Abstract
The aim of this study was to perform a comparative investigation of the actions of three mutagens that are widely used in plant mutagenesis using the comet-FISH technique. The comet-FISH technique was used for the analysis of DNA damage and the kinetics of repair within specific DNA sequences. FISH with rDNA and telomeric/centromeric DNA probes was applied to comets that were obtained from an alkaline/neutral comet assay. Migration within specific DNA sequences was analysed after treatment with two chemical mutagens-maleic hydrazide (MH) and N-nitroso-N-methylurea (MNU), and γ-rays. Barley was used as a model plant in this study. The possible utility of specific DNA sequences in a comparative assessment of the distribution of DNA damage within a plant genome was evaluated. This study proved that the comet-FISH technique is suitable for a detailed quantification of DNA damage and repair within specific DNA sequences in plant mutagenesis. The analysis of FISH signals demonstrated that the involvement of specific DNA sequences in DNA damage was different and was dependent on the mutagen used. We showed that 5S rDNA and telomeric DNA sequences are more sensitive to mutagenic treatment, which was expressed by a stronger fragmentation and migration in comparison to the other probes used in the study. We found that 5S rDNA and telomeric DNA probes are more suitable for testing the genotoxicity of environmental factors. A comparison of the involvement of specific chromosome domains in direct DNA breakage/repair and in chromosome aberration formation after mutagen treatment indicates the compatibility of the results.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The comet assay has been widely accepted as a reliable marker for DNA damage (Collins 2004). It has become an important tool in the assessment of environmental genotoxicity (Fairbairn et al. 1994; Cotelle and Férard 1999; Poli et al. 1999; Restivo et al. 2002). The comet-FISH technique enables the localisation of a specific chromosome, regions of chromosomes or specific genes within the comets (Collins 2004). Thus, it allows DNA damage and the repair rate within the specific DNA sequences and their sensitivity to various damaging agents to be measured. One of the most promising areas for comet-FISH is the study of the sensitivity of individual genes and chromosome regions, especially in human and animal cells.
Our previous work was the first report on the application of FISH to comet preparations from plants after mutagenic treatment. Following the comet-FISH technique, the quantification of the involvement of rDNA sequences was achieved through an analysis of the distribution of FISH signals between the head and the tail, as well as the morphology of the comets. The results helped DNA damage and repair distribution in a plant genome within rRNA genes to be understood (Kwasniewska et al. 2012).
By providing additional information regarding the distribution of DNA damage, comet-FISH may be helpful in better understanding the biological impact of genotoxic effects in plants. An analysis of the sensitivity of specific DNA sequences to mutagens and repair effectivity is very promising for plant mutagenesis and the assessment of environmental genotoxicity (Kwasniewska et al. 2012). The centromeric, telomeric and rDNA sequences representing repetitive DNA are most often used as probes for FISH in plant cytogenetics. Due to the lack of chromosome-specific DNA sequences, the application of region-specific sequences enables the analysis of breakpoints. These were previously used for the analysis of the specific localisation of mutagen-induced chromosome aberrations.
In our previous papers (Juchimiuk et al. 2007; Juchimiuk-Kwasniewska et al. 2011), we quantitatively analysed the involvement of specific DNA sequences in micronuclei induced using N-nitroso-N-methylurea (MNU), maleic hydrazide (MH) and γ-rays in order to compare the possible origin of the micronuclei induced by these mutagens in barley. The analysis of the micronuclei with signals of the investigated DNA probes showed differences between the frequencies of MH-, MNU- and γ-ray-induced micronuclei with specific DNA signals. Nowadays, the application of comet-FISH makes it possible to compare the chromatin domains that are involved in comet formation with the localisation of chromosome aberrations.
The aim of the present study was to compare the involvement of telomere, centromere and rDNA sequences in DNA damage and repair induced by MH, MNU and γ-rays using comet-FISH. The above mutagens, which are commonly used in plant mutagenesis, are characterised by different mechanisms of action. MH is a clastogenic agent that can lead to chromosome breaks. It can also cause spindle fibre defects. MNU is an alkylating agent that mainly induces gene mutations; however, it can also lead to chromosomal aberrations. A γ-ray, which causes breaks in one or two chains of DNA, is also routinely used in plant mutagenesis (Hagberg and Persson 1968) and most barley mutant varieties were developed by applying this type of radiation. An attempt was also made to check the usefulness of the above-mentioned DNA sequences as probes to FISH for the assessment of the distribution of the DNA damage, which is induced by the different mutagens within a plant genome.
Material and methods
Material and treatment
Barley (Hordeum vulgare, 2n = 14) seeds of the “Start” variety were used. Two chemical mutagens and one physical one were used. The mutagens doses used in the study were applied in previous experiments in which chromosome aberrations were estimated (Juchimiuk et al. 2007; Juchimiuk-Kwasniewska et al. 2011). Two gamma radiation doses were employed: 175 Gy and 225 Gy. The irradiation was performed at the International Atomic Energy Agency, Seibersdorf Laboratory, Austria. After irradiation, the seeds were pre-soaked in distilled water for 8 h and germinated in Petri dishes at 21 °C in the dark. Before chemical treatment, the seeds of barley were pre-soaked in distilled water for 8 h and then treated with MNU (2 mM or 3 mM; Sigma, CAS no. 684-93-5) or MH (3 mM or 4 mM; Sigma, CAS no. 123-33-1) for 3 h. After the treatment, the seeds were washed three times in distilled water and then germinated in Petri dishes in the light. The nuclei were isolated from the leaves of seedlings at 72 and 96 h post-treatment. Seventy-two hours post-treatment is the earliest point at which the leaves could be harvested. Ninety-six hours post-treatment represents the duration of two cell cycles in H. vulgare.
Comet assay
The procedure for preparing the comets was as described by Gichner and Plewa (1998), with modifications. After treatment, individual leaves were placed in a small Petri dish on ice and spread with 200 μl of a cold 400 mM Tris–HCl buffer, pH 7.5. Using a fresh razor blade, each leaf was gently sliced into the “fringe” to release nuclei into the buffer. Each slide, which had previously been coated with 1 % NMP agarose (Sigma) and dried, was covered with a mixture of 55 μl of a nuclear suspension and 55 μl of LMP agarose (Sigma, 1 % prepared with phosphate-buffered saline) at 40 °C and coverslipped. The slide was placed on ice for at least 5 min and the coverslip was removed. Then, 110 μl of LMP agarose (0.5 %) was placed on the slide and the coverslip was remounted. After 5 min on ice, the coverslip was removed.
The comet assay procedure based on A/N conditions (alkaline denaturation/neutral gel electrophoresis) was applied, according to the protocol previously established for barley (Jovtchev et al. 2001). The slides were subjected to unwinding in 0.03 M NaOH for 5 min. Then, the slides were washed three times in TBE for 3 min each. Unwinding was followed by electrophoresis in neutral conditions—in a 1× TBE buffer (0.09 M Tris-borate, 0.002 M EDTA, pH 7.5) at 16 V (0.64 V/cm) 17 mA at room temperature (RT) for 10 min. The electrophoresis conditions used in the study were optimal, as they have been proved to provide a low level of DNA damage in the control cells and a linear concentration–response for the induction of comets after chemical mutagenic treatment in these species in earlier studies (data not presented). Then, the gels were neutralised, washed, dehydrated and air-dried. The slides were stained with 40 μl DAPI (2 μg/ml).
For each slide, 50 randomly chosen cells were analysed under a fluorescence microscope with an excitation filter of 546 nm and a barrier filter of 590 nm. Each experiment was repeated twice and then three slides were analysed per each experimental group. The frequencies of H. vulgare nuclei with tails were estimated. Data were analysed using the OriginPro 8.5.1 software (OriginLab Corporation, Northampton, MA, USA). The mean values of frequencies of comets with tails were analysed by a one-way analysis of variance (ANOVA) test. If a significant F-value of P < 0.05 was obtained, a Dunnett’s pairwise comparison test between the treated and control groups and between treated groups with different post-incubation times was conducted.
Fluorescence in situ hybridisation (FISH) on comets
FISH was applied according to the method described by Kwasniewska et al. (2012), with some minor modifications. Four DNA probes were used: (1) HT100.3: Arabidopsis-type telomeric repeats ((TTTAGGG)n) labelled with rhodamine-4-dUTP by polymerase chain reaction (PCR) (Roche); (2) CCS1: centromere DNA isolated from Brachypodium sylvaticum (Aragón-Alcaide et al. 1996) labelled with digoxigenin-11-dUTP (Roche); (3) 5S rDNA isolated from Triticum aestivum: pTa 794 (Gerlach and Dyer 1980) labelled with rhodamine-4-dUTP using a PCR labelling kit (Amersham Life Sciences); and (4) 25S rDNA isolated from Arabidopsis thaliana (Unfriend and Gruendler 1990) labelled with digoxigenin-11-dUTP by nick translation (Roche). HT100.3 and CCS1 were used as probes in the first FISH experiment, whereas 25S and 5S rDNA were used in the second one. The DNA of the nuclei on the gels was denatured using a solution of 0.5 M NaOH and 1 M NaCl for 30 min at room temperature. Then, the gels were washed four times for 5 min in dH20 and neutralised in 0.4 M Tris–HCl for 30 min at room temperature. The denatured DNA was dehydrated in an ethanol series (70, 90, 100 %, 5 min each) and the gels were air-dried. The hybridisation mixture, containing 2.5 μg mL−1 of labelled DNA, 50 % (v/v) formamide, 10 % (w/v) dextran sulphate and 0.1 mg μL−1 salmon testes DNA in 2× SSC, was denatured at 75 °C for 10 min and immediately placed on ice for a few minutes. The hybridisation mixture (40 μl) was dropped onto each gel, covered with a coverslip and incubated for 24 h at 24° in a wet-chamber. Before the detection of the probes, the slides were washed for 10 min in 2× SSC, RT and 10 min in 0.1× SSC, RT. The digoxigenin-labelled probe was detected by using FITC-conjugated anti-digoxigenin antibodies (Roche). After dehydration in the ethanol series, the slides were mounted in a Vectashield medium (Vector) containing 6 μg mL−1 DAPI. Preparations were examined using an Olympus Provis epifluorescence microscope and the appropriate filter set. Images were captured using a Hamamatsu C5810 CCD camera and processed in Adobe Photoshop 4.0. The distribution of 5S/25S rDNA and centromeric/telomeric signals (comet head or comet tail) were recorded for each nucleus with a tail. Of the comets with a tail that were examined, four categories were distinguished based on the distribution of FISH signals between the head and the tail. From the three replicates of the treatment experiment, 30 comets were analysed for each from the five slides.
Results and discussion
Comet assay
MH, MNU and γ-ray treatment led to comet formation under the A/N procedure. Significantly higher frequencies of H. vulgare nuclei with tails were observed after treatment with the mutagens as compared to the controls (Fig. 1). The frequency of control nuclei with tails was very similar at 72 and 96 h post-treatment—about 20 %, whereas after mutagenic treatment, it was 56.6–100 %. Comparison of the frequencies of nuclei with tails varied for different mutagens in the doses applied. MNU induced DNA fragmentation in all of the cells analysed. The lowest frequency of cells with tails was observed after treatment with 175 Gy of γ-rays, whereas all of the nuclei had tails if 225 Gy was applied. The kinetics of DNA damage repair after chemical and physical treatment was analysed after the application at 72 and 96 h post-treatment. A significant decrease in the frequencies of nuclei with tails was observed after MH, γ-ray and 2 mM MNU treatment. An application of the post-treatment recovery time did not lead to a decrease in the frequencies of comets with tails that were induced by 3 mM MNU. The kinetics of DNA repair after the exposure of seedlings to alkylating agents and to γ-rays was previously determined by Gichner et al. (2000). DNA lesions in leaf cells induced by alkylating agents were not repaired within 4 weeks, whereas the leaf nuclei from cells exposed to γ-rays expressed complete DNA repair after 24 h post-treatment. In this study, DNA damage caused by γ-rays in barley was not completely repaired, even at 96 h post-treatment; however, only seeds not seedlings were treated. Nevertheless, the differences in the kinetics of DNA repair between γ-ray-induced versus alkylation-induced damage were also observed in our studies.
The frequencies of comets with tails in H. vulgare after treatment with MH, MNU and γ-rays at 72 and 96 h post-treatment. The errors bars represent the standard deviations of the mean. The capital letter A indicates that the frequencies of the comets with tails after treatments are significantly different (P < 0.05) from the frequency of comets of the control groups. The capital letters B or D indicate that the frequencies of the comets with tails are significantly different (P < 0.05) within the same treatment for different post-incubation times
Distribution of FISH signals on comets
Comet-FISH with 5S/25S rDNA and telomeric/centromeric probes was used to compare the distribution of these sequences into the comet tail and head after MH, MNU and γ-ray treatment. rDNA signals were distributed in both the head and the tail of the control and treated cells. A quantitative examination of the comet-FISH results was not possible due to the presence of a number of small signals, as well chains of signals. The number of FISH foci in control cells was as expected. The high number of small signals, more than expected from the barley chromosome number (2n = 14), indicates that fragmentation occurs often within the applied sequences. Due to the similar appearance of the comets with DNA sequences, scoring of the FISH signals was also not possible in our previous work (Kwasniewska et al. 2012).
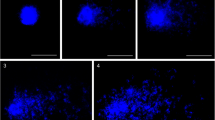
In this work, we classified the comets into four categories based on the distribution of FISH signals between the head and the tail. We only considered the comets with a tail. The classification was based on the distribution of 5S and 25S rDNA, independently. Comets with the 5S or 25S rDNA only in the head were classified as category I (Fig. 2a, e); comets with rDNA signals in the head and in the ‘halo’ were classified as category II (Fig. 2b, f); comets with rDNA in the head, ‘halo’ and the tail were classified as category III (Fig. 2c, g); and comets with the rDNA signals in the ‘halo’ and the tail were classified as category IV (Fig. 2d, h).
Only comets in categories I and II were observed in the control (Fig. 3). Differences in the frequencies of the comets in categories I–IV based on the distribution of 5S and 25S rDNA induced by different mutagens were observed. However, after all treatments, comets with 5S rDNA in the tail were observed more often than comets with 25S rDNA (Fig. 3). These results are in agreement with our previous statement about the different sensitivity of rDNA sequences (Kwasniewska et al. 2012). 5S rDNA was involved in comet formation more often than 25S rDNA using treatment with MH in Crepis capillaris cells. We can now extend the differences in the behaviour of 5S and 25S rDNA to other mutagens and plant species, thus emphasising that the concept is generalised. A very similar distribution of the comet categories with 25S rDNA was observed following treatment with MH and MNU at 72 h post-treatment. The same comet categories, namely, I–III (no comet category IV), were observed if γ-rays were applied; however, higher frequencies of comet category II and lower frequencies of comet category III were observed in comparison to both chemical treatments. Therefore, a lower sensitivity of 25S rDNA to a physical then to a chemical treatment can be suggested. The frequencies of comet categories were generally unchanged with respect to mutagen doses. However, following mutagenic treatment, the frequencies of comet categories at 72 h post-treatment differed from those at 96 h post-treatment. The reversibility of 25S rDNA damage depended on the mutagen dosages. Effective repair processes were confirmed only for lower doses of the mutagens.
A totally different behaviour of 5S rDNA in comparison to 25S rDNA was observed after the applied treatments. No comets in category I and high frequencies of comets in category III were observed at 72 h post-treatment. Moreover, in contrast to 25S rDNA, differences in the frequencies of 5S rDNA-bearing comets were observed for MH, MNU and γ-rays. This might suggest that the level of the fragmentation of 5S rDNA sequences is dependent on the mutagen type. In the MH-treated cells, only comets in category III were observed. Comets in categories II and III were observed in 2 mM MNU-treated cells, whereas comet categories III and IV were observed in 3 mM MNU-treated cells. Comet categories II and III occurred in the γ-ray-treated cells; however, a weaker fragmentation of 5S rDNA than after treatment with MNU was observed. Differences in the frequencies of the comet categories with 5S rDNA were seen at 72 and 96 h post-treatment for all mutagens at selected doses (Fig. 3), which indicates the reversibility of 5S rDNA damage.
We conclude that 5S rDNA sequences are more sensitive to mutagenic treatment than 25S rDNA. The effect of treatment within the 5S rDNA sequences is different for the applied mutagens. We suggest that 5S rDNA sequences are suitable to test the genotoxicity of mutagens, including environmental factors. In contrast, the predictable behaviour of 25S rDNA independent of treatment was observed. The differences in the response of 5S and 25S rDNA to mutagens may be explained by the involvement of 25S rDNA in the formation of the nucleolus and differences in chromatin structure. As a consequence, the involvement of 25S rDNA in comet formation does not seem to be a suitable indicator of the DNA-damaging effects of mutagens, especially as compared to the distribution of DNA damage within the plant genome that is induced by the different mutagens.
We also classified the comets into four categories based on the distribution of telomeric and centromeric DNA sequences, independently (Fig. 4). The classification was set like the one for rDNA. Only comet categories I and II were observed in the control (Fig. 5). The same comet categories based on the distribution of centromeric DNA were observed for all treatments with similar frequencies. In contrast, significant differences in the frequencies of the four categories based on the distribution of telomeric DNA were observed between the MH-, MNU- and γ-ray-induced comets. Comet categories II and III were the most common for all treatments. However, comet category IV occurred only in MNU- and γ-ray-treated cells. From the mutagens used in this study, γ-rays induced comet categories III and IV with the highest frequencies. Comets in category I were present only in MH-treated cells. To summarise, the telomere regions of chromosomes were the most fragile after treatment with γ-rays, then MNU and, finally, MH. Surprisingly, differences in the frequencies of the comet categories were seen at 72 and 96 h post-treatment, therefore, the irreversibility of DNA damage within telomeric and centromeric DNA caused by the mutagens is stated.
These results indicate that telomeric DNA sequences are involved in comet formation more often than centromeric ones, which indicates that breaks occur in/or near the telomere sequences. It needs to be kept in mind that telomeres need only one break to generate a fragment, which is then able to migrate in the electric field. By contrast, centromeres need two breaks to form a fragment that can migrate to the tail.
In view of the results, the organisation of the centromeric and telomeric DNA in the nuclei needs to be underlined. Rabl orientation with a polarity of centromeres and telomeres was observed in barley (Dong and Jiang 1998). It is known that telomeres are in close association with the nuclear membrane, whereas centromeres are not attached to the nuclear membrane. However, these features do not prove the better mobility of telomeric DNA than centromeric DNA. The differences can be explained by the presence of ‘hot spots’ in/near the telomere DNA sequences during a mutagenic attack or the specific location of these sequences at the end of chromosomes. The study using comet-FISH of Santos et al. (1997) showed that centromere probes hybridised in a completely different manner than that of telomere probes. Telomeres were concentrated on the main nuclear area near the nuclear membrane, probably due to the specific chromatin structure and the attachment to the nuclear membrane in plant cells (Rawlins et al. 1991). The telomere signals were also present in the tails—some pieces of DNA ran out of the nucleus. No differences in the distribution of centromeres and telomeres were observed in our studies. Our results give an insight into the breakage sensitivity of telomeres, which is of great importance for the stability of the genome. The loss of telomere sequences may lead to a destabilisation of the genome. Reports that indicate telomeres as points of mutagenic attacks are well known (Slijepcevic et al. 1998; Boei et al. 2000). Using comet-FISH, it was shown that telomeric repeats are more fragile compared to the total DNA (Arutyunyan et al. 2005).
Comet-FISH also makes it possible to compare the involvement of specific DNA sequences in comet formation with the localisation of other endpoints of genotoxicity, e.g. chromosome aberrations. It should be underlined that not all initially mutagen-induced chromatin breaks are observed as chromosome aberrations, as they can restitute due to repair processes. Comet-FISH hybridisation is as sensitive and reproducible as FISH on chromosome preparations (Rapp et al. 2000). An application of FISH with rDNA sequences as probes in a micronuclei (MN) characterisation showed that 5S rDNA-bearing chromosomes are involved in the formation of chromosome aberrations more often than NOR-bearing chromosomes (Juchimiuk-Kwasniewska et al. 2011). The results of this study confirmed that 5S rDNA was more frequently subjected to DNA damage.
Studies on the origin of MH-, MNU- and γ-ray-induced micronuclei using the MN test with FISH and telomere- and centromere-specific probes were also done previously by our group (Juchimiuk et al. 2007). We found that micronuclei most often originated from terminal fragments; however, the involvement of telomere-specific sequences differed between the applied mutagens. Micronuclei with telomeric DNA were most frequently observed after γ-ray treatment (81 %) than MNU (28 %) and less frequently in MH-treated cells (10 %). Similarly, in this study, the telomeric DNA sequences were most often involved in comet formation after treatment with γ-rays and less often in MH-treated cells.
The differences regarding the involvement of specific DNA sequences in DNA damage that is induced by the applied mutagens could be related to their different mechanisms of action. MH is a clastogenic agent that can lead to chromosome breaks. It can also cause spindle fibre defects. MNU is an alkylating agent that mainly induces gene mutations; however, it can also lead to chromosomal aberrations. The applied chemical mutagens act in different phases of the cell cycle: MH in the S-phase and MNU in the G2 stage (Maluszynska and Maluszynski 1983).
In conclusion, this is a report on a comparative investigation of the action of three mutagens using the comet-FISH technique in plant cells. We showed that the involvement of specific DNA sequences in DNA damage is dependent on the mutagen used. Among the DNA sequences used as probes for comet-FISH, 5S rDNA and telomeric DNA sequences should be preferentially used in any analysis of the DNA-damaging effects of environmental mutagens in order to gain a better understanding of their mechanisms of action. The comparison of the involvement of specific chromosome domains in direct DNA breakage/repair, and in the formation of chromosome aberrations after mutagen treatment, showed the compatibility of the results. However, it needs to be stressed that chromosome aberrations are the final result of mutagenic treatment, after taking into account the repair processes. Comet-FISH enables the analysis of direct DNA damage within specific DNA sequences. Additionally, the examination of this effect within the post-treatment time allows an analysis of the repair effectivity. To summarise, the comet-FISH approach is suitable for the detailed quantification of the DNA damage and repair within specific DNA sequences in plant mutagenesis.
References
Aragón-Alcaide L, Miller T, Schwarzacher T, Reader S, Moore G (1996) A cereal centromeric sequence. Chromosoma 105:261–268
Arutyunyan R, Rapp A, Greulich KO, Hovhannisyan G, Haroutiunian S, Gebhart E (2005) Fragility of telomeres after bleomycin and cisplatin combined treatment measured in human leukocytes with the Comet-FISH technique. Exp Oncol 27:38–42
Boei JJ, Vermeulen S, Natarajan AT (2000) Analysis of radiation-induced chromosomal aberrations using telomeric and centromeric PNA probes. Int J Radiat Biol 76:163–167
Collins AR (2004) The comet assay for DNA damage and repair: principles, applications, and limitations. Mol Biotechnol 26:249–261
Cotelle S, Férard JF (1999) Comet assay in genetic ecotoxicology: a review. Environ Mol Mutagen 34:246–255
Dong F, Jiang J (1998) Non-Rabl patterns of centromere and telomere distribution in the interphase nuclei of plant cells. Chromosome Res 6:551–558
Fairbairn D, Meyers D, O’Neill K (1994) Detection of DNA damaging agents in environmental water samples. Bull Environ Contam Toxicol 52:687–690
Gerlach WL, Dyer TA (1980) Sequence organization of the repeating units in the nucleus of wheat which contain 5S rRNA genes. Nucleic Acids Res 11:4851–4865
Gichner T, Plewa MJ (1998) Induction of somatic DNA damage as measured by single cell gel electrophoresis and point mutation in leaves of tobacco plants. Mutat Res 401:143–152
Gichner T, Ptácek O, Stavreva DA, Wagner ED, Plewa MJ (2000) A comparison of DNA repair using the comet assay in tobacco seedlings after exposure to alkylating agents or ionizing radiation. Mutat Res 470:1–9
Hagberg A, Persson G (1968) Induced mutations in barley breeding. Hereditas 59:396–412
Jovtchev G, Menke M, Schubert I (2001) The comet assay detects adaptation to MNU-induced DNA damage in barley. Mutat Res 493:95–100
Juchimiuk J, Hering B, Maluszynska J (2007) Multicolour FISH in an analysis of chromosome aberrations induced by N-nitroso-N-methylurea and maleic hydrazide in barley cells. J Appl Genet 48:99–106
Juchimiuk-Kwasniewska J, Brodziak L, Maluszynska J (2011) FISH in analysis of gamma ray-induced micronuclei formation in barley. J Appl Genet 52:23–29
Kwasniewska J, Grabowska M, Kwasniewski M, Kolano B (2012) Comet-FISH with rDNA probes for the analysis of mutagen-induced DNA damage in plant cells. Environ Mol Mutagen 53:369–375
Maluszynska J, Maluszynski M (1983) The influence of MNUA and MH on the cell cycle and DNA contents in meristematic cells of barley. Acta Biol 11:227–237
Poli P, Buschini A, Restivo FM, Ficarelli A, Cassoni F, Ferrero I, Rossi C (1999) Comet assay application in environmental monitoring: DNA damage in human leukocytes and plant cells in comparison with bacterial and yeast tests. Mutagenesis 14:547–556
Rapp A, Bock C, Dittmar H, Greulich KO (2000) UV-A breakage sensitivity of human chromosomes as measured by COMET-FISH depends on gene density and not on the chromosome size. J Photochem Photobiol B 56:109–117
Rawlins DJ, Highett MI, Shaw PJ (1991) Localization of telomeres in plant interphase nuclei by in situ hybridization and 3D confocal microscopy. Chromosoma 100:424–431
Restivo FM, Laccone MC, Buschini A, Rossi C, Poli P (2002) Indoor and outdoor genotoxic load detected by the comet assay in leaves of Nicotiana tabacum cultivars bel B and Bel W3. Mutagenesis 17:127–134
Santos SJ, Singh NP, Natarajan AT (1997) Fluorescence in situ hybridization with comets. Exp Cell Res 232:407–411
Slijepcevic P, Natarajan AT, Bryant PE (1998) Telomeres and radiation-induced chromosome breakage. Mutagenesis 13:45–49
Unfriend I, Gruendler P (1990) Nucleotide sequence of the 5.8S and 25S rRNA genes and of the internal transcribed spacers from Arabidopsis thaliana. Nucleic Acids Res 18:4011
Acknowledgements
The authors wish to thank Prof. J. Maluszynska for the valuable discussions during the preparation of this manuscript and MSc. Marta Hosiawa-Baranska for her technical assistance.
Funding
Financial support for this study was provided by the National Science Centre, Poland, under agreement 3178/B/P01/2011/40.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Kwasniewska, J., Kwasniewski, M. Comet-FISH for the evaluation of plant DNA damage after mutagenic treatments. J Appl Genetics 54, 407–415 (2013). https://doi.org/10.1007/s13353-013-0169-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13353-013-0169-6