Abstract
Anti-tumor necrosis factor (TNF) therapy is used to induce and maintain remission in Crohn’s disease (CD) patients. However, primary non-responders to initial treatment constitute 20–40% of cases. The causes of this phenomenon are still unknown. We aim to investigate the impact of the caspase 9 (CASP9) gene variants on the variable reactions of CD patients to anti-TNF therapy. The study group included 196 diagnosed and clinically characterized CD Polish patients following anti-TNF therapy. The sequence of the CASP9 gene was analyzed using next-generation and Sanger sequencing and was analyzed with the response to biological treatment. Using the RT-qPCR analysis, we estimated the CASP9 gene mRNA level in colon biopsies material from inflamed and non-inflamed tissue (21 CD patients: 14 responders and seven non-responders to anti-TNF therapy and six controls), as well as in vitro in a peripheral blood mononuclear cells (PBMCs) from CD patients (seven responders and seven non-responders to anti-TNF therapy) and eight controls. Our findings indicated association of variants rs1052571 and rs4645978 with response to anti-TNF monoclonal antibodies (mAbs). Moreover, we observed tendency for reduced expression after incubation with anti-TNF in the group of CD patients, in contrast to the control group. Our results suggest that response to anti-TNF therapy in CD patients may be an effect of variants of the CASP9 gene as a key effector of the internal pathway of apoptosis; however, further population and functional research are necessary.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With the development of societies industrialization, we observe an increase in the incidence of inflammatory bowel diseases (IBD). The onset of the disease usually falls around the age of 20, i.e., the period of the greatest life activity, which is why the challenge of modern medicine is to implement effective treatment returning patients to full agility. Crohn’s disease (CD) is a disorder that can lead to permanent disability. It has been proven that properly selected and intensive therapy, introduced at the earliest possible stage of the disease, can prevent its unfavorable development. Biological drugs, including anti-TNF mAbs, have become a hope for effective treatment. The Food and Drug Administration approved them for CD in 1998 which revolutionized the IBD treatment.
The cytokine TNFα is one of the primary cytokines responsible for activating and maintaining inflammation in the altered gastrointestinal tract tissues in patients with CD (Van Assche and Rutgeerts 2000). Increased secretion of it was found in the inflamed mucosa, the lamina propria, and the submucosa (Murch et al. 1993). TNF is involved, among others, in the accumulation of neutrophils, the formation of granulomas as well as increased permeability of the intestinal epithelium (Mullin and Snock 1990; Amiri et al. 1992). Anti-TNF drugs bind to soluble and transmembrane TNFα and exhibit a particular affinity for inflamed tissues. The TNF transmembrane form is attached to the target cell surface and anti-TNF binds to it, acting as a ligand through reverse signaling, thereby suppressing cytokines and activating apoptosis. Anti-TNF drugs also induce apoptosis of activated T lymphocytes in the lamina propria. On this way, they inhibit one of the key disability responsible for the chronicity of inflammation in CD, increased survival and resistance to apoptosis of T lymphocytes (Van den Brande et al. 2007; Itoh et al. 2001). Studies up to date showed an imbalance in the controlled death of peripheral blood lymphocytes in both CD and ulcerative colitis (UC) (Dudzinska et al. 2019). Additionally, it was shown that apoptosis is the main mechanism by which infliximab exerts a killing activity on lamina propria T cells (LPT) in CD (Di Sabatino et al. 2004). Although anti-TNF drugs have changed the approach to treating patients with CD, it is still challenging to predict an individual patient’s response. Approximately 30% of patients do not respond to this form of therapy—“primary non-response” and nearly 50% will lose this effectiveness within the first year of therapy—“secondary non-response.” While the production of anti-drug antibodies is primarily responsible for the loss of the secondary response, the mechanisms responsible for the lack of the primary response are still not fully understood. Due to the development of new biological molecules, in recent years, there is an increasing need to develop biomarkers that will allow the initiation of personalized medicine and, as a result, will lead to a greater effectiveness of therapy, reducing risk of complications, and costs (Lykowska-Szuber et al. 2023a, b).
Therefore, the aim of our study was to investigate whether variants of caspase 9 (CASP9) gene, one of the key proteinases responsible for the induction of the internal pathway of cell apoptosis, may be one of the reasons for the lack of primary response to anti-TNF alpha therapy in patients with CD. Our goal was to answer this question by conducting both studies of the CASP9 gene sequence and the tissue-specific CASP9 expression profile in the intestinal mucosa of CD patients treated with anti-TNF and in vitro studies in peripheral blood mononuclear cells (PBMCs) from CD patients cultured with anti-TNF mAbs. The current investigation in part of the CASP9 gene sequence analysis is complementary (includes the same set of the studied DNA samples) to the previously published research estimating selected variants within FCGR3A, IL1R, TNFSF1B, IL1B, FAS, and ADAM17 genes (Lykowska-Szuber et al. 2023a) and in part of CASP9 gene expression is complementary (includes the same set of cDNA material) to previously described research on FCGR3A, IL1R, TNFSF1B, IL1B, FAS, and ADAM17 genes expression genes (Lykowska-Szuber et al. 2021).
Material and methods
Clinical characterization and study
The study included in total 196 Polish patients hospitalized at the Department of Gastroenterology, Dietetics and Internal Diseases of the Medical University in Poznan with a confirmed diagnosis of CD based on a history, physical examination, endoscopy, and MR enterography. All subjects were treated with anti-TNF therapy under the therapeutic program of the National Health Fund (the official reimbursement program for all biological therapies in Poland) at the Gastroenterology Clinic in years 2017–2021. We included in the study biologically naïve patients > 18 years old with active CD and after treatment failure or intolerance to first-line therapies such as mesalamine, corticosteroids and/or immunosuppressants. The exclusion criteria were the presence of an ileostomy or colostomy and infectious complications (including intraabdominal infections). The diagnosis was based on predefined criteria (Gomollón et al. 2017) and clinical disease activity was assessed using the Crohn's Disease Activity Index (CDAI) (Best et al. 1976). Individuals who had never smoked or quit smoking for at least 10 years prior to participating in the study were considered non-smokers. Patients were administered with infliximab (IFX) infusions at a dose of 5 mg/kg body weight at weeks 0, 2, 6 (induction phase) and then every 8 weeks up to a year (54 weeks—maintenance phase). Adalimumab (ADA) was administered subcutaneously at week 0 at a dose of 160 mg, 80 mg at week 2, then 40 mg every other week for up to 1 year (54 weeks). Response to anti-TNF treatment was assessed after 12 weeks of treatment. The CDAI score was used to determine the clinical response. Clinical response was defined as a reduction in CDAI of ≥ 70 points. In patients with fistulas, a complete response was defined as complete cessation of drainage of all fistulas, while a partial response was defined as a reduction of at least 50%, but not drainage of all fistulas. We also assessed the biological parameter (C-reactive protein, CRP), endoscopic response (simple endoscopic assessment of Crohn’s disease, SES-CD) and MRI (simple assessment of enterographic activity in Crohn’s disease, SEAS-CD) (Daperno et al. 2004; Eder et al. 2013a). These parameters were assessed twice—before treatment and after 12 weeks of induction therapy. The scheme of our study consisted of four stages and it is shown in Fig. 1.
In the first step, after DNA isolation from collected peripheral blood samples, we performed sequencing of the CASP9 gene (from 5′UTR to exon 9 with 3′ untranslated region, UTR) in group of 96 CD patients (86 responders and 10 non-responders to anti-TNF mAbs) using NGS technology. In the second step of our research after NGS results interpretation and enlargement of CD patients group to 196 subjects treated with anti-TNF, we performed analysis of the CASP9 gene promotor with exons 1, 2 and 3. Table S1 in supplementary material presents whole patients group characteristics. The third step of current research involving CASP9 gene expression in CD patients in two types of material was carried out. The first group consisted of 19 individuals, for whom the research was carried out on tissue material collected during a routine colonoscopy. The second group included 13 patients, from whom peripheral blood samples for cell culture studies were collected. The detailed clinical characteristics of both studied groups are presented in supplementary material (Table S2 and Table S3). The control groups for CASP9 gene expression studies consisted of 6 healthy (2 female, 4 male) subjects with the average age of 46.7 years in mucosal studies and 8 healthy subjects (4 female, 4 male) with average age 40.2 years in PBMCs culture studies.
All subjects gave their written consent to carry out genetic testing, endoscopy, MR enterography, and serum testing for the assessment of biochemical parameters. The research was approved by the Bioethics Committee of the Medical University in Poznan under Resolution No. 762/13 approved on 9 November 2013 and Resolution No. 1042/18 approved on 11 October 2018. All experiments were performed in accordance with the principles of the 1964 Declaration of Helsinki with its later amendments.
DNA isolation, next generation, and Sanger sequencing analysis
Genomic DNA was isolated from the peripheral blood of all participants using the method with guanidine isothiocyanate and stored at 4 °C in a TE buffer containing 1 mM EDTA and 10 mM Tris–Cl. Next, the amplification of CASP9 gene regions were performed using PCR primers sequences presented in Table 1.
For NGS amplicon library preparation based on a total of 3 amplicons from each patient were prepared using previously described conditions (Walczak et al. 2019). According to the manufacturer’s protocol, 1 ng of the pooled DNA fragments was subjected to the Nextera XT procedure (Illumina) using transposome technology. Finally, using the Nextera XT DNA Sample Preparation Kit (Illumina) and the Nextera® XT Index Kit (96) (Illumina), we obtained one hundred and seven both-side indexed DNA libraries ready for high-throughput sequencing. The normalization of all libraries was carried out with magnetic beads, according to the Nextera XT procedure. Sequencing on the Illumina MiSeq platform was performed as paired-end targeted resequencing using the MiSeq Reagent Kit v2 (300 cycle) (Illumina).
PCR program for amplification of the promotor regions and exons 1, 2, and 3 for Sanger sequencing, started with initial denaturation at 95 °C for 4 min, followed by 32 cycles of denaturation at 94 °C for 30 s, annealing at a temperature shown in Table 1 for each fragment, respectively for 30 s, and extension at 72 °C for 1 min, as well as a final extension step at 72 °C for 7 min. Then, Sanger sequencing was performed in both directions on an Applied Biosystems 3500 Genetic Analyzer using a BigDye® Terminator v3.1 Cycle Sequencing Kit (Thermo Fisher Scientific, Waltham, MA, USA), according to the manufacturer’s instructions. The results were analyzed using the Sequencing Analysis Software system.
Biopsy preparation
Approximately 1–2 mg of biopsies were obtained from sites of inflammed and non-inflamed regions from treatment-naïve patients with CD and from healthy controls during a colonoscopy. Next, the collected biopsies were suspended in 300 μl of RNALatter® reagent (Sigma) and frozen at − 80 °C until RNA isolation started.
PBMCs isolation, culture, and treatment with the antibody
PBMCs were isolated from 9 ml samples of whole blood using LYMPHOSEP™ (MP Biomedicals LLC, OH, USA), according to the manufacturer’s instructions. The obtained pellet was suspended in 4 ml of a Lymphogrow medium (Cytogen-Polska Sp. z o.o., Zgierz, Poland) containing phytohemagglutinin (PHA) and recombinant IL-2 (4 ng, 100 U, BioLegend, San Diego, CA, USA). The suspension was then transferred to a 25-ml vessel for adherent culture. Cells were grown under standard conditions at 37 °C, 5% CO2 with shaking for 24 h. Non-adherent cells were washed with PBS and transferred to a 25-ml vessel for suspension culture with fresh Lymphogrow medium supplemented with IL2. After another 48 h, the cells were passaged and maintained in a culture using a standard RPMI-1630 medium supplemented with L-glutamine (2 mM), FBS (10%), penicillin (100 IU/ml), streptomycin (100 µg/ml), and with the addition of IL-2. Cell differentiation was measured by CD3, CD4, CD8, CD45, and HLA-DR by flow cytometry analysis. In the third passage, anti-TNF mAbs’ (Sigma) was added (10 µg/ml). In parallel, a control culture without the addition of the antibody was carried out. After 72 h of culture, cells were collected and suspended in 200 µl stayRNA solution (A&A Biotechnology, Gdansk, Poland) and frozen at − 80 °C for RNA isolation.
CASP9 gene expression studies
RNA isolation, cDNA synthesis, and quality control
Cells or mucosal tissue samples suspended in stayRNA™ (A&A BIOTECHNOLOGY, Gdansk, Poland) were homogenized with electric homogenizer and subjected to RNA isolation with TRIzol™ Reagent (Life Technologies, Carlsband, California), according to the manufacturer’s procedure. For all obtained RNA samples, a quantitative and qualitative evaluation was carried out using Agilent RNA 6000 Nano Kit and the Bioanalyzer 2.0 equipment (Agilent, Santa Clara, CA, USA). A 2 μg of total RNA with RIN ≥ 7 was converted to cDNA with an iScript Advanced Reverse Reaction kit (Bio-Rad) with the following conditions: 25 °C for 5 min—annealing step, 42 °C for 30 min—reverse transcription, and 95 °C for 1 min—inactivation.
Real-time quantitative PCR (RT-qPCR)
The mRNA level of the CASP9 gene was measured by a real-time quantitative polymerase chain reaction on a BioRad CFX Connect 96-well Thermal Cycler (Bio-Rad Laboratories, Inc., Hercules, CA, USA) using the iTaq UniverSYBR Green assay (Bio-Rad Laboratories, Inc., Hercules, CA, USA), according to the manufacturer’s instructions. Specific primers: (1) forward 5′- GAGAATTGACCCTGGGGACAG-3′, reverse 5′- GCAGGACGCATCTCCAACG-3′ for amplification of 108 bp-length fragment of CASP9 gene were designed by a Primer-BLAST tool. Primers for reference PPIA and RPLP0 genes were ordered as PrimePCRTM SYBR® Green Assay by Bio-Rad Laboratories, Inc. manufacturer. Results of qPCR reactions are presented as dCt = (dCtreference gene – dCtgene of interest). Every reaction was performed in duplicates.
Bioinformatic and statistical analysis
The NGS reads generated in analysis were aligned to the hg19 reference genome using a Burrows–Wheeler Aligner (BWA. version 0.7.5) (Li and Durbin 2009). For PCR, duplicates marking Picard (version 2.1.0) were used. Local realignment around indels and a base quality score recalibration were carried out, followed by calling the variants by the Genome Analysis Toolkit (GATK version 3.5), in accordance with the GATK best practice procedure (McKenna et al. 2010). Next, single nucleotide variants were identified by means of using a HaplotypeCaller module and annotated by the VariantAnnotator module.
The comparison of interval data between responders and non-responders was conducted by nonparametric Mann–Whitney test, since the data did not follow the normal distribution pattern (Shapiro–Wilk test). The chi-square test was used for comparing nominal data, as well as to determine whether the association between the allele frequencies and the response to treatment was significant. Those analyses were performed using STATISTICA 13.3 software (StatSoft, Inc.) and PQStat 1.8.4 (PQStat Software, Poland) and all tests were considered significant at p < 0.05. After selecting variants that identified as statistically significant in the percentage of particular allele distribution between the group of responders and non-responders to anti-TNF treatment, in the next step, we used an odds ratio (OR) to demonstrate how many times more often a particular variant occurred in non-responder patients comparing to responder patients. OR is considered statistically significant when its 95% confidence interval (95% CI) does not contain 1. The analysis of genotypes distribution concordance with Hardy–Weinberg equilibrium and calculations of odds ratios (OR) with confidence intervals (CI) have been performed using the online calculator of Court-lab HW calculator (Court Lab—HW Calculator, (scribd.com) accessed on date 25.07.2023).
For the RT-qPCR data, in the case of lymphocytes, the Mann–Whitney non-parametric test was used to compare median value of the three groups—controls, responders and non-responding patients in reference to conditions with and without anti-TNF infliximab antibody. Comparison between tissue samples from controls, responders (inflammed and non-inflammed) and non-responders (inflammed and non-inflammed tissue) were conducted with Kruskal–Wallis one-way analysis of variance. P-values < 0.05 were considered as statistically significant. All analyses were performed using R software 3.6.1 and RStudio.
Results
Based on NGS results of CASP9 gene in a studied group of 96 CD patients, we observed in total of 55 variants in exons 4–9. For the first amplicon containing 5′UTR and exons 1–3, the obtained NGS data did not meet quality filtering as sequencing depth > 30 reads; therefore, they were discarded and replicated using Sanger sequencing as shown below. Among identified variants, one was located in exon (rs1052576, c.662A > G), 51 in introns (one of them in splice region, rs2020902, c.453 + 8 T > C) and three in 3′UTR (Table S4). Compering alleles distribution in responders and non-response to anti-TNF treatment, 29 variants revealed statistical significance differences based on chi-square p-value < 0.05. However, after multiple corrections, the statistical significance was not rich (Table S4).
The standard Sanger sequencing method was used to verify the NGS results as well as to complete the promoter region and exons 1, 2, and 3 as well as extension the study group to 196 CD patients. Obtained data of Sanger sequencing showed significant association of two variants rs1052571 and rs4645981with response to anti-TNF as detailed in Table 2 (p < 0.05). The results for the third variant rs4645978 are near statistical significance (p = 0.07).
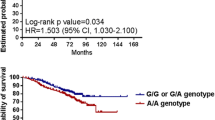
We observed that rs1052571 was non consistent with Hardy–Weinberg equilibrium (HWE) in both studied groups, whereas rs4645981 was deviations only in the responders patients’ group (p < 0.05). Detailed comparison of allelic and genotypic distributions between responders and non-responders to anti-TNF treatment is shown in Table 3 and Fig. 2.
In the last part of experiments, the CASP9 gene mRNA expression was estimated in CD patients colon biopsies and in PBMCs culture treated with anti-TNF. Based on results, no statistically significant differences in the expression of CASP9 gene were found in any of the subgroups studied; however, in the case of cell cultures, there is a tendency for reduced expression after incubation with anti-TNF in the group of patients, in contrast to the control group, in which expression increases (Fig. 3, Table S5, Table S6).
Discussion
Impaired adaptive immunity of cells of the intestinal immune system is crucial for the development and maintenance of inflammation in IBD. Lymphocyte apoptosis plays an important role in the breakdown of inflammation. Cell death is initiated by activation of ligands, such as TNF-α or FAS that bind to appropriate death receptors—in the external pathway or by the release of mitochondrial components, such as cytochrome c—in the internal pathway. Disruption of the apoptosis process can lead to many pathologies, such as cancer or autoimmune diseases. A characteristic feature of CD is the infiltration of T lymphocytes in the intestinal mucosa. In patients with IBD, an increased level of anti-apoptotic mediators in the intestinal mucosa and the abnormal response of T lymphocytes to pro-apoptotic signals is observed (Sturm et al. 2002). Interestingly, one of the modes of action of anti-TNF drugs is the ability to induce lymphocyte apoptosis (Makrygiannakis and Catrina 2012); however, the exact mechanism of the influence of these drugs on the phenomenon of apoptosis remains elusive.
The studies conducted so far showed that in the T lymphocytes of the lamina propria of patients with CD, increased activation of the anti-apoptotic protein Bcl-2, which results in the inhibition of the internal apoptotic pathway, is observed (Lindsay et al. 2011; Eder et al. 2013b). CASP-9 is one of the main factors of the internal apoptosis pathway. Cytochrome c, together with CASP-9 and Apaf-1, creates the so-called apoptosome, which activates caspase-3, the main enforcer of apoptosis. Hlavaty et al. observed that patients with infliximab-resistant luminal and fistula form of the disease, carried the rs4645983 genotype CC and CT of the CASP9 with higher frequency (p = 0.04; OR = 1.50; 95% CI [1.34–1.68]) (Hlavaty et al. 2005). In our study, we did not confirm this association. However, we identified two other variants related to response to anti-TNF: rs1052571 and rs4645981. Results for the third variant, rs4645978 are near statistical significance (p = 0.07). Interestingly, the variant rs1052571 described by us is located at position c.83 of the CASP9 gene, i.e., 10 nucleotides prior to exon 1 than rs4645983 described by Hlavaty et al. and leads to the substitution of the amino acid alanine to valine at position 28 (p.Ala28Val). We found that homozygote TT occurred with lower frequency in responders compared to non-responders (28.2% vs. 48.5%) and may promote response to anti-TNF therapy (OR = 2.40, 95% CI [1.12–5.14], p = 0.0224, Table 3). Therefore, homozygote CC is associated with non-response (OR = 3.28, 95% CI [1.25–8.61], p = 0.0125). Guo and colleagues studied this variant in a group of 555 patients with CD and 651 patients with UC, who attempted to assess the correlation between CASP9 gene haplotypes and susceptibility to IBD. They showed that rs1052571 in the CASP9 gene was associated with severe UC (p = 0.0034, OR = 1.957, 95% CI [1.240–3.088]). These findings suggest that the CASP9 gene may be another susceptibility gene for severe IBD (Guo et al. 2011). It cannot be ruled out that the polymorphism of this gene is also related to non-response to drugs, which we showed in our research. In addition to this relationship, we also observed that two promoter variants are associated with response to anti-TNF treatment. T allele of rs4645978 (c.-239 + 652G > T) was detected much more often in responders (60.4% vs. 47% respectively), and the GG and GT genotype was three times more common in non-responders (OR = 3.00, 95% CI [1.17–7.68], p = 0.0177), and the TT genotype conditioned a better response to drugs (OR = 0.34, 95% CI [0.13–0.91], p = 0.0259). The second variant of the promoter region shown by us in this study is rs4645981 (c.-239 + 1203C > G), where the presence of heterozygote CG was associated with non-response to anti-TNF treatment in patients with CD. Although we observed a statistical dependence in the distribution of genotypes, the share of C and G alleles is similar in both study groups. Therefore, it is difficult to postulate the significance of this variant in response to anti-TNF drugs, in contrast to the two variants described above. The location of the rs4645978 variant in the gene promoter may affect the expression level of the CASP9 gene and the protein level. Therefore, the continuation of our observations was the evaluation of CASP-9 protein expression in colon tissues from patients with CD and healthy individuals. We found no differences in CASP9 mRNA expression between these groups. Our observations suggest no change in the induction of the internal apoptosis pathway in active CD. However, it cannot be ruled out that the polymorphisms described by us have a negative impact on the function of the CASP-9 protein and its expression. The limitation of our observation is the small number of examined tissues.
It might not be excluded that the variants described by us have a negative impact on the function and expression of the CASP-9 protein. The limitation of our observation is a small number of examined tissues. However, we observed that in patients with active Crohn’s disease, the internal apoptosis pathway is not significantly enhanced, especially in the group of non-responders (Fig. 3). Our observations are consistent with the results obtained by Neubauer et al. They assessed CASP-9 concentration in peripheral blood lymphocytes of patients with IBD by immunoenzymatic method and showed significantly lower concentrations of CASP-9 in patients with active IBD compared to the control group (Neubauer et al. 2018). A follow-up study of our experiment was to determine CASP-9 mRNA expression in cell cultures of peripheral blood lymphocytes that were treated with IFX. We showed that CASP-9 expression is reduced under the influence of IFX in non-responding patients. No correlation was observed in healthy subjects and in responders. Our observations are consistent with the observations of Eder et al., who, using immunohistochemical methods, showed that in patients with active CD responding to infliximab treatment, there is a significant increase in the expression of active caspase-3 in the mononuclear cells of the lamina propria, which correlated with an increase in the pro-apoptotic Bax/Bcl-2 pathway. These differences were not observed in non-responding patients (Eder et al. 2013a). Similar results were obtained by other researchers. Ten Hove et al. demonstrated in an in vitro model that IFX induces apoptosis and increases the Bax/Bcl-2 ratio of CD3/CD28-stimulated Jurkat T cells (Ten Hove et al. 2002).
Although many researchers suggest that anti-TNF drugs do not directly induce apoptosis of inflammatory cells, it seems that damage to the internal pathway may be critical in resistance to biological treatment. Undoubtedly, the limitation of our study is the small number of patients. However, there is a clear tendency that patients who did not respond to the treatment did not show an increase in CASP9 gene expression when compared to the control group and the group of patients responding to the drug.
In summary, our results suggest that response to anti-TNF therapy in CD patients could be an effect of variants of CASP9 gene as a key effector of the internal pathway of apoptosis. However, more detailed research is needed in this area.
Data availability
All primary data are available from the corresponding author upon the reasonable request.
References
Amiri P, Locksley RM, Parslow TG, Sadick M et al (1992) Tumour necrosis factor alpha restores granulomas and induces parasite egg-laying in schistosome-infected SCID mice. Nature 356:604–607. https://doi.org/10.1038/356604a0
Best WR, Becktel JM, Singleton JW et al (1976) Development of a Crohn’s disease activity index. National Cooperative Crohn’s Disease Study. Gastroenterology 70:439–444. https://doi.org/10.1016/S0016-5085(76)80163-1
Daperno M, D’Haens G, Van Assche G et al (2004) Development and validation of a new, simplified endoscopic activity score for Crohn’s disease: the SES-CD. Gastrointest Endosc 60:505–512. https://doi.org/10.1016/s0016-5107(04)01878-4
Di Sabatino A, Ciccocioppo R, Cinque B et al (2004) Defective mucosal T cell death is sustainably reverted by infliximab in a caspase dependent pathway in Crohn’s disease. Gut 53:70–77. https://doi.org/10.1136/gut.53.1.70
Dudzinska E, Szymona K, Gil-Kulik P et al (2019) Imbalance of controlled death in peripheral blood lymphocytes in Crohn’s disease and ulcerative colitis. Medicina (kaunas) 55:231. https://doi.org/10.3390/medicina55060231
Eder P, Katulska K, Lykowska-Szuber L et al (2013a) Simple enterographic activity score for Crohn’s disease: comparison with endoscopic, biochemical, and clinical findings. Pol Arch Intern Med 123:378–385. https://doi.org/10.20452/pamw.1825
Eder P, Lykowska-Szuber L, Krela-Kazmierczak I et al (2013b) The influence of infliximab and adalimumab on the expression of apoptosis-related proteins in lamina propria mononuclear cells and enterocytes in Crohn’s disease—an immunohistochemical study. J Crohns Colitis 7:706–716. https://doi.org/10.1016/j.crohns.2012.09.006
Gomollón F, Dignass A, Annese V et al (2017) 3rd European evidence-based consensus on the diagnosis and management of Crohn’s disease 2016: part 1: diagnosis and medical management. J Crohns Colitis 11:3–25. https://doi.org/10.1093/ecco-jcc/jjw168
Guo C, Ahmad T, Beckly J et al (2011) Association of caspase-9 and RUNX3 with inflammatory bowel disease. Tissue Antigens 77:23–29. https://doi.org/10.1111/j.1399-0039.2010.01569.x
Hlavaty T, Pierik M, Henckaerts L et al (2005) Polymorphisms in apoptosis genes predict response to infliximab therapy in luminal and fistulizing Crohn’s disease. Aliment Pharmacol Ther 22:613–626. https://doi.org/10.1111/j.1365-2036.2005.02635.x
Itoh J, de La Motte C, Strong SA et al (2001) Decreased Bax expression by mucosal T cells favours resistance to apoptosis in Crohn’s disease. Gut 49:35–41. https://doi.org/10.1136/gut.49.1.35
Li H, Durbin R (2009) Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25:1754–1760. https://doi.org/10.1093/bioinformatics/btp324
Lindsay J, Esposti MD, Gilmore AP (2011) Bcl-2 proteins and mitochondria–specificity in membrane targeting for death. Biochim Biophys Acta 1813:532–539. https://doi.org/10.1016/j.bbamcr.2010.10.017
Lykowska-Szuber L, Walczak M, Skrzypczak-Zielinska M et al (2021) Effect of anti-TNF therapy on mucosal apoptosis genes expression in Crohn’s disease. Front Immunol 12:615539. https://doi.org/10.3389/fimmu.2021.615539
Lykowska-Szuber L, Walczak M, Skrzypczak-Zielinska M, Dobrowolska (2023a) Apoptosis and inflammatory genes variants in primary non-response to anti-TNF therapy in Crohn’s disease patient. Eur J Gastroenterol Hepatol In print. https://doi.org/10.1097/MEG.0000000000002618
Lykowska-Szuber L, Skrzypczak-Zielinska M, Zuraszek et al (2023b) Association of the ILR1 and FAS genes variants with a primary non-response to Anti-TNF therapy in Crohn’s disease patients. Pol Arch Intern Med 16461. Advance online publication. https://doi.org/10.20452/pamw.16461
Makrygiannakis D, Catrina AI (2012) Apoptosis as a mechanism of action of tumor necrosis factor antagonists in rheumatoid arthritis. J Rheumatol 39:679–685. https://doi.org/10.3899/jrheum.110974
McKenna A, Hanna M, Banks E et al (2010) The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res 20:1297–1303. https://doi.org/10.1101/gr.107524.110
Mullin JM, Snock KV (1990) Effect of tumor necrosis factor on epithelial tight junctions and transepithelial permeability. Cancer Res 50:2172–2176
Murch SH, Braegger CP, Walker-Smith JA, MacDonald TT (1993) Location of tumour necrosis factor alpha by immunohistochemistry in chronic inflammatory bowel disease. Gut 34:1705–1709. https://doi.org/10.1136/gut.34.12.1705
Neubauer K, Woźniak-Stolarska B, Krzystek-Korpacka M (2018) Peripheral lymphocytes of patients with inflammatory bowel disease have altered concentrations of key apoptosis players: preliminary results. Biomed Res Int 2018:4961753. https://doi.org/10.1155/2018/4961753
Satsangi J, Silverberg MS, Vermeire S et al (2006) The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut 55:749–753. https://doi.org/10.1136/gut.2005.082909
Sturm A, Itoh J, Jacobberger JW et al (2002) p53 negatively regulates intestinal immunity by delaying mucosal T cell cycling. Journal Clin Invest 109:1481–1492. https://doi.org/10.1172/JCI14967
Ten Hove T, van Montfrans C, Peppelenbosch MP et al (2002) Infliximab treatment induces apoptosis of lamina propria T lymphocytes in Crohn’s disease. Gut 50:206–211. https://doi.org/10.1136/gut.50.2.206
Van Assche G, Rutgeerts P (2000) Anti-TNF agents in Crohn’s disease. Expert Opin Investig Drugs 9:103–111. https://doi.org/10.1517/13543784.9.1.103
Van den Brande JM, Koehler TC, Zelinkova Z et al (2007) Prediction of antitumour necrosis factor clinical efficacy by real-time visualisation of apoptosis in patients with Crohn’s disease. Gut 56:509–517. https://doi.org/10.1136/gut.2006.105379
Walczak M, Skrzypczak-Zielinska M, Plucinska M et al (2019) Long-range PCR libraries and next-generation sequencing for pharmacogenetic studies of patients treated with anti-TNF drugs. Pharmacogenomics J 19:358–367. https://doi.org/10.1038/s41397-018-0058-9
Funding
Kamila Stawczyk-Eder was the recipient of a fellowship for young researchers from Poznan Medical University, Poland (grant no. 502–14–02223359– 10715, 502–14–02223800– 10715) This work was supported by the Polish National Science Centre (grant no. 2016/23/D/NZ2/01620).
Author information
Authors and Affiliations
Contributions
Conceptualization: Liliana Lykowska-Szuber and Marzena Skrzypczak-Zielinska. Methodology and validation: Liliana Lykowska-Szuber and Michal Walczak. Investigation: Liliana Lykowska-Szuber, Michal Walczak, Kamila Stawczyk-Eder, Iwona Krela-Kazmierczak, Piotr Eder, and Oliwia Zakerska-Banaszak. Formal analysis: Liliana Lykowska-Szuber and Michal Walczak and Marzena Skrzypczak-Zielinska. Writing—original draft preparation: Liliana Lykowska-Szuber and Marzena Skrzypczak-Zielinska. Critical revision of the manuscript: Piotr Eder and Agnieszka Dobrowolska. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
The research was approved by the Bioethics Committee of the Medical University in Poznan under Resolution No. 762/13 approved on 9 November 2013 and Resolution No. 1042/18 approved on 11 October 2018. All experiments were performed in accordance with the principles of the 1964 Declaration of Helsinki with its later amendments.
Competing interests
The authors declare no competing interests.
Additional information
Communicated by Ewa Ziętkiewicz
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lykowska-Szuber, L., Walczak, M., Stawczyk-Eder, K. et al. Variants of the CASP9 gene as candidate markers for primary response to anti-TNF therapy in Crohn’s disease patients. J Appl Genetics 64, 759–768 (2023). https://doi.org/10.1007/s13353-023-00783-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13353-023-00783-7