISPR system is typically combined with Cas9 nuclease, which is derived from the bacterial CRISPR-associated protein nine nucleases from Streptococcus pyogenes (Sander and Joung 2014). However, other systems that use Cas12 nucleases have shown promising results in the treatment of NSCLC (Feng et al. 2022). Bijoya et al. suggests that CRISPR-Cas12a can be used as a multiplex genome-editing tool and a system to promote HDR instead of NHEJ, which can lead to more precise DNA targeting (Paul and Montoya 2020). In 2020, Sreedurgalakshmi et al. comprehensively described the use of CRISPR-Cas in NSCLC treatment, including several issues that may lead to treatment resistance, such as mutations, molecular rearrangement, and conformational changes in ATP-binding domains (Sreedurgalakshmi et al. 2021). Therefore, CRISPR holds promise in different strategies, such as drug target validation and screening, generating mutant cell models, and discovering new targets for chemotherapy.
Today, one of the most common approaches in cancer research is investigating the roles of specific enzymes and domains in tumour growth to find new therapeutic targets to support chemotherapy. CRISPR is efficient in overcoming acquired drug resistance, as indicated by mutations in EGFR, KRAS, NRAS, B-RAF, and other genes. Therefore, it is necessary to investigate mutations responsible for modulating the sensitivity of these targets (Tsukumo et al. 2020; Terai et al. 2021; Floc’h et al. 2018; Park et al. 2021; Raoof et al. 2019; Gammelgaard et al. 2019; Wang et al. 2016; Caiola et al. 2020; Guerra et al. 2020).
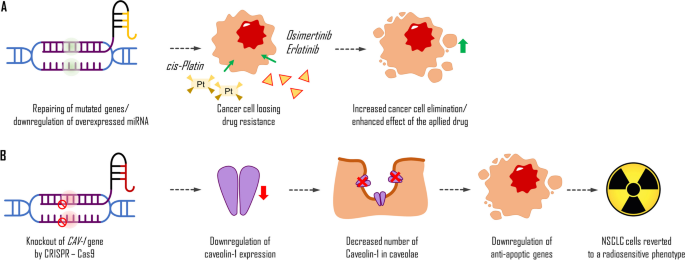
Most patients diagnosed with advanced stages of NSCLC with EGFR mutations are treated with TKIs, but they often develop secondary resistance to drugs such as dasatinib, erlotinib, and osimertinib. Studies have shown the potential of the CRISPR system to overcome these issues or predict therapeutic outcomes by revealing genes and mediators, such as microRNA, responsible for drug sensitivity (Fig. 4A). For example, the efficacy of dasatinib may be evaluated by revealing molecular alterations of YES1 (Garmendia et al. 2019). Erlotinib resistance might be caused by the presence of microRNA-214 (miR-214) or depletion of insulin-like growth factor 1 receptor (Liao et al. 2017; Hussmann et al. 2017). Acquired resistance to osimertinib might be overcome with ERK inhibition (Li et al. 2020). Enforced expression of miR-204 can downregulate caveolin-1 (CAV-1) expression and resensitise NSCLC cells for cisplatin, another commonly used drug that interferes with DNA replication in fast-proliferating cells.
Potential application of Crisp-Cas system in the treatment of NSCLC. Increasing the possibilities of drugs used in the cancer treatment (A); caveolin-1 is a structural protein in caveolae. Its overexpression in cancer cells leads to the upregulation of anti-apoptotic genes. Thus, the knockout of the caveolin-1 (CAV-1) gene by CRISPR-Cas9-mediated gene editing leads to a decrease in its synthesis and a decrease in its number in caveolae. This process causes the downregulation of anti-apoptotic gene transcription in targeted NSCLC cells which reverts them to be sensitive to radiation (B)
Moreover, the CRISPR-Cas system can also be used to improve more traditional treatment methods, such as radiotherapy, by targeting and suppressing factors responsible for radio-resistance, such as focal adhesion kinase in KRAS-mutant cells or gene knockout, which might revert NSCLC cells to a radiosensitive phenotype (Leiser et al. 2021; Moreno Roig et al. 2019; Tang et al. 2016). Recent research indicates that CAV-1 is highly expressed in some NSCLC cells, and the CRISPR-Cas9 knockout of that protein’s gene can revert such cells to a radiosensitive phenotype (Fig. 4B) (Leiser et al. 2021).
Overall, CRISPR has demonstrated great potential in different strategies such as drug target validation and screening, generating mutant cell models, discovering new targets for chemotherapy, and improving traditional treatment methods like radiotherapy. Further research using the CRISPR system could provide valuable insights into the mechanisms of cancer and help develop more effective therapies for NSCLC. The abovementioned studies were all pre-clinical and conducted mostly in vitro using human cell models or in vivo on mouse models incorporating xenografts. Such mouse xenograft models involve the transplantation of cells, e.g. modified by CRISPR-Cas or human tumour cells into immunodeficient or humanised mice.
Recent research has shown the potential of CRISPR in improving diagnostic tools for NSCLC, such as next-generation sequencing (NGS). Wang et al. have demonstrated the benefits of using the CRISPR-Cas9 system in the early detection of circulating tumour DNA in NSCLC (Wang et al. 2020b). This is crucial because late diagnosis is a significant challenge in treating this neoplasm. The ultimate treatment goal is to halt tumour metastasis, particularly to the brain, which is associated with a poor prognosis. The activated leukocyte cell adhesion molecule has been identified as a critical factor in brain metastasis formation, and its gene knockout using CRISPR has been suggested as a new therapeutic target (Justine et al. 2020). Other factors, such as myocardin, have been shown to promote pathways that enhance the epithelial-mesenchymal transition of NSCLC cells (Tong et al. 2020). Prior to the publication by Sreedurgalakshmi et al., only in vitro or in vivo studies using mouse models had been published. In 2020, the results of the first-in-human phase I clinical trial of CRISPR–Cas9 PD-1-edited T cells in patients with advanced NSCLC were reported (Lu et al. 2020). Twelve patients whose tumours were unresponsive to other available therapies were treated using this approach. The results showed that the treatment was generally safe and feasible, with a low frequency of off-target mutations (median 0.05%) (Lu et al. 2020).
To conclude, as indicated by the aforementioned reports, CRISPR-Cas9 technology holds great promise in the field of cancer research and treatment. It can contribute significantly to our understanding of cancer genomics by enabling the creation of genetically modified models of cancer within the laboratory setting. These models, in turn, aid researchers in comprehending the genetic mutations and molecular pathways underpinning various cancer types. This knowledge is pivotal in the development of targeted therapies. Furthermore, CRISPR-Cas9 can be employed to precisely target and edit the genes responsible for cancer initiation or progression. This capability has the potential to deactivate oncogenes or activate tumour suppressor genes, thus presenting an innovative approach to cancer treatment. Moreover, CRISPR technology can be effectively utilised to enhance immunotherapy by genetically modifying immune cells, such as T cells, to improve their efficiency in recognising and combating cancer cells. Another valuable application lies in addressing the significant challenge of drug resistance in cancer treatment. CRISPR technology plays a crucial role in elucidating how cancer cells develop resistance to chemotherapy or targeted therapies. This understanding can pave the way for the development of strategies to overcome drug resistance, ultimately enhancing the efficacy of cancer treatments. Additionally, this approach has the potential to usher in the era of personalised medicine. CRISPR-Cas9 could facilitate the development of personalised cancer treatments by analysing a patient’s tumour DNA and utilising CRISPR to target specific genetic mutations. This allows for the tailoring of treatments to align with an individual’s unique cancer profile. Lastly, in certain cases, genetic mutations directly contribute to cancer development. In such instances, CRISPR-Cas9 can be effectively applied in gene therapy approaches to correct these mutations or replace faulty genes, potentially leading to a genetic-level cure for cancer.
The convergence of cutting-edge technology and cancer research presents a promising future for more effective and personalised cancer treatments.
Nanoparticles
Nanotechnology is rapidly becoming one of the most promising therapeutic strategies for treating widespread diseases such as diabetes, bacterial infections, cardiovascular diseases, and cancer (Mukherjee et al. 2019). Nanoparticles have been utilised for diagnosis and treatment, including active drug delivery and CRISPR-Cas systems (Mukherjee et al. 2019). Mukherjee et al. identified several nanomaterials, such as liposomal, polymeric, metal, and bio-nanoparticles, with promising theranostic effects in their 2019 study. These materials have been shown to improve drug delivery by increasing stability and site specificity, reducing the toxic effect on non-cancerous cells, and enabling a reduction in dosage frequency (Ngema et al. 2021). These benefits are significant for developing the CRISPR-Cas system for in vivo delivery and activation. When it comes to NSCLC treatment, albumin-based nanoparticles are currently being utilised across various stages of clinical trials to transport paclitaxel and cisplatin.
Three groups of carriers can be used for CRISPR-Cas delivery: biological, physical, and chemical. Among the chemical group, nanoparticles such as gold nanoparticles (AuNPs) and lipid nanoparticles (LNPs) have been demonstrated as a possible approach to the treatment of NSCLC (Taha et al. 2022). This strategy is expected to overcome the limitations of biological and physical methods, which can be immunogenic or toxic and mainly limited to ex vivo manipulations. AuNPs are bound with thiol-modified oligonucleotides, hybridised with a single-stranded donor oligonucleotide, complexed with Cas9 RNP, and coated with the endosomal disruptive polymer PAsp, which allows specific cell internalisation (Taha et al. 2022). However, AuNPs have a low level of HDR system induction.
Meanwhile, LNP technology is becoming increasingly popular due to its effectiveness in RNA-based vaccines against COVID-19. The method employs a combination of four types of lipids: cationizable (or ionisable) lipid, PEG lipid, helper phospholipid, and cholesterol. Its pH dependency and lack of proteins or peptides are thought to be responsible for its lower toxic or immunogenic level. Following the advancement of LNP technology, the first phase 1 clinical trial employing LNP-CRISPR took place in 2020 and a targeted organ was a human liver. Thus, such an approach holds promise for extending the scope of organs and genetic conditions amenable to targeting.
Given that most patients with NSCLC are diagnosed at advanced stages and develop secondary resistance to therapeutic methods, the development of both CRISPR and nanoparticle technology is essential for improving treatment outcomes.
Conclusion
Conventional therapies, including surgery, radiotherapy, and chemotherapy, are still the primary treatments for NSCLC. However, despite significant development and improvement in these treatments, OS and PFS remain unsatisfactory, especially for more advanced stages of cancer. Therefore, developing new and more advanced therapies and studying them is crucial.
Emerging experimental methods in medicine, such as CRISPR and nanotechnology, are essential for developing highly personalised therapies. The CRISPR-Cas9 and Cas12a systems provide a variety of approaches in both therapeutic and diagnostic processes. It is worth mentioning that CRISPR can be used for drug target validation, generating mutant cell models, discovering new targets for chemotherapy, and developing screening tests. It is significant that CRISPR-Cas9 already shows promising results in in-human clinical trials using modified T cells of patients. Nanomedicine focuses on improving drug delivery systems, but its application extends to the precise transport of the abovementioned genome-editing tools. However, more comprehensive studies are required to evaluate the safety and efficacy of these techniques.
Immunotherapy has gained popularity over the years and has become one of the most promising approaches to NSCLC treatment. It provides an increase in PFS and OS while still being relatively safe for most patients. Its nature incorporates a patient’s immune system instead of relying on aggressive cytotoxic drugs, allowing combination with other primary NSCLC therapies, such as chemotherapy.
Conventional therapies are based on resecting a tumour or using cytotoxic agents. In contrast, modern methodology targets the cause of tumorigenesis, resulting in a lower impact on surrounding non-cancerous tissue. Current technology and its continuous improvement allow for a diverse approach to LC care. Combining well-established and innovative forms of treatment enables oncologists to exploit individual variables, such as mutations or genetic landscapes, and increase a patient’s chance of survival. Future research should focus on integrating these forms of treatment and taking advantage of knowledge about another molecular pathway for challenging of lung tumour development.
Abbreviations
- AE:
-
Adverse effect
- ALK:
-
Anaplastic lymphoma kinase
- AuNP:
-
Gold nanoparticle
- B-RAFi:
-
B-RAF inhibitors
- CRISPR:
-
Clustered regularly interspaced short palindromic repeat
- CTLA-4:
-
Cytotoxic T-lymphocyte associated protein-4
- EGFR:
-
Epidermal growth factor receptor
- HDR:
-
Homology-directed repair
- HIF:
-
Hypoxia-induced factor
- ICI:
-
Immune checkpoint inhibitor
- IGRT:
-
Image-guided radiotherapy
- KRAS:
-
Kirsten rat sarcoma virus
- LC:
-
Lung cancer
- LNP:
-
Lipid nanoparticle
- LUAD:
-
Adenocarcinoma of the lung
- mAb:
-
Monoclonal antibody
- MEKi:
-
MEK inhibitors
- NGS:
-
Next-generation sequencing
- NHEJ:
-
Non-homologous end-joining
- NSCLC:
-
Non-small cell lung cancer/carcinoma
- ORR:
-
Objective response rate
- OS:
-
Overall survival
- PD-1:
-
Programmed death receptor-1
- PD-L1:
-
Programmed death ligand-1
- PD-L2:
-
Programmed death ligand-2
- PFS:
-
Progression-free survival
- PS:
-
Performance status
- RATS:
-
Robot-assisted thoracic surgery
- RGRT:
-
Respiratory-gated radiotherapy
- SBRT:
-
Stereotactic body radiation therapy
- TK:
-
Tyrosine kinase
- TKI:
-
Tyrosine kinase inhibitor
- TSG:
-
Tumor suppressor gene
- VATS:
-
Video-assisted thoracic surgery
- VEGF:
-
Vascular endothelial growth factor
- VEGFR:
-
Vascular endothelial growth factor receptor
References
Abdelgalil AA, Al-Kahtani HM, Al-Jenoobi FI (2020) Erlotinib. Profiles Drug Subst Excip Relat Methodol 45:93–117. https://doi.org/10.1016/BS.PODRM.2019.10.004
Aguiar D, Aguiar J, Bohn U (2005) Alternating weekly administration of paclitaxel and gemcitabine: a phase II study in patients with advanced non-small-cell lung cancer. Cancer Chemother Pharmacol 55(2):152–158. https://doi.org/10.1007/S00280-004-0897-8
Akopyan G, Bonavida B (2006) Understanding tobacco smoke carcinogen NNK and lung tumorigenesis (review). Int J Oncol 29(4):745–752. https://doi.org/10.3892/IJO.29.4.745/HTML
Baik CS, Myall NJ, Wakelee HA (2017) Targeting BRAF-mutant nonsmall cell lung cancer: from molecular profiling to rationally designed therapy. Oncologist 22(7):786–796. https://doi.org/10.1634/THEONCOLOGIST.2016-0458
Binder D et al (2007) Docetaxel/gemcitabine or cisplatin/gemcitabine followed by docetaxel in the first-line treatment of patients with metastatic nonsmall cell lung cancer (NSCLC): results of a multicentre randomised phase II trial. Cancer Chemother Pharmacol 60(1):143–150. https://doi.org/10.1007/S00280-006-0358-7
Bonnesen B, Pappot H, Holmstav J, Skov BG (2009) Vascular endothelial growth factor A and vascular endothelial growth factor receptor 2 expression in nonsmall cell lung cancer patients: relation to prognosis. Lung Cancer 66(3):314–318. https://doi.org/10.1016/J.LUNGCAN.2009.02.013
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6):394–424. https://doi.org/10.3322/CAAC.21492
Caiola E et al (2020) LKB1 deficiency renders NSCLC cells sensitive to ERK inhibitors. J Thorac Oncol 15(3):360–370. https://doi.org/10.1016/J.JTHO.2019.10.009
Camidge DR et al (2018) Brigatinib versus crizotinib in ALK-positive non-small-cell lung cancer. N Engl J Med 379(21):2027–2039. https://doi.org/10.1056/NEJMOA1810171
Camidge DR et al (2019) Updated efficacy and safety data and impact of the EML4-ALK fusion variant on the efficacy of alectinib in untreated ALK-positive advanced nonsmall cell lung cancer in the global phase III ALEX Study. J Thorac Oncol 14(7):1233–1243. https://doi.org/10.1016/j.jtho.2019.03.007
Camidge DR et al (2020) Brigatinib versus crizotinib in advanced ALK inhibitor-naive ALK-positive nonsmall cell lung cancer: second interim analysis of the phase III ALTA-1L trial. J Clin Oncol 38(31):3592–3603. https://doi.org/10.1200/JCO.20.00505
Carmeliet P (2000) Mechanisms of angiogenesis and arteriogenesis. Nat Med 6(4):389–395. https://doi.org/10.1038/74651
Carmeliet P (2005) VEGF as a key mediator of angiogenesis in cancer. Oncology 69 Suppl 3(SUPPL. 3):4–10. https://doi.org/10.1159/000088478
Castellanos E, Feld E, Horn L (2017) Driven by mutations: the predictive value of mutation subtype in EGFR-mutated non–small cell lung cancer. J Thorac Oncol 12(4):612–623. https://doi.org/10.1016/J.JTHO.2016.12.014
Cébe-Suarez S, Zehnder-Fjällman A, Ballmer-Hofer K (2006) The role of VEGF receptors in angiogenesis; complex partnerships. Cell Mol Life Sci 63(5):601–615. https://doi.org/10.1007/S00018-005-5426-3
Chang JY et al (2008) Image-guided radiation therapy for nonsmall cell lung cancer. J Thorac Oncol 3(2):177–186. https://doi.org/10.1097/JTO.0B013E3181622BDD
Chiappori A et al (2005) Phase II study of first-line sequential chemotherapy with gemcitabine-carboplatin followed by docetaxel in patients with advanced nonsmall cell lung cancer. Oncology 68(4–6):382–390. https://doi.org/10.1159/000086979
Clark JI et al (2001) Pilot study of sequential vinorelbine and cisplatin followed by docetaxel for selected IIIB and stage IV nonsmall cell lung cancer. Lung Cancer 34(2):271–277. https://doi.org/10.1016/S0169-5002(01)00251-3
Cohen MH, Johnson JR, Chen Y-F, Sridhara R, Pazdur R (2005) FDA drug approval summary: erlotinib (Tarceva) tablets. Oncologist 10(7):461–466. https://doi.org/10.1634/THEONCOLOGIST.10-7-461
Conforti F et al (2018) Cancer immunotherapy efficacy and patients’ sex: a systematic review and meta-analysis. Lancet Oncol 19(6):737–746. https://doi.org/10.1016/S1470-2045(18)30261-4
Cristescu R et al (2018) Pan-tumor genomic biomarkers for PD-1 checkpoint blockade-based immunotherapy. Science 362(6411). https://doi.org/10.1126/SCIENCE.AAR3593
De Castro J et al (2002) Sequential combination chemotherapy in patients with advanced nonsmall cell lung carcinoma. Carboplatin and gemcitabine followed by paclitaxel. Cancer 94(10):2797–2798. https://doi.org/10.1002/CNCR.10514
Dela Cruz CS, Tanoue LT, Matthay RA (2011) Lung cancer: epidemiology, etiology, and prevention. Clin Chest Med 32(4):605–644. https://doi.org/10.1016/j.ccm.2011.09.001
Dobosz P and Dzieciątkowski T (2019) The intriguing history of cancer immunotherapy. Front Immunol. https://doi.org/10.3389/FIMMU.2019.02965
Dogan S et al (2012) Molecular epidemiology of EGFR and KRAS mutations in 3026 lung adenocarcinomas: higher susceptibility of women to smoking-related KRAS-mutant cancers. Clin Cancer Res 18(22):6169. https://doi.org/10.1158/1078-0432.CCR-11-3265
Donovan EK, Swaminath A (2018) Stereotactic body radiation therapy (SBRT) in the management of non-small-cell lung cancer: clinical impact and patient perspectives. Lung Cancer (auckl) 9:13–23. https://doi.org/10.2147/LCTT.S129833
Du X, Shao Y, Qin HF, Tai YH, Gao HJ (2018) ALK-rearrangement in non-small-cell lung cancer (NSCLC). Thorac Cancer 9(4):423–430. https://doi.org/10.1111/1759-7714.12613
Dudley AC (2012) Tumor endothelial cells. Cold Spring Harb Perspect Med 2(3). https://doi.org/10.1101/CSHPERSPECT.A006536
Duffy AM, Bouchier-Hayes DJ, Harmey JH (2013) Vascular endothelial growth factor (VEGF) and its role in non-endothelial cells: autocrine signalling by VEGF. Accessed: Jun. 06, 2023. [Online]. Available: https://www.ncbi.nlm.nih.gov/books/NBK6482/
Dugay F et al (2017) Clinicopathological characteristics of ROS1- and RET-rearranged NSCLC in caucasian patients: data from a cohort of 713 non-squamous NSCLC lacking KRAS/EGFR/HER2/BRAF/PIK3CA/ALK alterations. Oncotarget 8(32):53336. https://doi.org/10.18632/ONCOTARGET.18408
Dziedzic DA, Zbytniewski M, Gryszko GM, Cackowski MM, Langfort R, Orlowski TM (2021) Video-assisted versus open thoracotomy lobectomy: comparison on lymphadenectomy and survival in early stage of lung cancer. J Thorac Dis 13(1):101–112. https://doi.org/10.21037/JTD-20-2251
Edelman MJ et al (2004) Randomised phase II trial of sequential chemotherapy in advanced nonsmall cell lung cancer (SWOG 9806): carboplatin/gemcitabine followed by paclitaxel or cisplatin/vinorelbine followed by docetaxel. Clin Cancer Res 10(15):5022–5026. https://doi.org/10.1158/1078-0432.CCR-04-0002
Feng C et al (2022) A simple and highly sensitive naked-eye analysis of EGFR 19del via CRISPR/Cas12a triggered no-nonspecific nucleic acid amplification. ACS Synth Biol 11(2):867–876. https://doi.org/10.1021/ACSSYNBIO.1C00521
Ferrara N (2004) Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev 25(4):581–611. https://doi.org/10.1210/ER.2003-0027
Ferrara N, Gerber HP, LeCouter J (2003) The biology of VEGF and its receptors. Nat Med 9(6):669–676. https://doi.org/10.1038/NM0603-669
Floc’h N et al (2018) Antitumor activity of osimertinib, an irreversible mutant-selective EGFR tyrosine kinase inhibitor, in NSCLC harboring EGFR exon 20 insertions. Mol Cancer Ther 17(5):885–896. https://doi.org/10.1158/1535-7163.MCT-17-0758
Formenti SC et al (2018) Radiotherapy induces responses of lung cancer to CTLA-4 blockade. Nat Med 24(12):1845–1851. https://doi.org/10.1038/S41591-018-0232-2
Gammelgaard KR et al (2019) Up-regulated FGFR1 expression as a mediator of intrinsic TKI resistance in EGFR-mutated NSCLC. Transl Oncol 12(3):432–440. https://doi.org/10.1016/J.TRANON.2018.11.017
Garcia J et al (2020) Bevacizumab (Avastin®) in cancer treatment: a review of 15 years of clinical experience and future outlook. Cancer Treat Rev 86:102017. https://doi.org/10.1016/J.CTRV.2020.102017
Garmendia I et al (2019) YES1 drives lung cancer growth and progression and predicts sensitivity to dasatinib. Am J Respir Crit Care Med 200(7):888–899. https://doi.org/10.1164/RCCM.201807-1292OC
Garon EB et al (2014) Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet 384(9944):665–673. https://doi.org/10.1016/S0140-6736(14)60845-X
Gelatti ACZ, Drilon A, Santini FC (2019) Optimising the sequencing of tyrosine kinase inhibitors (TKIs) in epidermal growth factor receptor (EGFR) mutation-positive nonsmall cell lung cancer (NSCLC). Lung Cancer 137:113–122. https://doi.org/10.1016/J.LUNGCAN.2019.09.017
Gerber DE, Schiller JH (2013) Maintenance chemotherapy for advanced non-small-cell lung cancer: new life for an old idea. J Clin Oncol 31(8):1009–1020. https://doi.org/10.1200/JCO.2012.43.7459
Giraud P et al (2011) Respiratory gating techniques for optimisation of lung cancer radiotherapy. J Thorac Oncol 6(12):2058–2068. https://doi.org/10.1097/JTO.0B013E3182307EC2
Godwin JL et al (2017) Nivolumab-induced autoimmune diabetes mellitus presenting as diabetic ketoacidosis in a patient with metastatic lung cancer. J Immunother Cancer 5(1). https://doi.org/10.1186/S40425-017-0245-2
Gridelli C et al (2004) Treatment of advanced non-small-cell lung cancer patients with ECOG performance status 2: results of an European Experts Panel. Ann Oncol 15(3):419–426. https://doi.org/10.1093/ANNONC/MDH087
Gridelli C et al (2007) Single-agent pemetrexed or sequential pemetrexed/gemcitabine as front-line treatment of advanced nonsmall cell lung cancer in elderly patients or patients ineligible for platinum-based chemotherapy: a multicenter, randomised, phase II trial. J Thorac Oncol 2(3):221–229. https://doi.org/10.1097/JTO.0B013E318031CD62
Grossi F et al (2004) Sequential chemotherapy with paclitaxel plus cisplatin, followed by vinorelbine, followed by gemcitabine in advanced nonsmall cell lung cancer: an Alpe-Adria Thoracic Oncology Multidisciplinary group study (ATOM 001). Lung Cancer 46(1):99–106. https://doi.org/10.1016/j.lungcan.2004.03.003
Grossi F et al (2007) Sequential, alternating, and maintenance/consolidation chemotherapy in advanced nonsmall cell lung cancer: a review of the literature. Oncologist 12(4):451–464. https://doi.org/10.1634/THEONCOLOGIST.12-4-451
Guerra SL et al (2020) A deregulated HOX gene axis confers an epigenetic vulnerability in KRAS-mutant lung cancers. Cancer Cell 37(5):705-719.e6. https://doi.org/10.1016/J.CCELL.2020.03.004
Guo F, Ma D, Li S, Adamek M (2019) Compare the prognosis of Da Vinci robot-assisted thoracic surgery (RATS) with video-assisted thoracic surgery (VATS) for nonsmall cell lung cancer: A Meta-analysis. Medicine 98(39):e17089. https://doi.org/10.1097/MD.0000000000017089
Hamrah P, Chen L, Cursiefen C, Zhang Q, Joyce NC, Dana MR (2004) Expression of vascular endothelial growth factor receptor-3 (VEGFR-3) on monocytic bone marrow-derived cells in the conjunctiva. Exp Eye Res 79(4):553–561. https://doi.org/10.1016/J.EXER.2004.06.028
Holla VR et al (2017) ALK: a tyrosine kinase target for cancer therapy. Cold Spring Harb Mol Case Stud 3(1):a001115. https://doi.org/10.1101/MCS.A001115
Holm C, Gineitis D, McConville GS, Kazlauskas A (1996) Expression of PDGF, VEGF and their receptors in nonsmall cell lung tumor cell lines. Int J Oncol 9(5):1077–1086. https://doi.org/10.3892/IJO.9.5.1077
Hosoe S et al (2003) Gemcitabine and vinorelbine followed by docetaxel in patients with advanced non-small-cell lung cancer: a multi-institutional phase II trial of nonplatinum sequential triplet combination chemotherapy (JMTO LC00-02). Br J Cancer 88(3):342–347. https://doi.org/10.1038/SJ.BJC.6600723
Huang MY, Jiang XM, Wang BL, Sun Y, Lu JJ (2021) Combination therapy with PD-1/PD-L1 blockade in nonsmall cell lung cancer: strategies and mechanisms. Pharmacol Ther 219:107694. https://doi.org/10.1016/J.PHARMTHERA.2020.107694
huiJia X et al (2020) Efficacy and safety of neoadjuvant immunotherapy in resectable nonsmall cell lung cancer: a meta-analysis. Lung Cancer 147:143–153. https://doi.org/10.1016/J.LUNGCAN.2020.07.001
Hussmann D, Madsen AT, Jakobsen KR, Luo Y, Sorensen BS, Nielsen AL (2017) IGF1R depletion facilitates MET-amplification as mechanism of acquired resistance to erlotinib in HCC827 NSCLC cells. Oncotarget 8(20):33300–33315. https://doi.org/10.18632/ONCOTARGET.16350
Iwama S, Arima H (2017) Clinical practice and mechanism of endocrinological adverse events associated with immune checkpoint inhibitors. Nihon Rinsho Meneki Gakkai Kaishi 40(2):90–94. https://doi.org/10.2177/JSCI.40.90
Johnson DH et al (2004) Randomised phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol 22(11):2184–2191. https://doi.org/10.1200/JCO.2004.11.022
Judd J et al (2021) Characterisation of KRAS mutation subtypes in nonsmall cell lung cancer. https://doi.org/10.1158/1535-7163.MCT-21-0201
Justine M et al (2020) ALCAM contributes to brain metastasis formation in non-small-cell lung cancer through interaction with the vascular endothelium. Neuro Oncol 22(7):955–966. https://doi.org/10.1093/NEUONC/NOAA028
Kamba T, McDonald DM (2007) Mechanisms of adverse effects of anti-VEGF therapy for cancer. Br J Cancer 96(12):1788–1795. https://doi.org/10.1038/SJ.BJC.6603813
Kepka L, Socha J (2021) Dose and fractionation schedules in radiotherapy for nonsmall cell lung cancer. Transl Lung Cancer Res 10(4):1969–1982. https://doi.org/10.21037/TLCR-20-253
Kilburn JM et al (2016) Image guided radiotherapy may result in improved local control in locally advanced lung cancer patients. Pract Radiat Oncol 6(3):e73. https://doi.org/10.1016/J.PRRO.2015.10.004
Kowanetz M, Ferrara N (2006) Vascular endothelial growth factor signaling pathways: therapeutic perspective. Clin Cancer Res 12(17):5018–5022. https://doi.org/10.1158/1078-0432.CCR-06-1520
Langevin SM, Kratzke RA, Kelsey KT (2015) Epigenetics of lung cancer. Transl Res 165(1):74–90. https://doi.org/10.1016/J.TRSL.2014.03.001
Larsen JE, Cascone T, Gerber DE, Heymach JV, Minna JD (2011) Targeted therapies for lung cancer: clinical experience and novel agents. Cancer J 17(6):512–527. https://doi.org/10.1097/PPO.0B013E31823E701A
Lee JH, Hong JI, Kim HK (2021) Robot-assisted thoracic surgery in nonsmall cell lung cancer. J Chest Surg 54(4):266. https://doi.org/10.5090/JCS.21.070
Leiser D et al (2021) Role of caveolin-1 as a biomarker for radiation resistance and tumor aggression in lung cancer. PLoS One 16(11):e0258951. https://doi.org/10.1371/JOURNAL.PONE.0258951
Leonetti A, Sharma S, Minari R, Perego P, Giovannetti E, Tiseo M (2019) Resistance mechanisms to osimertinib in EGFR-mutated nonsmall cell lung cancer. Br J Cancer 121(9):725. https://doi.org/10.1038/S41416-019-0573-8
Li H et al (2014) Addition of bevacizumab enhances antitumor activity of erlotinib against nonsmall cell lung cancer xenografts depending on VEGF expression. Cancer Chemother Pharmacol 74(6):1297–1305. https://doi.org/10.1007/S00280-014-2610-X
Li Y, Zang H, Qian G, Owonikoko TK, Ramalingam SR, Sun SY (2020) ERK inhibition effectively overcomes acquired resistance of epidermal growth factor receptor-mutant nonsmall cell lung cancer cells to osimertinib. Cancer 126(6):1339–1350. https://doi.org/10.1002/CNCR.32655
Liao J et al (2017) Down-regulation of miR-214 reverses erlotinib resistance in non-small-cell lung cancer through up-regulating LHX6 expression. Sci Rep 7(1). https://doi.org/10.1038/S41598-017-00901-6
Lohela M, Bry M, Tammela T, Alitalo K (2009) VEGFs and receptors involved in angiogenesis versus lymphangiogenesis. Curr Opin Cell Biol 21(2):154–165. https://doi.org/10.1016/J.CEB.2008.12.012
Lu Y et al (2020) Safety and feasibility of CRISPR-edited T cells in patients with refractory non-small-cell lung cancer. Nat Med 26(5):732–740. https://doi.org/10.1038/S41591-020-0840-5
Ma J, Li X, Zhao S, Wang J, Zhang W, Sun G (2021) Robot-assisted thoracic surgery versus video-assisted thoracic surgery for lung lobectomy or segmentectomy in patients with nonsmall cell lung cancer: a meta-analysis. BMC Cancer 21(1). https://doi.org/10.1186/S12885-021-08241-5
Mao Y, Wu S (2017a) ALK and ROS1 concurrent with EGFR mutation in patients with lung adenocarcinoma. Onco Targets Ther 10:3399. https://doi.org/10.2147/OTT.S133349
Mao Y, Wu S (2017b) OncoTargets and Therapy Dovepress ALK and ROS1 concurrent with EGFR mutation in patients with lung adenocarcinoma. Onco Targets Ther 10:3399. https://doi.org/10.2147/OTT.S133349
Masters GA et al (2015) Systemic therapy for stage IV non-small-cell lung cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol 33(30):3488–3515. https://doi.org/10.1200/JCO.2015.62.1342
Mattson K et al (2000) Phase II study of docetaxel alternating with cisplatin in chemotherapy-naïve patients with advanced nonsmall cell lung cancer. Anticancer Drugs 11(1):7–13. https://doi.org/10.1097/00001813-200001000-00002
McKeage K (2015) Alectinib: a review of its use in advanced ALK-rearranged nonsmall cell lung cancer. Drugs 75(1):75–82. https://doi.org/10.1007/S40265-014-0329-Y
Mehta A, Dobersch S, Romero-Olmedo AJ, Barreto G (2015) Epigenetics in lung cancer diagnosis and therapy. Cancer Metastasis Rev 34(2):229–241. https://doi.org/10.1007/S10555-015-9563-3
Moreno Roig E et al (2019) HIF-1α and HIF-2α differently regulate the radiation sensitivity of NSCLC cells. Cells 8(1):45. https://doi.org/10.3390/CELLS8010045
Moro-Sibilot D et al (2019) Crizotinib in c-MET- or ROS1-positive NSCLC: results of the AcSé phase II trial. Ann Oncol 30(12):1985–1991. https://doi.org/10.1093/ANNONC/MDZ407
Mukherjee A, Paul M, Mukherjee S (2019) Recent progress in the theranostics application of nanomedicine in lung cancer. Cancers (Basel) 11(5):597. https://doi.org/10.3390/cancers11050597
Nakagawa K et al (2019) Ramucirumab plus erlotinib in patients with untreated, EGFR-mutated, advanced non-small-cell lung cancer (RELAY): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 20(12):1655–1669. https://doi.org/10.1016/S1470-2045(19)30634-5
Nasser NJ, Gorenberg M, Agbarya A (2020) First line Immunotherapy for nonsmall cell lung cancer. Pharmaceuticals (Basel) 13(11):1–24. https://doi.org/10.3390/PH13110373
Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z (1999) Vascular endothelial growth factor (VEGF) and its receptors. FASEB J 13(1):9–22. https://doi.org/10.1096/FASEBJ.13.1.9
Ngema LM, Adeyemi SA, Marimuthu T, Choonara YE (2021) A review on engineered magnetic nanoparticles in non-small-cell lung carcinoma targeted therapy. Int J Pharm 606:120870. https://doi.org/10.1016/j.ijpharm.2021.120870
O’Leary CG et al (2019) Targeting BRAF mutations in nonsmall cell lung cancer. Transl Lung Cancer Res 8(6):1119–1124. https://doi.org/10.21037/TLCR.2019.10.22
Oiseth SJ, Aziz MS (2017) Cancer immunotherapy: a brief review of the history, possibilities, and challenges ahead. J Cancer Metastasis Treat 3(10):250–261. https://doi.org/10.20517/2394-4722.2017.41
Park HR et al (2021) Acquired resistance to third-generation EGFR tyrosine kinase inhibitors in patients with de novo EGFR T790M-mutant NSCLC. J Thorac Oncol 16(11):1859–1871. https://doi.org/10.1016/J.JTHO.2021.06.013
Paul B, Montoya G (2020) CRISPR-Cas12a: functional overview and applications. Biomed J 43(1):8–17. https://doi.org/10.1016/J.BJ.2019.10.005
Piperdi B, Merla A, Perez-Soler R (2014) Targeting angiogenesis in squamous nonsmall cell lung canceR. Drugs 74(4):403. https://doi.org/10.1007/S40265-014-0182-Z
Planchard D et al (2017) Dabrafenib plus trametinib in patients with previously untreated BRAFV600E-mutant metastatic non-small-cell lung cancer: an open-label, phase 2 trial. Lancet Oncol 18(10):1307–1316. https://doi.org/10.1016/S1470-2045(17)30679-4
Puzanov I et al (2017) Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer 5(1). https://doi.org/10.1186/S40425-017-0300-Z
Raoof S et al (2019) Targeting FGFR overcomes EMT-mediated resistance in EGFR mutant nonsmall cell lung cancer. Oncogene 38(37):6399–6413. https://doi.org/10.1038/S41388-019-0887-2
Reck M et al (2021) Five-year outcomes with pembrolizumab versus chemotherapy for metastatic non-small-cell lung cancer with PD-L1 tumor proportion score ≥ 50. J Clin Oncol 39(21):2339–2349. https://doi.org/10.1200/JCO.21.00174
Reck M et al (2021) First-line nivolumab plus ipilimumab with two cycles of chemotherapy versus chemotherapy alone (four cycles) in advanced non-small-cell lung cancer: CheckMate 9LA 2-year update. ESMO Open 6(5). https://doi.org/10.1016/J.ESMOOP.2021.100273
Recondo G, Facchinetti F, Olaussen KA, Besse B, Friboulet L (2018) Making the first move in EGFR-driven or ALK-driven NSCLC: first-generation or next-generation TKI? Nat Rev Clin Oncol 15(11):694–708. https://doi.org/10.1038/S41571-018-0081-4
Rinaldi M et al (2005) Preliminary results of a multicenter randomised phase II trial of two different combinations of docetaxel (D) and gemcitabine (G) and of cisplatin/gemcitabine (CG) followed by docetaxel as first line therapy for locally advanced or metastatic non small cell lung cancer (NSCLC). https://doi.org/10.1200/jco.2005.23.16_suppl.7124 23(16_suppl):7124–7124. https://doi.org/10.1200/JCO.2005.23.16_SUPPL.7124
Rizvi NA et al (2015) Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in nonsmall cell lung cancer. Science 348(6230):124–128. https://doi.org/10.1126/SCIENCE.AAA1348
Rosen LS (2002) Clinical experience with angiogenesis signaling inhibitors: focus on vascular endothelial growth factor (VEGF) blockers. Cancer Control 9(2 Suppl):36–44. https://doi.org/10.1177/107327480200902S05
Rosenberg SA (2014) IL-2: the first effective immunotherapy for human cancer. J Immunol 192(12):5451–5458. https://doi.org/10.4049/JIMMUNOL.1490019
Rosenberg SA et al (1984) Biological activity of recombinant human interleukin-2 produced in Escherichia coli. Science 223(4643):1412–1415. https://doi.org/10.1126/SCIENCE.6367046
Rosenberg SA et al (1985) Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N Engl J Med 313(23):1485–1492. https://doi.org/10.1056/NEJM198512053132327
Rude Voldborg B, Damstrup L, Spang-Thomsen M, Skovgaard Poulsen H (1997) Epidermal growth factor receptor (EGFR) and EGFR mutations, function and possible role in clinical trials. Ann Oncol 8(12):1197–1206. https://doi.org/10.1023/A:1008209720526
Salgia R, Pharaon R, Mambetsariev I, Nam A, Sattler M (2021) The improbable targeted therapy: KRAS as an emerging target in nonsmall cell lung cancer (NSCLC). Cell Rep Med 2(1):100186. https://doi.org/10.1016/J.XCRM.2020.100186
Sander JD, Joung JK (2014) CRISPR-Cas systems for editing, regulating and targeting genomes. Nat Biotechnol 32(4):347–355. https://doi.org/10.1038/nbt.2842
Shaw AT et al (2014) Crizotinib in ROS1-rearranged non–small-cell lung cancer. N Engl J Med 371(21):1963. https://doi.org/10.1056/NEJMOA1406766
Shaw AT et al (2019) Crizotinib in ROS1-rearranged advanced non-small-cell lung cancer (NSCLC): updated results, including overall survival, from PROFILE 1001. Ann Oncol 30(7):1121. https://doi.org/10.1093/ANNONC/MDZ131
Siegel R, Ma J, Zou Z, Jemal A (2014) Cancer statistics, 2014. CA Cancer J Clin 64(1):9–29. https://doi.org/10.3322/CAAC.21208
Skobe M, Rockwell P, Goldstein N, Vosseler S, Fusenig NE (1997) Halting angiogenesis suppresses carcinoma cell invasion. Nat Med 3(11):1222–1227. https://doi.org/10.1038/NM1197-1222
Skoulidis F et al (2021) Sotorasib for lung cancers with KRAS p. G12C mutation. N Engl J Med 384(25):2371–2381. https://doi.org/10.1056/NEJMOA2103695
Socinski MA et al (2018) Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med 378(24):2288–2301. https://doi.org/10.1056/NEJMOA1716948
Soria J-C et al (2018) Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med 378(2):113–125. https://doi.org/10.1056/NEJMOA1713137
Sreedurgalakshmi K, Srikar R, Rajkumari R (2021) CRISPR-Cas deployment in nonsmall cell lung cancer for target screening, validations, and discoveries. Cancer Gene Ther 28(6):566–580. https://doi.org/10.1038/S41417-020-00256-7
Steven A, Fisher SA, Robinson BW (2016) Immunotherapy for lung cancer. Respirology 21(5):821–833. Blackwell Publishing. https://doi.org/10.1111/resp.12789
Sunderkotter C, Steinbrink K, Goebeler M, Bhardwaj R, Sorg C (1994) Macrophages and angiogenesis. J Leukoc Biol 55(3):410–422. https://doi.org/10.1002/JLB.55.3.410
Sung H et al (2021) Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3):209–249. https://doi.org/10.3322/CAAC.21660
Suresh K, Naidoo J, Lin CT, Danoff S (2018) Immune checkpoint immunotherapy for nonsmall cell lung cancer: benefits and pulmonary toxicities. Chest 154(6):1416–1423. https://doi.org/10.1016/J.CHEST.2018.08.1048
Taha EA, Lee J, Hotta A (2022) Delivery of CRISPR-Cas tools for in vivo genome editing therapy: trends and challenges. J Control Release 342:345–361. https://doi.org/10.1016/J.JCONREL.2022.01.013
Tandberg DJ, Tong BC, Ackerson BG, Kelsey CR (2018) Surgery versus stereotactic body radiation therapy for stage I nonsmall cell lung cancer: a comprehensive review. Cancer 124(4):667–678. https://doi.org/10.1002/CNCR.31196
Tang KJ et al (2016) Focal adhesion kinase regulates the DNA damage response and its inhibition radiosensitizes mutant KRAS lung cancer. Clin Cancer Res 22(23):5851–5863. https://doi.org/10.1158/1078-0432.CCR-15-2603
Taniguchi T et al (1983) Structure and expression of a cloned cDNA for human interleukin-2. Nature 302(5906):305–310. https://doi.org/10.1038/302305A0
Terai H et al (2021) SHOC2 is a critical modulator of sensitivity to EGFR-TKIs in nonsmall cell lung cancer cells. Mol Cancer Res 19(2):317–328. https://doi.org/10.1158/1541-7786.MCR-20-0664
Thandra KC, Barsouk A, Saginala K, Aluru JS, Barsouk A (2021) Epidemiology of lung cancer. Wspolczesna Onkologia 25(1):45–52. https://doi.org/10.5114/WO.2021.103829
Tong X et al (2020) MYOCD and SMAD3/SMAD4 form a positive feedback loop and drive TGF-β-induced epithelial-mesenchymal transition in nonsmall cell lung cancer. Oncogene 39(14):2890–2904. https://doi.org/10.1038/S41388-020-1189-4
Tsukumo Y, Naito M, Suzuki T (2020) Influence of EGFR-activating mutations on sensitivity to tyrosine kinase inhibitors in a KRAS mutant nonsmall cell lung cancer cell line. PLoS One 15(3):e0229712. https://doi.org/10.1371/JOURNAL.PONE.0229712
Uzel EK, Figen M, Uzel Ö (2019) Radiotherapy in lung cancer: current and future role. Sisli Etfal Hastan Tıp Bul 53(4):353. https://doi.org/10.14744/SEMB.2019.25991
Veronesi G (2015) Robotic lobectomy and segmentectomy for lung cancer: results and operating technique. J Thorac Dis 7(Suppl 2):S122. https://doi.org/10.3978/J.ISSN.2072-1439.2015.04.34
Veronesi G, Bianchi F, Inamura K (2017) Article 193 Citation: Inamura K (2017) Lung cancer: understanding its molecular pathology and the 2015 WHO classification. Front Oncol 7:193. https://doi.org/10.3389/fonc.2017.00193
Wang H et al (2016) Tankyrase inhibitor sensitizes lung cancer cells to endothelial growth factor receptor (EGFR) inhibition via stabilizing angiomotins and inhibiting YAP signaling. J Biol Chem 291(29):15256–15266. https://doi.org/10.1074/JBC.M116.722967
Wang L, Liu D, Lu J, Zhang S, Yang X (2017) The feasibility and advantage of uniportal video-assisted thoracoscopic surgery (VATS) in pulmonary lobectomy. BMC Cancer 17(1):1–7. https://doi.org/10.1186/S12885-017-3069-Z/FIGURES/2
Wang C et al (2020a) The landscape of immune checkpoint inhibitor plus chemotherapy versus immunotherapy for advanced non-small-cell lung cancer: a systematic review and meta-analysis. J Cell Physiol 235(5):4913–4927. https://doi.org/10.1002/JCP.29371
Wang L et al (2020b) Improved EGFR mutation detection sensitivity after enrichment by Cas9/sgRNA digestion and PCR amplification. Acta Biochim Biophys Sin (shanghai) 52(12):1316–1324. https://doi.org/10.1093/ABBS/GMAA123
Wang Z et al (2019) Video-assisted thoracoscopic surgery versus muscle-sparing thoracotomy for nonsmall cell lung cancer: a systematic review and meta-analysis. BMC Surg 19(1). https://doi.org/10.1186/S12893-019-0618-1
Wherry EJ, Kurachi M (2015) Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol 15(8):486–499. https://doi.org/10.1038/NRI3862
Wiedenheft B, Sternberg SH, Doudna JA (2012) RNA-guided genetic silencing systems in bacteria and archaea. Nature 482(7385):331–338. https://doi.org/10.1038/nature10886
Wu H, Jin R, Yang S, Park BJ, Li H (2021) Long-term and short-term outcomes of robot- versus video-assisted anatomic lung resection in lung cancer: a systematic review and meta-analysis. Eur J Cardiothorac Surg 59(4):732–740. https://doi.org/10.1093/EJCTS/EZAA426
Xia L, Liu Y, Wang Y (2019) PD-1/PD-L1 blockade therapy in advanced non-small-cell lung cancer: current status and future directions. Oncologist 24(S1):S31–S41. https://doi.org/10.1634/theoncologist.2019-IO-S1-s05
Yamazaki Y, Morita T (2006) Molecular and functional diversity of vascular endothelial growth factors. Mol Divers 10(4):515–527. https://doi.org/10.1007/S11030-006-9027-3
Zappa C, Mousa SA (2016) Nonsmall cell lung cancer: current treatment and future advances. Transl Lung Cancer Res 5(3):288–300. https://doi.org/10.21037/TLCR.2016.06.07
Zarogoulidis P et al (2017) Possible adverse effects of immunotherapy in nonsmall cell lung cancer; treatment and follow-up of three cases. Respir Med Case Rep 22:101–105. https://doi.org/10.1016/J.RMCR.2017.07.004
Zhang Y et al (2022) Platelet-derived PDGFB promotes recruitment of cancer-associated fibroblasts, deposition of extracellular matrix and Tgfβ signaling in the tumor microenvironment. Cancers (Basel) 14(8). https://doi.org/10.3390/CANCERS14081947/S1
Zhao Z, Gao Y, Xue Q, Gao S, He J (2021) Safety and efficacy of neoadjuvant immune checkpoint inhibitor therapy in patients with resectable non-small-cell lung cancer: a systematic review. Target Oncol 16(4):425–434. https://doi.org/10.1007/S11523-021-00818-1
Zhou C et al (2021) Camrelizumab plus carboplatin and pemetrexed versus chemotherapy alone in chemotherapy-naive patients with advanced non-squamous non-small-cell lung cancer (CameL): a randomised, open-label, multicentre, phase 3 trial. Lancet Respir Med 9(3):305–314. https://doi.org/10.1016/S2213-2600(20)30365-9
Author information
Authors and Affiliations
Contributions
Andrzej Jachowski: writing—original draft (“Introduction”), review and editing, literature searching; Mikołaj Marcinkowski: writing—original draft (“Immunotherapy”), review and editing, literature searching; Jakub Szydłowski: writing—original draft (“Pathway targeted therapy”), review and editing, literature searching; Oskar Grabarczyk: writing—original draft (“Introduction – conventional therapies”), literature searching; Zuzanna Nogaj: writing—original draft, conceptualization (“CRISPR-Cas”), literature searching; Marcin Łaz: writing—original draft (“Antiangiogenic therapy”), literature searching; Andrzej Pławski: writing—review and editing, supervision; Paweł Piotr Jagodziński: writing—review and editing, supervision; Bartosz Kazimierz Słowikowski: conceptualization, article idea, supervision, writing—review and editing, figure preparation.
Corresponding author
Ethics declarations
Ethics approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent to participate
Not applicable/not required.
Consent for publication
Not applicable/not required.
Competing interests
The authors declare no competing interests.
Additional information
Communicated by Ewa Ziętkiewicz
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jachowski, A., Marcinkowski, M., Szydłowski, J. et al. Modern therapies of nonsmall cell lung cancer. J Appl Genetics 64, 695–711 (2023). https://doi.org/10.1007/s13353-023-00786-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13353-023-00786-4