Abstract
Breast cancer (BC) prevails as a major burden on global healthcare, being the most prevalent form of cancer among women. BC is a complex and heterogeneous disease, and current therapies, such as chemotherapy and radiotherapy, frequently fall short in providing effective solutions. These treatments fail to mitigate the risk of cancer recurrence and cause severe side effects that, in turn, compromise therapeutic responses in patients. Over the last decade, several strategies have been proposed to overcome these limitations. Among them, RNA-based technologies have demonstrated their potential across various clinical applications, notably in cancer therapy. However, RNA therapies are still limited by a series of critical issues like off-target effect and poor stability in circulation. Thus, novel approaches have been investigated to improve the targeting and bioavailability of RNA-based formulations to achieve an appropriate therapeutic outcome. Lipid nanoparticles (LNPs) have been largely proven to be an advantageous carrier for nucleic acids and RNA. This perspective explores the most recent advances on RNA-based technology with an emphasis on LNPs’ utilization as effective nanocarriers in BC therapy and most recent progresses in their clinical applications.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer (BC) defines an array of malignancies of the mammary glands. According to GLOBCAN, in 2022, it stood as the most common neoplasm in women, making it one of the most frequently diagnosed cancers worldwide [1]. BC displays high heterogeneity and classifies into four subtypes based on immunohistochemical features: Estrogen receptor positive (ER +); progesterone receptor positive (PR +); human epidermal growth factor receptor positive (HER2 +); and triple negative breast cancer (TNBC) in which none of the previous receptors are expressed [2, 3]. Both ER and PR are diagnostic and prognostic factors. High expression of ER is found in ~ 70–75% of invasive breast carcinomas, and women with an ER + BC show an increased overall survival with respect to the ER- [4]. The ER expression is commonly used to choose the proper treatment because it has predictive value in prognosis and endocrine therapy responsiveness [5]. High levels of PR are a good prognostic factor associated with a better overall survival, while lower levels are generally linked with more severe forms and poor prognosis [6, 7].
HER2 is a protooncogene that encodes epidermal growth factor receptor with tyrosine kinase activity. In BC, HER2 gene is overexpressed in about 20% of invasive forms, and as a prognostic factor, HER2 + is associated with high rates of recurrence and mortality, being a predictive factor for treatment benefits from HER2-targeting agents like trastuzumab, lapatinib, and pertuzumab [8]. TNBC accounts for 10–20% of all breast cancers and it is characterised by the absence of the markers listed above it is therefore untreatable with target therapies. This, together with the moderate/high aggressiveness and highly proliferative profile makes TNBC associated with the poorest prognosis [9].
BC is marked by several risk factors classified as modifiable and non-modifiable (Fig. 1). Modifiable factors include lifestyle, physical activity, body weight, alcohol consumption, and exposure to artificial light [10, 11]. Non-modifiable factors encompass genetic mutations, sex, age, and family history [12]. Moreover, numerous studies have proved a strong connection between exposure to sex hormones and BC. Events like first menstruation, pregnancy, breastfeeding, menopause and the age of their appearance, as well as their duration, influence the occurrence of BC [13]. The first full-term pregnancy before the age of 30, the number of pregnancies, early menopause are linked to a lower risk of BC [14]. In contrast, early age menarche increases the risk of disease’s development [15].
Overall BC is a complex disease, where both the combination of genetic susceptibility and the interaction with the environment affect the determination of the phenotype. As reported in the latest WHO epidemiological data report, in 2022, BC represented 23.8% of all cancer cases and 15.4% of cancer-related deaths among women. Globally, this disease caused more than 666,000 fatalities with 2.3 million new cases of BC diagnosed every year [16]. Figure 2 shows the cancer incidence and mortality age standardised rates (ASRs) worldwide, in 2022. The fatality of BC is still high for all the reported countries, with the highest mortality numbers registered in Europe, Africa and Oceania. Moreover, by 2050, the number of cases diagnosed annually is expected to reach 3.36 million, with deaths accounting for 1.06 million yearly. To date, BC remains one of the major burdens on the global healthcare system [17].

Source: GLOBOCAN 2022 [16]
Scatter plot of the incidence and mortality age-standardized rates (ASRs) worldwide, in 2022. The graph shows that the higher rates are reported for Europe and Africa.
The current treatments for BC include surgery, chemo-, radio-, hormone, and immuno- therapy [18]. Patients with resectable lesions undergo surgery, usually followed by pharmacological treatment. When possible, the surgery is breast-conserving, but depending on the spreading of the lesion and its staging, the resection can often imply the complete removal of the breast tissue. Chemotherapy and radiotherapy are still the conventional pharmacological treatments. All the above therapies are often effective and prolong the lifespan of patients [19]. However, they do not prevent risks of recurrence and come with strong side effects, like hair loss, fatigue, increased susceptibility to infections, bone marrow suppression, leucopenia, and anemia [20, 21]. Additionally, drug-specific side effects can also lead to cardiotoxicity and pulmotoxicity [22].
A more recent therapeutic approach is represented by immunotherapy. Cancer immunotherapy harnesses the body's own immune system to target tumor cells, with various approaches [23, 24]; currently, a few of them are also exploited as BC treatment options [25]. These strategies include the use of monoclonal antibodies, immune checkpoint inhibitors, and vaccines designed to prime the immune system against cancer cells [26]. Moreover, over the last decade, adoptive cellular therapies have been implemented, they include the introduction of engineered immune cells into the body to recognise and kill cancer cells [27, 28].
About one third of patients affected by BC exhibit upregulation of HER2 which makes the receptor a good molecular target for selective immunotherapy. Hence, many types of monoclonal antibodies have been developed and demonstrated effectiveness by inhibiting signalling pathways involved in tumor growth [29]. By combining monoclonal antibodies, several pathways may be blocked simultaneously, leading to potential synergism. Among them trastuzumab and pertuzumab − often utilized in combination − are now used in clinical practice for the treatment of those patients [30].
Immune checkpoints are a set of regulatory proteins, crucial in the adaptive immune response. They are essential for maintaining self-tolerance and immune homeostasis. Immune checkpoints also act as immunosuppressors. For example, proteins like programmed cell death protein 1 (PD-1), its ligand (PD-L1), and CTLA-4 (cytotoxic T-lymphocyte-associated protein 4) are exploited by cancer cells to evade the immune system [31]. By blocking these checkpoints with specific inhibitors, it is possible to unleash the immune system's full potential for cancer cell recognition and destruction, leading to improved outcomes for patients [31, 32]. Cancer vaccines are under investigation as a form of active immunotherapy, designed to stimulate the immune system to recognize tumor cells as non-self [33]. As an example, NeuVax™ (a peptide vaccine targeting HER2) is currently undergoing a clinical trial [34]. Furthermore, adoptive cellular therapies involve the manipulation of immune cells obtained from either the tumor or the peripheral blood of patients. These therapies include three main approaches using (I) tumor-infiltrating lymphocytes (TILs), (II) TCR-modified lymphocytes, and (III) chimeric antigen receptor (CAR) T cells [35]. Ongoing clinical trials are investigating the feasibility, safety, and effectiveness of CAR-T cells in targeting HER2, GD2, and CD44v6 surface antigens in BC cells [36]. As an example, the clinical trial Multiple 4SCAR-T Cell Therapy Targeting BC (GIMI-IRB-20005) is currently in phase II, attesting the clinical applicability of this therapeutic platform. Adoptive T-cell therapy holds promise, however, to date, it shows poor functionality and persistence, due to the inter-individual heterogeneity of the T cell population of the tumor microenvironment, inter-tumoral heterogeneity, and resistance mechanisms [37]. Furthermore, high costs and methodologic complexity significantly affect the extensive application of T-cell therapy.
Overall, immunotherapy is not very effective for solid tumors, due to several factors characterising the complex and immunosuppressive cancer microenvironment. Firstly, the dense extracellular matrix (ECM) represents an additional layer, hard to infiltrate. Moreover, the cellular components play a key role in the immune response impairment: myeloid-derived suppressor cells (MDSCs) can prevent specific anti-tumour adaptive immune responses by inhibiting T cells function. In addition, MDSCs produce increased levels of immunosuppressive cytokines like IL-10 and TGF-β [38].
All the above hinders the efficacy of immunotherapy in solid lesions. Moreover, an increased risk of developing autoimmune diseases is a side effect of immunotherapy [39]. Considering all the discussed aspects, novel therapeutic strategies are still needed to obtain a proper treatment of patients affected by BC, especially for the TNBC. The emergence of cancer nano therapeutics opened up many new opportunities by providing some effective tools in cancer treatment, shifting the focus from conventional approaches to personalized medicine [40, 41].
In the last decade, nanoparticles (NPs) have emerged as innovative and promising tools for both diagnostics and therapy; they offer all the physicochemical advantages of nanosystems, and they can be easily modified to suit the desired purpose. As engineered nanomaterials, they proved to be effective drug delivery systems due to their size and their capacity to enter several biological barriers like gastrointestinal walls, blood brain barrier, and cell membranes, overcoming the limitations classically related to traditional drugs [42, 43]. Given the promising characteristics of the NPs, several fabrication strategies and a plethora of materials have been explored to the preparation of new nanosystems for BC treatment [24, 44, 45]. Among them, lipid NPs (LNPs) present major advantages as drug delivery agents by demonstrating precise targeting, reduced side effects, efficient pharmacokinetics and low toxicity, also overcoming the challenge of multidrug resistance [46,47,48,49,50]. Moreover, LNPs production is easily scalable, as demonstrated by several studies and by the massive production of anti-covid vaccines during the last pandemic.
These are just a few reasons why LNPs have been extensively used as delivery platforms, now standing as the most efficient means of RNA delivery [51,52,53,54,55], exemplified by the approval of Patisiran for amyloidosis therapy in 2018 [56, 57]. In the last decade,
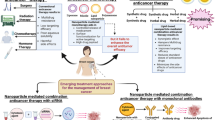
RNA-LNPs based therapy has also shown effectiveness in BC treatment (Fig. 3), providing a smart, alternative approach and increasing the success rate of BC cure, including the most resistant forms [56, 58].
Conventional treatments vs. RNA-LNPs therapeutics for BC. Conventional therapies encompass both local interventions, such as surgery and radiation, and systemic treatments, including chemotherapy and hormone therapy. In contrast, RNA-LNP-based therapeutics specifically target tumor sites (Image created with Biorender)
But can RNA-LNPs therapy be a standalone approach for BC treatment? This perspective provides an overview of the most recent, mostly within the last 5-years, RNA-based LNPs for BC gene therapy. It focuses on the different types of RNAs used, by giving a perspective on whether RNA-LNPs therapies can be a viable strategy for BC treatment.
Lipid NPs as drug delivery vehicles
Over the years, the gradually optimized large-scale production of LNPs successfully moved them from the laboratory to the clinic, making them as pioneer in commercialized nanomedicines. Especially, after the massive administration of Moderna (mRNA-1273) and Pfizer/BioNTech (BNT162b2) COVID-19 vaccines [59,60,61], LNPs demonstrate their safety and effectiveness as delivery vehicles for mRNAs [62]. The synthesis of LNPs includes two major methods: (1) Bottom-up [63] and (2) top-down approaches [64,65,66,67]. The most utilized bottom-up techniques are chemical vapor deposition, laser pyrolysis, microfluidics, and plasma or flame spraying synthesis. Commonly used methods for the top-down fabrication are mechanical milling, electro-explosion, thermal decomposition, chemical etching, nanolithography, etc. Therefore, by modulating the synthetic procedures and reaction conditions, desirable alterations in the LNPs' morphological and chemical attributes can be induced [68]. A typical FDA-approved LNP consists of four different types of lipid components. These include ionizable lipids, phospholipids, cholesterol, and PEGylated lipids [69] (Table 1 and Fig. 4). Each lipid component provides the NPs distinctive properties and specific advantages. Therefore, compositions, ratios, and concentrations of lipids, also contribute to obtaining a tailored nanosystem for specific applications.
The possibility to modulate the physical–chemical characteristics of LNPs makes them particularly suitable for a stable delivery of small molecules and nucleic acids, contributing to successful cell targeting [69, 84, 85]. LNPs have emerged as efficient tools for advancing the technology for vaccination, immunotherapy, and diagnostics [69, 86]. This is due to the LNPs’ properties like effective stability, high encapsulation efficiency, biocompatibility, limiting off-target effects, protection from the mononuclear phagocyte system (MPS), and other pharmacokinetic and pharmacodynamic attributes [87]. For example, Doxil®, the first FDA-approved nano drug [88] has been formulated as a liposomal nanosystem containing doxorubicin. The Doxil® composition contains hydrogenated phosphatidylcholine, cholesterol, and PEG-lipid in a specific ratio (56:39:5) [82, 89]. Since then, research in the development of LNPs has seen an exponential increase. The bibliometric searches conducted in WorldCat indicated that the number of scientific articles on LNPs has exceeded 51,300 in the past three decades (1995–2024). More than 1,900 of these have reported LNPs specifically for the treatment of BC, and to date many undruggable pathways have become treatable thanks to the use of LNPs.
The effect of structure, composition and surface modification on delivery efficacy
As mentioned above, the structure modulation heavily affects the efficiency of LNPs as delivery system. The inclusion of phospholipids and cholesterol, for example, confers great stability and integrity [65, 66]. The phospholipids, commonly called “helper lipids,” improve the biodistribution of LNPs by binding to specific plasma proteins, thus facilitating cellular uptake and cytosolic entry [90]. In contrast to some cationic lipids, phospholipids are not positively charged and therefore may help reduce inflammation and cytotoxicity potentially induced by cationic lipids [91]. Currently, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE) and 1,2-distearoyl-sn-glycero-3-phosphoethanolamine (DSPC) [92] are the two most used phospholipids, DSPC has been successfully used in clinical applications, like siRNA therapeutics and the mRNA vaccines against SARS-CoV-2 [93]. DSPC possesses two saturated aliphatic tails, that confer a higher melting point and a solid state at room temperature. This saturation characteristic makes the LNPs membrane composed of DSPC highly rigid and stable. DOPE includes two aliphatic tails, each tail contain a cis-double bond, and when used for gene delivery, it increases transfection efficiency [94]. In addition, the tapered structure of DOPE helps to adjust the size of LNPs and the curvature of the membrane, favouring the cargo encapsulation [95]. Including different helper lipids in the formula of LNPs is expected to achieve the co-delivery of different types of genes. The research of Hamilton et al. [28] showed that the encapsulation efficiency of DOPE-incorporated LNPs was significantly improved (88–97%) compared to LNPs containing only DSPC (63%) [96].
Cholesterol plays multiple key roles in LNPs formulations. It can stabilize the surface membrane of LNPs, preventing premature release of nucleic acids by maintaining the integrity of the membrane, and promoting its circulation in the blood to extend the half-life of LNPs [90, 97]. A study by Kim et al. showed cholesterol enhanced gene transfection and biodistribution of mRNA-LNPs [98]. Furthermore, cholesterol concentration affects various LNP properties, including particle size, polydispersity, internalization, and overall structure. Very low cholesterol levels can reduce encapsulation efficiency [99]. Conversely, increasing cholesterol levels at the expense of ionisable or cationic lipids result in smaller particle size and reduced polydispersity while maintaining particle integrity [100]. Another study by Kotoucek et al. investigated the role of cholesterol in liposome formulations. They found that, when the molar ratio of cholesterol to DSPC was mixed from 0 to 50%, the size of the liposomes also increased. It shows a size-dependent association of liposomes with cholesterol amounts [96]. Therefore, optimizing the ratio between cholesterol and other lipid components is critical to tailor LNP properties to specific formulation requirements.
In the first instance, permanently positively charged lipids such as DOTMA or DOTAP were included in LNPs formulation to facilitate RNA electrostatic interactions with the negatively charged RNA backbone. However, these lipids induce toxicity by destroying cellular and nuclear membranes and by increasing the production of reactive oxygen species [101]. To address this problem, ionizable lipids with pH-sensitive tertiary amines were developed. The ionizable lipids are typically defined by the presence of tertiary amines that are deprotonated at a neutral pH and undergo protonation at lower pHs [63, 69]. Consequently, under lower pH, a positively charged LNPs layer is formed. The latter, in turn, can establish electrostatic interaction with negative charged molecules like nucleic acids [102], allowing an efficient encapsulation. This electrostatic interaction also enhances the biocompatibility of the LNPs, thereby preventing nonspecific binding [103]. The pH in mature endosomes is also acidic [104]. Under such conditions, the protonation of ionisable lipids leads to destabilization of the endosomal membrane, allowing LNPs to escape from the endosome [69]. Notably, the pKa value has proven critical for efficient LNPs delivery and transfection potency. Jayaraman et al. firstly identified DLin-MC3-DMA from an amino lipids' library [105], and showed that it has a high transfection efficiency with an optimal pKa value between 6.2 and 6.5. This lipid was later successfully used in Patisiran (Onpattro). In another study, Whitehead identified four key parameters for the efficacy of siRNA delivery in vivo, highlighting the importance of pKa of LNPs containing ionizable lipids [106]. The conclusion of their results showed that the pKa value should be higher than 5.4 to get efficient gene silencing [107].
PEGylated lipids (PEG-lipids) usually represent the smallest portion (typically ∼1.5 mol%) of LNPs. Despite that, these influence several key properties, like size and zeta potential [75]. This directly impacts the delivery efficacy of the LNPs. Usually, PEG with a higher molar mass (20–50 kDa) is used for small-sized drugs. This increases the size of the carrier, which helps them prevent renal clearance. Conversely, larger drugs like antibodies and nanoparticle drugs use a lower molar mass of PEG, like 1 to 5 kDa [108]. These also have roles in preventing the aggregation of LNPs and enhancing the LNPs' blood circulation time. In a very interesting study, Lokugamage et al. [75] demonstrated the PEGylation-mediated stability and long-term distribution of LNPs [75]. They loaded high- and low-PEG LNPs with luciferase to determine their stability. They found that LNPs with a lower mol% of PEG exceeded the quality control cut-off (200 nm in size), indicating the necessity of PEG for delivering high-quality LNPs. Moreover, high PEGylated LNPs showed improved long-term distribution effects, as indicated by their luciferase intensities after 48 h. This proves a lower degradation of luciferase and, consequently, higher stability [109].
In addition, PEGylated lipids are also involved in protecting LNPs through mononuclear phagocyte system (MPS) clearance [102, 110]. Further studies have cumulatively determined their roles in improving encapsulation of nucleic acids, increasing circulatory half-lives, in vivo distribution, and immune response. Conclusively, these decrease fusogenicity and increase the overall stability [85] and bioavailability [103].
Besides structure, surface modification is another key approach in modulating the characteristics of LNPs to specific target cell types. As discussed above, the off-target effect associated with many nanomedicines remains a limitation that needs to be overcome, as it implies an increased dose of drug to achieve the desired therapeutic effect and reduces the bioavailability of the drug. Affinity targeting of the LNPs to the BC lesions can be easily achieved due to several chemical surface modifications [23, 111]. By attaching smart molecules to the available functional groups of the lipids the selectivity and effectiveness can be certainly improved. In a very recent study, Kim et al. engineered LNPs for pTEN mRNA delivery in TNBC cells [112]. They presented a PD-L1-targeting LNP integrating peptides with strong PD-L1 binding affinity onto the LNP surface by conjugating them with PEGylated lipid. This led to an increased affinity for the target, by obtaining an augmented selectivity. These results clearly indicate how the targeting therapy leads to an improved, personalized and more effective treatment.
Cellular uptake and endosomal escape
LNPs overcome several limits of conventional delivery systems, such as low delivery efficiency and immune responses triggered by naked nucleic acids. Currently, LNPs are considered the most effective tool in RNA-based therapies. However, the release into the cytoplasm of loaded nucleic acids is very limited and it still represents an issue. LNPs enter the cell mainly through macropinocytosis and clathrin-mediated endocytosis [84]. Then they are incorporated in early endosomes, which subsequently mature into late endosomes and finally into lysosomes (Fig. 5). Efficient delivery is achieved if the nucleic acid is released from LNPs into the cytoplasm before endosomes become lysosomes, a process known as endosomal escape [113,114,115]. It is widely acknowledged that modulating the composition of LNPs is a promising strategy to improve endosomal escape and thereby increase transfection efficiency. In a recent work, Tang et al. introduced a novel mRNA vaccine based on sialic acid (SA) cholesterol derivatives and cleavable PEG lipid modification, which efficiently targets dendritic cells (DCs) and achieves effective endosomal/lysosomal escape. The SA modification enhances DCs uptake of LNPs by 2 times, facilitates rapid escape from early endosomes (EEs), prevents entry into lysosomes, and promotes mRNA translation in both cytoplasm and endoplasmic reticulum (ER). Additionally, the incorporation of cleavable PEG-lipids enhances cellular uptake and DCs targeting. SA-modified mRNA vaccines exhibit superior efficacy in DCs targeting, enhanced endosomal/lysosomal escape, improved transfection efficiency in DCs, superior tumour treatment effects, and lower side effects compared to commercially formulated mRNA vaccines [116]. In addition, identifying the exact endosomal compartment of escape is also crucial to improve cellular uptake [117] and it still is a debatable aspect. Hence, to understand how the uptake and the transport to endosomal compartments can affect the cargo’s delivery and release, nanocarriers with different properties have been investigated [118,119,120]. For instance, Paramasivam et al. compared the uptake and endosomal distribution of six mRNA-LNPs formulations, with similar size and RNA contents, but diverse chemical composition. The results obtained from different biological assays in primary human adipocytes, fibroblasts, and HeLa cells showed that the LNPs have different uptake efficiencies and endosomal distributions. This demonstrated that multiple mechanisms affect the delivery efficacy, including differences in LNPs composition and surface properties, loaded macromolecules, and their intracellular transport [120].
Schematic representation of endocytosis mechanisms for RNA-LNPs. Image adapted from ref [117], and created with Biorender
Overall, the main mechanism of endosomal escape has not yet been clearly determined. However, studying the highly dynamic nature and complex molecular interactions of the endosomal system is critical to clarify the intracellular action site and release kinetics. This would allow to improve the efficacy of LNPs as delivery system of nucleic acid drugs.
RNA therapeutics: a potent therapeutic approach against BC
In the last decade, RNA technologies have demonstrated their potential in a wide range of applications [121,122,123]. Recently, mRNA-based vaccines have successfully become a promising substitute for traditional treatments, due to their high immunogenicity and safety profile. Furthermore, the production processes are low-cost and can be easily scaled-up [124]. Data obtained from population-wide administration of anti-SARS-CoV-2 mRNA vaccines have confirmed tolerability and safety of RNA based technology in the clinical field, paving the way to extend its use both in diagnostics and therapeutics, being particularly suited for personalized medicine strategies [125].
RNA therapeutics include different approaches based on diverse mechanisms and implied molecules like messenger RNAs (mRNA), antisense oligonucleotides (ASOs), aptamers, etc. This perspective focuses on two therapeutic strategies: (1) RNA interference (RNAi) and (2) messenger RNA (mRNA) [126]. RNAi is a post-transcriptional regulation of gene expression, initially discovered in plants [127]. In this process, short, non-coding RNAs bind the target endogenous RNA, by altering the transcript processing. In contrary, mRNAs are long, coding sequences, that encode protein products. Once introduced in the cytoplasm, mRNAs can produce coded protein by exploiting the intracellular translation machine. Thus, as therapeutic agents, they are administered to produce peptides or proteins in order to replace defective proteins or presenting antigens for vaccination [128, 129].
Modulating the expression of genes involved in infections, as in the pathogenesis of genetic diseases like cancer, opens a range of therapeutic opportunities: mRNA-based anti-cancer vaccines are already proving effective for cancer immunotherapy, while RNAi proved a powerful tool for targeted gene silencing in cancer gene therapy [130]. RNAi through small interfering RNAs (siRNAs) shows advantages in patients who are resistant to classic treatments [131, 132] and consequently is particularly suitable in personalized medicine.
Despite its potential, clinical applications of RNAi are still limited by a series of critical issues: (i) being negatively charged, RNAs are not able to cross cell membranes; (ii) they are highly unstable in the systemic circulation, where they are easily degraded by enzymes like serum RNases and endonucleases; and (iii) their therapeutic use is affected by off-target effects. So far, different strategies have been explored to overcome these limitations, for example, chemical modifications, conjugation to carbohydrates, and inclusion in liposomal particles have all been proved to increase selective targeting and prevent RNA degradation. Moreover, NPs have also proved an effective system to overcome these limitations and have shown to be great nano-carriers for stable RNA delivery. Among them, LNPs have been largely proven to be excellent RNA carriers, showing several advantages, as described in the "The effect of structure, composition and surface modification on delivery efficacy" section. In this part the most recent advances on RNAi technology will be discussed, with a focus on LNPs application as effective nanocarriers for RNAs [133].
Recent advances in microRNA technology and clinical applications
MicroRNAs (miRNAs) are endogenous, single stranded, non-coding RNAs of about 22 nucleotides in length, which function at post-transcriptional level as modulators of gene expression. Over 2500 human miRNA sequences have been identified so far to regulate mammalian gene expression. Additionally, over 60% of human protein-coding genes have been found to contain at least one binding site for miRNA, demonstrating the significant contribution of miRNA-mediated regulation in the human genome [134]. miRNAs are key regulators of several pathophysiological processes, including cellular proliferation, and differentiation [135]. Several miRNAs are exclusively expressed or downregulated in certain cancers. It has been proven that miRNAs can act as oncogenes (oncomiRs) or onco-suppressors. Moreover, they are used as therapeutic agents to silence overexpressed genes and to revert the phenotype in cancer cells. Several studies have focused on targeting cancer by either inhibiting oncomiRs or overexpressing tumor suppressor miRNAs as a promising cancer treatment strategy [136]. Among the studied suppressive miRNAs, miR-34a has been proven to have strictly suppressive features in different cancer types [137]. Additionally, miR-34a is involved in the regulation of drug-resistance in BC, both in vitro and in vivo. Down regulation of miR-34a has been reported in MDR-MCF-7 cells compared with MCF-7 cells. Moreover, transfecting miR-34a mimics into MDR-MCF-7 cells led to partial MDR reversion. Additionally, by comparing the expression levels of miR-34a in a cohort of 113 patients with BC, the authors demonstrated the low expression of miR-34a is related with poor both overall and disease-free survival [138]. Another suppressive miRNA, miR-200c, is downregulated in BC tissues and cell lines, and its low expression is associated with poor prognosis [139]. In the case of oncogenic miRNAs, miR-182-3p is significantly upregulated in TNBC tissues and cell lines, indicating its potential oncogenic role in breast cancer [140].
Although some issues have still to be addressed, over the last decade the development of miRNA therapeutics has significantly improved demonstrating that nanotechnology can lead to clinical application [141].
LNPs for miRNA delivery
LNPs are effective delivery agents for miRNA molecules. In the case of BC, several studies have been performed to combine LNPs with miRNA as displayed in Table 2. In a pioneering study, Liu et al. developed a LNPs-based system for the simultaneous delivery of paclitaxel (PTX), and miR-200c [142]. Another study by Hayward et al. used LNPs to deliver miRNA125a-5p to 21MT-1 cells silencing HER2. They coated LNPs with hyaluronic acid as targeting agent. This improved the targeting of cancer cells, endosomal escape, and homogenous dispersal of miRNA in the cytoplasm. Additionally, the HER2 proto-oncogene was knocked down at both the transcriptional and translational levels, as demonstrated by qRT-PCR and western blot analyses [143].
In a recent study, Dinami et al., utilized the miRNA-based strategy to inhibit the telomeric protein Telomere repeat binding factor 2 (TRF2), by inducing cancer cell death in TNBC. The proposed LNPs’ composition consisted of a lipid mixture of DSPC/CHOL/DLODAP/PEG2000‐Cer16 (25/45/20/10 w/w). This system reduced TRF2 levels in HeLa, HCT116, Saos-2, U2-OS, MDA-MB-231, MDA-MB-436 and MDA-MB-436 growth. It was found that the TRF2 silencing by miR‐182‐3p led to DNA damage at the telomere and pericentromeric sites, thus resulting in genomic instability. Moreover, the study demonstrated that using LNPs to encapsulate miR‐182‐3p decreased tumor volume in vivo in multiple TNBC models, including the Olaparib‐resistant tumor xenografts derived from the patients. Lastly, LNPs‐miR‐182‐3p were also able to cross the blood–brain barrier, eliciting their potential against brain metastasis [144].
Nevskaya et al. proposed a LNP encapsulating three different microRNAs, namely miR-195 − 5p, miR-520a, and miR-630. These miRNAs downregulated the expression of stemness genes such as SOX2, MYC, TERT, and FZD9 in human breast cell lines. The system was intended to prevent metastasis by inhibiting the dedifferentiation of CD44 − cancer cells. Data show that the system effectively inhibited the ability of cells to form mammospheres in vitro and prevented lung metastasis in a C57L/6 mouse model (Fig. 6) [145].
Impact of three miRNAs (miR-195–5p/miR-520a/miR-630) encapsulated in LNPs on the formation of metastasis in the lungs of LLC-bearing mice. (A) Cartoon depiction of the workflow; s.c.: subcutaneous injection. (B) The area and (C) number of subpleural metastases in the lungs of mice after three-time injection of Lipo-miRNAmix in a dose of 10, 20, or 45 nmol (RNA)/kg. (D) The MII and metastasis inhibition rate (MIR) in the three experimental groups of mice. (E) Representative images of a lung excised from mice of the different groups. Data were subjected to one-way ANOVA with post hoc Tukey’s test. Data are presented as mean ± SD *p < 0.05; **p < 0.01; p < 0.001; **** p < 0.0001. Reprinted with permission from ref. [145]
Recent advances in siRNA technology and clinical applications
siRNAs, approximately 25 nucleotides in length, belong to a class of double-stranded RNAs that act as mediators in the RNAi. After being processed by dedicated enzymes in the cytoplasm, siRNAs bind a sequence of a complementary mRNA by inducing its degradation and preventing the translation in the corresponding protein product. To date, four siRNA-based treatments have been approved by FDA, demonstrating the potential efficacy and safety of this therapeutic approach [146]. Particularly, RNAi through siRNAs appears to be a promising tool to perform a selective gene silencing in the context of targeting tumor-specific mRNAs [84, 147]. Unlike microRNAs, siRNAs only bind to a target mRNA sequence, reducing the off-target effect that often leads to the silencing of multiple genes. Despite this, certain aspects, like degradation prevention and effective transportation of the siRNA still pose existing challenges. As mentioned above, these limitations are partially solved by using LNPs as carriers [148].
LNPs for siRNA delivery
Over the last decade, siRNAs have emerged as potent therapeutics against cancer due to their ability to silence genes involved in tumorigenesis and metastasis [149]. However, the delivery of siRNAs to the target remains a major inhibition [150]. LNPs have emerged as a suitable tool for an effective delivery of siRNAs; LNPs protect the siRNA from degradation and favour a selective delivery to the target cells, by increasing their bioavailability and reducing the off-target effect. Therefore, numerous studies have focused on LNPs as siRNAs delivery systems for therapeutic applications [151,152,153,154,155]. In 2018, the results of a clinical trial based on the use of a siRNA drug, Patisiran, were published, showing breakthrough results in the field [57]. The drug was used to treat patients suffering from hereditary transthyretin (TTR) amyloidosis. The disease is caused by the accumulation of misfolded TTR as amyloid fibrils in various tissues, including the heart, nerves, and gastrointestinal tract. Interestingly, the corresponding mRNA is primarily expressed in the liver. Thus, Adams et al. used a LNP nano-carrier to deliver siRNA to the liver, successfully inducing the degradation of mRNA encoding for TTR via RNAi [156]. Patisiran is currently present on the market as the first siRNA drug FDA approved. Recently, Kiaie et al. formulated siP2X7-LNPs, a lipid nano carrier for the delivery of siRNA targeting P2X7 receptor (P2X7R) [157]. The system included a novel symmetric branched ionizable lipid (SIL), able to deliver the loaded siRNA, and to target P2X7R in mouse mammary carcinoma cells (4T1). The findings suggest that SIL effectively transfected siP2X7, and siP2X7-LNPs notably suppressed migration by inducing apoptosis in 4 T-1 cells. These data demonstrated therapeutic efficacy of siRNA drugs in a triple-negative BC cell line.
To achieve synergistically enhanced tumour treatment effects, co-delivery of chemotherapeutic drugs and siRNA represents a promising opportunity. However, incorporating the drugs into siRNA-LNPs may disrupt their structure and provoke low encapsulation efficiency. To address those issues, in 2023 Butowska et al. proposed a surface modified siRNA-loaded LNP, obtaining a potent knockdown of Bcl-2 transcript in vitro and in vivo experiments. These authors used a sulfur-containing phospholipid to conjugate a (6-maleimidocaproyl) hydrazone derivative of DOX (DOX-EMCH) on the surface of the LNPs. They showed a successful delivery of DOX-EMCH to the cell nucleus while achieving potent knockdown of Bcl-2 in Raji cells. In vivo studies, conducted in Raji Luc + tumor-bearing NSG mice, further demonstrated the effectiveness of this system in inhibiting tumor growth. As shown in Fig. 7, they intratumorally injected different formulations, and they demonstrated a reduction in mice treated with siBcl-2 DOX LNP with the respect the used controls.
In vivo delivery of DOX-conjugated siRNA LNPs to treat a mouse model of lymphoma (A) Drug treatment schedules for in vivo experiments. The treatment doses for Bcl-2 siRNA and DOX were 1 μg and 0.1 μg per mouse, respectively (B) Representative bioluminescence images of tumor-bearing mice (C) Tumor growth curves for different treatment groups. All data is normalized to bioluminescence radiant efficiency (photons/sec/cm2/sr) on day 0. Data are plotted as mean ± SD, n = 5; ∗∗ P < 0.005, ∗∗∗∗ P < 0.0001 vs. Control (D) Average body weight of different treatment groups during the treatment schedule. Reprinted from ref. [158] under the CC BY-NC-ND license
These findings suggest that LNPs conjugated with doxorubicin could serve as a platform for co-delivering nucleic acids and chemotherapy drugs in combination cancer therapies [158]. Since overexpression of Bcl-2 occurs in breast tumour leading to increased survival rate, the investigated system was presented as a potential tool for BC therapy [159]. Based on the discussed results it is expected that the LNPs-siRNA technology will be increasingly applied for BC treatment and have outlets in clinical practice with enormous benefits for the patients.
Recent advances in mRNA technology and clinical applications
mRNA is a single strand nucleic acid sequence, and it is the molecule resulting from the transcription of a template strand of DNA. As mature transcript, it carries genetic information from the nucleus to the cytoplasm, where it is translated by ribosomes, leading to proteins’ synthesis. Due to its physiological function and properties, mRNA can be exploited as therapeutics, by producing proteins whose function is lost or whose production is deficient, depending on the disease [160].
Additionally, mRNA is easily designed, exhibiting minimal off-target effects, and compared to antibodies or proteins, it is considerably simpler to purify and scale up. [161, 162]. Also, mRNA activity is confined to cytoplasmatic compartment, and its expression is transient, dramatically decreasing the possibility to cause genetic mutations or integration processes. During the last pandemic the potential application of mRNA as nucleic acid became clear, and data collected following its administration confirmed its robustness and safety. This paved the way for exploring new applications of mRNA therapeutics.
For example, Karikó, Weissman, and co-workers developed approaches for modifications in the nucleotides that incrementally increased the stability, immunogenicity, and translation efficacy of mRNA [163]. All these attributes display the strong potential of mRNA to be used not just in vaccines but also for targeting cancer cells.
In cancer therapy, mRNAs encoding for tumour suppressor proteins antigens or cytokines are transfected to inhibit cancer cells proliferation, or enhance the immune response. Moreover, mRNA-encoded genome-editing proteins can destroy targeted genes by improving the treatment efficacy [122]. LNPs have also been proven to be the most efficient platform for mRNA delivery.
LNPs for mRNA delivery
Advances in drug delivery systems have contributed to the pre-clinical development of mRNA therapeutics, proposing them as a new class of drugs [107]. Out of a variety of materials like lipids, lipid-like polymers, proteins, etc., LNPs have emerged successfully in clinics for the delivery of mRNAs [164, 165]. To evaluate their efficacy in vitro and in vivo, El-Mayta et al. recently demonstrated the mRNA encapsulation efficiency to be 92.3% for C12-200 mRNA-LNPs with a molar ratio of 35/16/46.5/2.5 (ionizable lipid: helper lipid: cholesterol:lipid-PEG) [166]. This gives insights into the promising perspectives on the use of LNPs as mRNA carriers.
A study by Zhang et al. developed PTX amino lipid (PAL) derived NPs to integrate both chemotherapeutics and p53 mRNA to develop new treatment approaches for TNBC. The PAL p53 mRNA LNPs were found to show efficient PTX loading capacity (94.7% ± 6.8%) and mRNA encapsulation efficiency (88.7% ± 0.7%), in comparison to clinically used drugs like Abraxane® and Lipusu®. The LNPs also demonstrated the cytotoxicity of PTX and p53 mRNA in TNBC cells. Additionally, in vivo, anti-tumor efficacy was elicited in the TNBC mouse models. This provides a new platform for integrating chemotherapy with personalized medicine for treating TNBC [167].
Another in vitro study from Nakashima et al. aimed at developing a novel treatment strategy for refractory BC. It combined the tumour-tropic LNPs with inducible caspase9 (iC9) mRNA. The study demonstrated that the LNPs efficiently entered BC cells by delivering the mRNA cargo. Therefore, cytotoxicity mediated by LNP-mRNA was observed on three different BC cell lines (MDA-MB231, SKBR3, and MCF-7) loaded with iC9 mRNA. These effects resulted from iC9-mediated apoptosis through the activation of downstream caspase-9 and caspase-3/7 [168].
Recently, Wang et al. developed a universal stimulator of interferon genes mimic (uniSTING) based on a polymeric architecture, capable of activating STING signalling in various mouse and human cell types. Unlike traditional STING agonists, uniSTING selectively stimulates pathways associated with tumour control while avoiding tumour progression. Additionally, uniSTING enhances dendritic cell maturation and antigen-specific CD8 + T-cell responses, further bolstering its therapeutic potential. Extracellular vesicles (EVs) released from uniSTING-treated tumour cells contribute to dendritic cell sensitization, while combination therapy with α-Wnt2b antibodies shows synergistic inhibition of tumour growth and prolonged animal survival (Fig. 8).
The results of systemic uniSTING treatment exerting potent antitumor effects on orthotopic/metastatic tumours. a, Treatment scheme for the 4T1-Luc2 liver metastatic tumour model with the indicated formulations. b, Spider plots of individual tumour growth curves measured by bioluminescence intensity. n = 5. c, In vivo bioluminescence imaging of mice bearing liver metastatic tumours on days 1 and 10 following treatment. n = 5. d, Kaplan–Meier survival curves of mice treated with indicated formulations. n = 8. e, Treatment scheme for orthotopic Hepa1-6 HCC tumour-bearing mice with the indicated formulations. f, Average tumour weight in HCC tumour models after indicated treatments. n = 7. g, Kaplan–Meier survival curves of mice treated with indicated formulations. n = 8. For survival studies, 5 × 107 of bioluminescence intensity was used as the endpoint criteria in the 4T1 liver metastatic tumour model and a 30% weight loss was used as the endpoint criteria in the HCC tumour model. Each line represents one survival curve for each group; log-rank (Mantel–Cox) test. Significant differences were assessed using a one-way ANOVA and Tukey’s multiple-comparisons test. Results are presented as mean ± s.d. Reprinted with permission from ref. [169]
Delivery of uniSTING-mRNA via LNPs demonstrated potent efficacy in diverse tumours, including TNBCs, LLC1, B16-F10 and advanced orthotopic/metastatic liver tumour models surpassing existing STING agonists. Intravenous administration of uniSTING drastically inhibited 4T1 liver metastatic tumour growth and eradicated metastatic tumours in 40% of mice compared with the 2′3′-cGAMP-treated group which showed negligible effect (Fig. 8a–d). Moreover, mice with the orthotopic Hepa1-6 liver cancer exhibited reduced tumour burden and prolonged survival following intravenous injection of LNP-uniSTING-mRNA than controls (Fig. 8e–g). Overall, these findings highlight the promising role of LNP-mediated delivery of uniSTING-mRNA in overcoming therapeutic barriers associated with STING agonist therapy, particularly in cancers with downregulated or absent STING expression [169]. Table 2 summarises the most recent studies on LNPs for RNAs delivery in BC treatment.
Conclusions and future perspectives
BC is the most common cancer in women. Being a heterogeneous disease, different therapeutic regimens are currently used, depending on BC subtype, staging, and patients’ compliance. However, current available treatments are not in all cases effective; particularly, TNBC is the less responsive subtype. Hence, patients can experience recurrences over time, with serious health complications. Recently the scientific community has made considerable efforts to find more efficient approaches. By exploiting nanotechnology and RNA-based therapies, promising alternatives for the treatment of BC have been developed.
LNPs have shown great potential as delivery platform: they are, indeed, easily prepared and purified, and they can be smoothly scaled-up. LNPs are successfully used as nanocarriers in immunotherapy applications, proving their safety in clinical use. Furthermore, their surface can be easily modified with targeting molecules like peptides or antibodies to enhance the selectivity, the therapeutic effect, by increasing their bioavailability and decreasing the off-target effect and the effective dose.
Moreover, LNPs are the most effective delivery platform for RNAs, as largely testified by the worldwide administration of mRNA anti-COVID vaccines. Additionally, LNPs encapsulating RNA cargos have been proven effective in BC treatment [170,171,172,173], both in vitro and in vivo, demonstrating efficacy also for the treatment of metastatic forms of BC [174]. However, more clinical studies would be required to better understand the potential clinical applications and the safety profile of these systems applied to BC therapy.
The results of the last few years emphasized by the extraordinary outputs recorded after the worldwide administration of mRNA anti-COVID vaccines, confirm that the therapeutic potential of LNPs, combined with RNA technologies, will reach outstanding result in cancer gene therapy, including neurological and skin cancers [175,176,177,178].
Data availability
Data sharing not applicable to this article as no data set was generated or analyzed during the current study.
Availability of data and materials
Not applicable.
References
World Health organization. Breast Cancer. WHO. 2022. Available from: https://www.who.int/news-room/fact-sheets/detail/breast-cancer.
Rakha EA, Green AR. Molecular classification of breast cancer: what the pathologist needs to know. Pathology (Phila). 2017;49(2):111–9. Available from: https://www.sciencedirect.com/science/article/pii/S003130251640365X.
Orrantia-Borunda E, Anchondo-Nuñez P, Acuña-Aguilar LE, Gómez-Valles FO, Ramírez-Valdespino CA. Subtypes of Breast Cancer. In: Mayrovitz HN, editor. Breast Cancer. Brisbane (AU): Exon Publications; 2022. Available from: http://www.ncbi.nlm.nih.gov/books/NBK583808/.
Miah S, Bagu E, Goel R, Ogunbolude Y, Dai C, Ward A, et al. Estrogen receptor signaling regulates the expression of the breast tumor kinase in breast cancer cells. BMC Cancer. 2019 19(1):78. Available from: https://doi.org/10.1186/s12885-018-5186-8.
Wei S. Hormone receptors in breast cancer: An update on the uncommon subtypes. Pathol - Res Pract. 2023;250:154791. Available from: https://www.sciencedirect.com/science/article/pii/S0344033823004910.
Giulianelli S, Lamb CA, Lanari C. Progesterone receptors in normal breast development and breast cancer. Vol. 65, Essays in Biochemistry. 2021. p. 951–69. Available from: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85120976432&doi=10.1042%2fEBC20200163&partnerID=40&md5=acd08f222df8513967faaa0ed8b83661.
Purdie CA, Quinlan P, Jordan LB, Ashfield A, Ogston S, Dewar JA, et al. Progesterone receptor expression is an independent prognostic variable in early breast cancer: a population-based study. Br J Cancer. 2014 110(3):565–72. Available from: https://doi.org/10.1038/bjc.2013.756.
Ahn S, Woo JW, Lee K, Park SY. HER2 status in breast cancer: changes in guidelines and complicating factors for interpretation. J Pathol Transl Med. 2020;54(1):34–44. Available from: http://jpatholtm.org/journal/view.php?doi=10.4132/jptm.2019.11.03.
L Yin JJ Duan XW Bian SC Yu. Triple-negative breast cancer molecular subtyping and treatment progress. Breast Cancer Res. 2020 22(1):61 Available from: https://doi.org/10.1186/s13058-020-01296-5.
Mussallem D. Lifestyle for breast cancer risk reduction. Vol. 29, Menopause. 2022. p. 979–81. Available from: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85135421168&doi=10.1097%2fGME.0000000000002006&partnerID=40&md5=31985b3c3807840eaccb3c35c55983f5.
Daly AA, Rolph R, Cutress RI, Copson ER. A review of modifiable risk factors in young women for the prevention of breast cancer. Vol. 13, Breast Cancer: Targets and Therapy. 2021. p. 241–57. Available from: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85104776317&doi=10.2147%2fBCTT.S268401&partnerID=40&md5=e3ac32d105c5d6d8502ed82199a958ba.
Vogel VG. Epidemiology of breast cancer. The breast: comprehensive management of benign and malignant diseases. 2018. Available from: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85054344161&doi=10.1016%2fB978-0-323-35955-9.00015-5&partnerID=40&md5=e76c02b2aea40c1dca8b170479cce5fc.
Łukasiewicz S, Czeczelewski M, Forma A, Baj J, Sitarz R, Stanisławek A. Breast cancer-epidemiology, risk factors, classification, prognostic markers, and current treatment strategies-an updated review. Cancers. 2021;13(17):4287.
Toss A, Grandi G, Cagnacci A, Marcheselli L, Pavesi S, De Matteis E, et al. The impact of reproductive life on breast cancer risk in women with family history or BRCA mutation. Vol. 8, Oncotarget. 2017. p. 9144–54. Available from: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85011968931&doi=10.18632%2foncotarget.13423&partnerID=40&md5=8a37d5de6e499622877a398d502747e3.
Dall GV, Britt KL. Estrogen effects on the mammary gland in early and late life and breast cancer risk. Vol. 7, Frontiers in Oncology. 2017. Available from: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85020099244&doi=10.3389%2ffonc.2017.00110&partnerID=40&md5=1a8813b5d371e1b0b3c9d91e83460635.
Ferlay J, Ervik M, Lam F, Laversanne M, Colombet M, Mery L, Piñeros M, Znaor A, Soerjomataram I, Bray F (2024). Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer. Available from: https://gco.iarc.who.int/today.
WHO 2022. Breast Cancer TODAY. Global Cancer Observatory. 2022. Available from: https://gco.iarc.who.int/media/globocan/factsheets/cancers/20-breast-fact-sheet.pdf.
F Ye S Dewanjee Y Li NK Jha ZS Chen A Kumar Advancements in clinical aspects of targeted therapy and immunotherapy in breast cancer. Mol Cancer. et al 2023 22(1):105 Available from: https://doi.org/10.1186/s12943-023-01805-y.
Haq BU, Aisha S, Sofi S, Mir MA. Chemotherapy in combination with surgery and radiotherapy in breast cancer. combination therapies and their effectiveness in breast cancer treatment. 2021. Available from: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85129037124&doi=10.52305%2fZMNJ6932&partnerID=40&md5=be23a021805a79b4ae9d9266428ea73a.
Rose L, Lustberg M, Ruddy KJ, Cathcart-Rake E, Loprinzi C, Dulmage B. Hair loss during and after breast cancer therapy. Vol. 31, Supportive Care in Cancer. 2023. Available from: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85148936617&doi=10.1007%2fs00520-023-07634-5&partnerID=40&md5=6d7f27a7934f5f3202e2031aa5334df3.
Singh KP, Kober KM, Ernst B, Sachdev J, Brewer M, Zhu Q, et al. Multiple gastrointestinal symptoms are associated with chemotherapy-induced nausea in patients with breast cancer. Vol. 45, Cancer Nursing. 2022. p. 181–9. Available from: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85124124568&doi=10.1097%2fNCC.0000000000000976&partnerID=40&md5=2364ebccb9478ff089fbf51e61234009.
Salata C, deAlmeida CE, Ferreira-Machado SC, Barroso RC, Nogueira LP, Mantuano A, et al. Preliminary pre-clinical studies on the side effects of breast cancer treatment. Vol. 97, International Journal of Radiation Biology. 2021. p. 877–87. Available from: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85105387534&doi=10.1080%2f09553002.2021.1919782&partnerID=40&md5=fd79f5870924b4a43d935f0f9cd57429.
Han H, Chen J, Li J, Correia A, Bártolo R, Shahbazi MA, et al. Enhancing apoptosome assembly via mito-biomimetic lipid nanocarrier for cancer therapy. Adv Funct Mater. 2023;33(46):2305316. Available from: https://doi.org/10.1002/adfm.202305316.
Tapeinos C, Torrieri G, Wang S, Martins JP, Santos HA. Evaluation of cell membrane-derived nanoparticles as therapeutic carriers for pancreatic ductal adenocarcinoma using an in vitro tumour stroma model. J Controlled Release. 2023;362:225–42. Available from: https://www.sciencedirect.com/science/article/pii/S016836592300545X.
Javid H, Attarian F, Saadatmand T, Rezagholinejad N, Mehri A, Amiri H, et al. The therapeutic potential of immunotherapy in the treatment of breast cancer: Rational strategies and recent progress. J Cell Biochem. 2023;124(4):477–94. Available from: https://onlinelibrary.wiley.com/doi/10.1002/jcb.30402.
Li J, Huang D, Cheng R, Figueiredo P, Fontana F, Correia A, et al. Multifunctional biomimetic nanovaccines based on photothermal and weak-immunostimulatory nanoparticulate cores for the immunotherapy of solid tumors. Adv Mater. 2022;34(9):2108012. Available from: https://doi.org/10.1002/adma.202108012.
Alaluf E, Shalamov MM, Sonnenblick A. Update on current and new potential immunotherapies in breast cancer, from bench to bedside. Front Immunol. 2024;15. Available from: https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2024.1287824.
Hamilton AG, Swingle KL, Joseph RA, Mai D, Gong N, Billingsley MM, et al. Ionizable Lipid Nanoparticles with Integrated Immune Checkpoint Inhibition for mRNA CAR T Cell Engineering. Adv Healthc Mater. 2023;12(30): e2301515.
Sheikh MS, Huang Y. Antibody-drug conjugates for breast cancer treatment. Recent Patents Anticancer Drug Discov. 2023;18(2):108–13. Available from: https://www.eurekaselect.com/207119/article.
Phillips GDL, Fields CT, Li G, Dowbenko D, Schaefer G, Miller K, et al. Dual targeting of HER2-positive cancer with trastuzumab emtansine and pertuzumab: critical role for neuregulin blockade in antitumor response to combination therapy. Clin Cancer Res. 2014 20(2):456–68. Available from: https://doi.org/10.1158/1078-0432.CCR-13-0358.
Ghahremani Dehbokri S, Alizadeh N, Isazadeh A, Baghbanzadeh A, Abbaspour-Ravasjani S, Hajiasgharzadeh K, et al. CTLA-4: As an Immunosuppressive Immune Checkpoint in Breast Cancer. Curr Mol Med. 2023;23(6):521–6. Available from: http://www.eurekaselect.com/article/124331.
Mariela A. Moreno Ayala AS Maria Florencia Gottardo, Antonela S Asad, Camila Zuccato, Alejandro Nicola, Candolfi M. Immunotherapy for the treatment of breast cancer. Expert Opin Biol Ther. 2017;17(7):797–812. Available from: https://doi.org/10.1080/14712598.2017.1324566.
Abbaspour M, Akbari V. Cancer vaccines as a targeted immunotherapy approach for breast cancer: an update of clinical evidence. Expert Rev Vaccines. 2022;21(3):337–53. Available from: https://doi.org/10.1080/14760584.2022.2021884.
Mittendorf EA, Lu B, Melisko M, Price Hiller J, Bondarenko I, Brunt AM, et al. Efficacy and safety analysis of nelipepimut-s vaccine to prevent breast cancer recurrence: a randomized, multicenter, phase III clinical trial. Clin Cancer Res. 2019 Jul;25(14):4248–54. Available from: https://doi.org/10.1158/1078-0432.CCR-18-2867.
Du S, Yan J, Xue Y, Zhong Y, Dong Y. Adoptive cell therapy for cancer treatment. Vol. 3, Exploration. 2023. Available from: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85177753477&doi=10.1002%2fEXP.20210058&partnerID=40&md5=3d687479248404c8c1711f669c31ffa7.
Jiao C, Zvonkov E, Lai X, Zhang R, Liu Y, Qin Y, et al. 4SCAR2.0: a multi-CAR-T therapy regimen for the treatment of relapsed/refractory B cell lymphomas. Blood Cancer J. 2021;11(3):59. Available from: https://www.nature.com/articles/s41408-021-00455-x.
Wen P, Wu W, Wang F, Zheng H, Liao Z, Shi J, et al. Cell delivery devices for cancer immunotherapy. JCR. 2023. Vol. 353 p. 875–88. Available from: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85144370755&doi=10.1016%2fj.jconrel.2022.11.041&partnerID=40&md5=5178e82f34aaadeca1f4cf715da2bf30.
Yaseen MM, Abuharfeil NM, Darmani H, Daoud A. Mechanisms of immune suppression by myeloid-derived suppressor cells: the role of interleukin-10 as a key immunoregulatory cytokine. Open Biol. 2020;10(9):200111. Available from: https://doi.org/10.1098/rsob.200111.
Edechi CA, Ikeogu N, Uzonna JE, Myal Y. Regulation of immunity in breast cancer. Vol. 11, Cancers. 2019. Available from: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85071155275&doi=10.3390%2fcancers11081080&partnerID=40&md5=6aa94c630dfd7f5cb36b4976fee2a3f4.
Gonzalez-Valdivieso J, Girotti A, Schneider J, Arias FJ. Advanced nanomedicine and cancer: Challenges and opportunities in clinical translation. Int J Pharm Vol. 599, 2021. Available from: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85102083296&doi=10.1016%2fj.ijpharm.2021.120438&partnerID=40&md5=f9eb0ee3464f2e3017b5d034e7897fcd.
Feng C, Chan D, Joseph J, Muuronen M, Coldren WH, Dai N, et al. Light-activated chemical probing of nucleobase solvent accessibility inside cells. Nat Chem Biol. 2018;14(3):276–83.
Wang J, Li Y, Nie G. Multifunctional biomolecule nanostructures for cancer therapy. Nat Rev Mater. 2021;6(9):766–83. Available from: https://doi.org/10.1038/s41578-021-00315-x.
Mundekkad D, Cho WC. Nanoparticles in clinical translation for cancer therapy. Int J Mol Sci Vol. 23, 2022. Available from: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85123689428&doi=10.3390%2fijms23031685&partnerID=40&md5=17bc6fd3dd91f335b2aface81687564a.
Tahir N, Madni A, Correia A, Rehman M, Balasubramanian V, Khan MM, et al. Lipid-polymer hybrid nanoparticles for controlled delivery of hydrophilic and lipophilic doxorubicin for breast cancer therapy. Int J Nanomedicine. 2019 Volume 14:4961–74. Available from: https://www.dovepress.com/lipid-polymer-hybrid-nanoparticles-for-controlled-delivery-of-hydrophi-peer-reviewed-article-IJN.
Li J, Zhang W, Gao Y, Tong H, Chen Z, Shi J, et al. Near-infrared light and magnetic field dual-responsive porous silicon-based nanocarriers to overcome multidrug resistance in breast cancer cells with enhanced efficiency. J Mater Chem B. 2020;8(3):546–57. Available from: https://doi.org/10.1039/C9TB02340B.
Basu B, Pal T, Mukherjee S, Prajapati BG. Bioactive lipids for the treatment of cancer. Therapeutic Platform of Bioactive Lipids: Focus on Cancer. 2023. Available from: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85163216200&partnerID=40&md5=c17c409150d7d5b8ac98296227a561ed.
Veiga N, Diesendruck Y, Peer D. Targeted lipid nanoparticles for RNA therapeutics and immunomodulation in leukocytes. Adv Drug Deliv Rev. 2020;159:364–76. Available from: https://www.sciencedirect.com/science/article/pii/S0169409X20300223.
Ramishetti S, Kedmi R, Goldsmith M, Leonard F, Sprague AG, Godin B, et al. Systemic Gene Silencing in Primary T Lymphocytes Using Targeted Lipid Nanoparticles. ACS Nano. 2015 9(7):6706–16. Available from: https://doi.org/10.1021/acsnano.5b02796.
Elia U, Ramishetti S, Rosenfeld R, Dammes N, Bar-Haim E, Naidu GS, et al. Design of SARS-CoV-2 hFc-Conjugated Receptor-Binding Domain mRNA Vaccine Delivered via Lipid Nanoparticles. ACS Nano 2021;15(6):9627–37. Available from: https://doi.org/10.1021/acsnano.0c10180.
Swingle KL, Safford HC, Geisler HC, Hamilton AG, Thatte AS, Billingsley MM, et al. Ionizable Lipid Nanoparticles for In Vivo mRNA Delivery to the Placenta during Pregnancy. J Am Chem Soc. 2023 145(8):4691–706. Available from: https://doi.org/10.1021/jacs.2c12893.
Yan J, Zhang H, Li G, Su J, Wei Y, Xu C. Lipid nanovehicles overcome barriers to systemic RNA delivery: Lipid components, fabrication methods, and rational design. Vol. 14, Acta Pharmaceutica Sinica B. 2024. p. 579–601. Available from: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85175617186&doi=10.1016%2fj.apsb.2023.10.012&partnerID=40&md5=0552afa1e811f8be4b6103eb178cb7f7.
Xue L, Gong N, Shepherd SJ, Xiong X, Liao X, Han X, et al. Rational Design of Bisphosphonate Lipid-like Materials for mRNA Delivery to the Bone Microenvironment. J Am Chem Soc. 2022 Jun 8;144(22):9926–37. Available from: https://doi.org/10.1021/jacs.2c02706.
Seo H, Jeon L, Kwon J, Lee H. High-Precision Synthesis of RNA-Loaded Lipid Nanoparticles for Biomedical Applications. Adv Healthc Mater. 2023;12(13):2203033. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1002/adhm.202203033.
Shepherd SJ, Warzecha CC, Yadavali S, El-Mayta R, Alameh MG, Wang L, et al. Scalable mRNA and siRNA lipid nanoparticle production using a parallelized microfluidic device. Nano Lett. 2021 21(13):5671–80. Available from: https://doi.org/10.1021/acs.nanolett.1c01353.
Shepherd SJ, Han X, Mukalel AJ, El-Mayta R, Thatte AS, Wu J, et al. Throughput-scalable manufacturing of SARS-CoV-2 mRNA lipid nanoparticle vaccines. Proc Natl Acad Sci. 2023;120(33):e2303567120. Available from: https://www.pnas.org/doi/abs/10.1073/pnas.2303567120.
Jung HN, Lee SY, Lee S, Youn H, Im HJ. Lipid nanoparticles for delivery of RNA therapeutics: Current status and the role of in vivo imaging. Theranostics. 2022;12(17):7509–31. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9691360/.
Patisiran, an RNAi therapeutic for the treatment of hereditary transthyretin-mediated amyloidosis \textbar Neurodegenerative Disease Management. 2024. Available from: https://www.futuremedicine.com/doi/10.2217/nmt-2018-0033.
Chaudhuri A, Kumar DN, Shaik RA, Eid BG, Abdel-Naim AB, Md S, et al. Lipid-based nanoparticles as a pivotal delivery approach in triple negative breast cancer (TNBC) Therapy. Int J Mol Sci. Vol. 23, 2022. Available from: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85137548958&doi=10.3390%2fijms231710068&partnerID=40&md5=14d907311c4c873ca9a18e8cd371e316.
Walsh EE, Frenck RWJ, Falsey AR, Kitchin N, Absalon J, Gurtman A, et al. Safety and immunogenicity of two RNA-Based Covid-19 Vaccine Candidates. N Engl J Med. 2020;383(25):2439–50.
Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384(5):403–16.
Andresen JL, Fenton OS. Nucleic acid delivery and nanoparticle design for COVID vaccines. MRS Bull. 2021 Sep 1;46(9):832–9. Available from: https://doi.org/10.1557/s43577-021-00169-2.
B Li AY Jiang I Raji C Atyeo TM Raimondo AGR Gordon Enhancing the immunogenicity of lipid-nanoparticle mRNA vaccines by adjuvanting the ionizable lipid and the mRNA. Nat Biomed Eng. et al 2023 Sep 7 Available from: https://doi.org/10.1038/s41551-023-01082-6.
Han X, Zhang H, Butowska K, Swingle KL, Alameh MG, Weissman D, et al. An ionizable lipid toolbox for RNA delivery. Nat Commun. 2021;12(1):7233. Available from: https://www.nature.com/articles/s41467-021-27493-0.
R Pattipeiluhu Y Zeng MMRM Hendrix IK Voets A Kros TH Sharp Liquid crystalline inverted lipid phases encapsulating siRNA enhance lipid nanoparticle mediated transfection. Nat Commun. 2024 15(1):1303 Available from: https://doi.org/10.1038/s41467-024-45666-5.
Gavas S, Quazi S, Karpiński TM. Nanoparticles for cancer therapy: current progress and challenges. Nanoscale Res Lett. 2021;16(1):173.
Allen TM, Cullis PR. Liposomal drug delivery systems: From concept to clinical applications. Adv Drug Deliv Rev. 2013 65(1):36–48. Available from: https://www.sciencedirect.com/science/article/pii/S0169409X12002980.
Huang Y, Liu C, Feng Q, Sun J. Microfluidic synthesis of nanomaterials for biomedical applications. Nanoscale Horiz. 2023;8(12):1610–27.
Lassalle V, Ferreira ML. PLA Nano- and microparticles for drug delivery: an overview of the methods of preparation. Macromol Biosci. 2007;7(6):767–83. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1002/mabi.200700022.
Hald Albertsen C, Kulkarni JA, Witzigmann D, Lind M, Petersson K, Simonsen JB. The role of lipid components in lipid nanoparticles for vaccines and gene therapy. Adv Drug Deliv Rev. 2022 S;188:114416. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9250827/.
Jung HN, Lee SY, Lee S, Youn H, Im HJ. Lipid nanoparticles for delivery of RNA therapeutics: Current status and the role of in vivo imaging. Theranostics. 2022;12(17):7509–31.
Zhang L, More KR, Ojha A, Jackson CB, Quinlan BD, Li H, et al. Effect of mRNA-LNP components of two globally-marketed COVID-19 vaccines on efficacy and stability. NPJ Vaccines. 2023;8(1):156.
Escalona-Rayo O, Zeng Y, Knol RA, Kock TJF, Aschmann D, Slütter B, et al. In vitro and in vivo evaluation of clinically-approved ionizable cationic lipids shows divergent results between mRNA transfection and vaccine efficacy. Biomed Pharmacother. 2023;165:115065. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0753332223008569.
Kim J, Jozic A, Lin Y, Eygeris Y, Bloom E, Tan X, et al. Engineering lipid nanoparticles for enhanced intracellular delivery of mRNA through inhalation. ACS Nano. 2022;16(9):14792–806. Available from: https://pubs.acs.org/doi/https://doi.org/10.1021/acsnano.2c05647.
Zhigaltsev IV, Tam YK, Leung AKK, Cullis PR. Production of limit size nanoliposomal systems with potential utility as ultra-small drug delivery agents. J Liposome Res. 2015;1–7. Available from: http://www.tandfonline.com/doi/full/10.3109/08982104.2015.1025411.
Lokugamage MP, Vanover D, Beyersdorf J, Hatit MZC, Rotolo L, Echeverri ES, et al. Optimization of lipid nanoparticles for the delivery of nebulized therapeutic mRNA to the lungs. Nat Biomed Eng. 2021;5(9):1059–68.
Mihailescu M, Worcester DL, Carroll CL, Chamberlin AR, White SH. DOTAP: Structure, hydration, and the counterion effect. Biophys J. 2023;122(6):1086–93. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0006349523000474.
Lou G, Anderluzzi G, Schmidt ST, Woods S, Gallorini S, Brazzoli M, et al. Delivery of self-amplifying mRNA vaccines by cationic lipid nanoparticles: The impact of cationic lipid selection. J Controlled Release. 2020;325:370–9. Available from: https://linkinghub.elsevier.com/retrieve/pii/S016836592030362X.
Herrera M, Kim J, Eygeris Y, Jozic A, Sahay G. Illuminating endosomal escape of polymorphic lipid nanoparticles that boost mRNA delivery. Biomater Sci. 2021;9(12):4289–300. Available from: http://xlink.rsc.org/?DOI=D0BM01947J.
Patel S, Ashwanikumar N, Robinson E, Xia Y, Mihai C, Griffith JP, et al. Naturally-occurring cholesterol analogues in lipid nanoparticles induce polymorphic shape and enhance intracellular delivery of mRNA. Nat Commun. 2020;11(1):983. Available from: https://www.nature.com/articles/s41467-020-14527-2.
Radmand A, Kim H, Beyersdorf J, Dobrowolski CN, Zenhausern R, Paunovska K, et al. Cationic cholesterol-dependent LNP delivery to lung stem cells, the liver, and heart. Proc Natl Acad Sci. 2024;121(11):e2307801120. Available from: https://pnas.org/doi/10.1073/pnas.2307801120.
Patel SK, Billingsley MM, Frazee C, Han X, Swingle KL, Qin J, et al. Hydroxycholesterol substitution in ionizable lipid nanoparticles for mRNA delivery to T cells. J Controlled Release. 2022;347:521–32. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0168365922002826.
Nsairat H, Khater D, Sayed U, Odeh F, Al Bawab A, Alshaer W. Liposomes: structure, composition, types, and clinical applications. Heliyon. 2022;8(5): e09394.
Tenchov R, Sasso JM, Zhou QA. PEGylated Lipid Nanoparticle formulations: immunological safety and efficiency perspective. Bioconjug Chem. 2023;34(6):941–60. Available from: https://pubs.acs.org/doi/10.1021/acs.bioconjchem.3c00174.
Gokita K, Inoue J, Ishihara H, Kojima K, Inazawa J. Therapeutic potential of LNP-Mediated delivery of miR-634 for cancer therapy. Mol Ther Nucleic Acids. 2019;19:330–8. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6938807/.
Tanaka H, Takata N, Sakurai Y, Yoshida T, Inoue T, Tamagawa S, et al. Delivery of oligonucleotides using a self-degradable lipid-like material. Pharmaceutics. 2021;13(4):544. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8070490/.
Sahin U, Karikó K, Türeci Ö. mRNA-based therapeutics–developing a new class of drugs. Nat Rev Drug Discov. 2014;13(10):759–80.
Schoenmaker L, Witzigmann D, Kulkarni JA, Verbeke R, Kersten G, Jiskoot W, et al. mRNA-lipid nanoparticle COVID-19 vaccines: Structure and stability. Int J Pharm. 2021;601:120586. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8032477/.
Barenholz Y. Doxil®–the first FDA-approved nano-drug: lessons learned. J Control Release Off J Control Release Soc. 2012;160(2):117–34.
Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46(12 Pt 1):6387–92.
Cheng X, Lee RJ. The role of helper lipids in lipid nanoparticles (LNPs) designed for oligonucleotide delivery. Adv Drug Deliv Rev. 2016;99:129–37. Available from: https://www.sciencedirect.com/science/article/pii/S0169409X16300539.
Wang C, Zhao C, Wang W, Liu X, Deng H. Biomimetic noncationic lipid nanoparticles for mRNA delivery. Proc Natl Acad Sci U S A. 2023;120(51): e2311276120.
Kim J, Eygeris Y, Gupta M, Sahay G. Self-assembled mRNA vaccines. Adv Drug Deliv Rev. 2021;170:83–112. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7837307/.
Corbett KS, Edwards DK, Leist SR, Abiona OM, Boyoglu-Barnum S, Gillespie RA, et al. SARS-CoV-2 mRNA Vaccine Design Enabled by Prototype Pathogen Preparedness. Nature. 2020;586(7830):567–71. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7581537/.
Ahmad A, Khan JM, Haque S. Strategies in the design of endosomolytic agents for facilitating endosomal escape in nanoparticles. Biochimie. 2019;160:61–75.
Fasbender A, Marshall J, Moninger T, Grunst T, Cheng S, Welsh M. Effect of co-lipids in enhancing cationic lipid-mediated gene transfer in vitro and in vivo. Gene Ther. 1997;4(7):716–25. Available from: https://doi.org/10.1038/sj.gt.3300459.
Kotouček J, Hubatka F, Mašek J, Kulich P, Velínská K, Bezděková J, et al. Preparation of nanoliposomes by microfluidic mixing in herring-bone channel and the role of membrane fluidity in liposomes formation. Sci Rep. 2020;10(1):5595. Available from: https://www.nature.com/articles/s41598-020-62500-2.
Semple SC, Chonn A, Cullis PR. Influence of cholesterol on the association of plasma proteins with liposomes. Biochemistry. 1996;35(8):2521–5.
Kim BK, Doh KO, Hwang GB, Seu YB. Transfection property of a new cholesterol-based cationic lipid containing tri-2-hydroxyethylamine as gene delivery vehicle. J Microbiol Biotechnol. 2012;22(6):866–71.
Kulkarni JA, Witzigmann D, Leung J, Tam YYC, Cullis PR. On the role of helper lipids in lipid nanoparticle formulations of siRNA. Nanoscale. 2019;11(45):21733–9.
Roces CB, Lou G, Jain N, Abraham S, Thomas A, Halbert GW, et al. Manufacturing considerations for the development of lipid nanoparticles using microfluidics. Pharmaceutics. 2020;12(11).
Yun CH, Bae CS, Ahn T. Cargo-Free Nanoparticles Containing Cationic Lipids Induce Reactive Oxygen Species and Cell Death in HepG2 Cells. Biol Pharm Bull. 2016;39(8):1338–46.
Semple SC, Akinc A, Chen J, Sandhu AP, Mui BL, Cho CK, et al. Rational design of cationic lipids for siRNA delivery. Nat Biotechnol. 2010;28(2):172–6. Available from: https://www.nature.com/articles/nbt.1602.
Kowalski PS, Rudra A, Miao L, Anderson DG. Delivering the messenger: advances in technologies for therapeutic mRNA Delivery. Mol Ther. 2019;27(4):710–28. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6453548/.
Hou X, Zaks T, Langer R, Dong Y. Lipid nanoparticles for mRNA delivery. Nat Rev Mater. 2021;6(12):1078–94.
Jayaraman M, Ansell SM, Mui BL, Tam YK, Chen J, Du X, et al. Maximizing the potency of siRNA lipid nanoparticles for hepatic gene silencing in vivo. Angew Chem Int Ed Engl. 2012;51(34):8529–33.
Whitehead KA, Dorkin JR, Vegas AJ, Chang PH, Veiseh O, Matthews J, et al. Degradable lipid nanoparticles with predictable in vivo siRNA delivery activity. Nat Commun. 2014;5:4277.
Hajj KA, Whitehead KA. Tools for translation: non-viral materials for therapeutic mRNA delivery. Nat Rev Mater. 2017;2(10):1–17. Available from: https://www.nature.com/articles/natrevmats201756.
Allen TM. The use of glycolipids and hydrophilic polymers in avoiding rapid uptake of liposomes by the mononuclear phagocyte system. Adv Drug Deliv Rev. 1994;13(3):285–309. Available from: https://www.sciencedirect.com/science/article/pii/0169409X94900167.
Zhu X, Tao W, Liu D, Wu J, Guo Z, Ji X, et al. Surface De-PEGylation Controls Nanoparticle-Mediated siRNA Delivery In Vitro and In Vivo. Theranostics. 2017;7(7):1990–2002.
Zheng L, Bandara SR, Tan Z, Leal C. Lipid nanoparticle topology regulates endosomal escape and delivery of RNA to the cytoplasm. Proc Natl Acad Sci. 2023;120(27):e2301067120. Available from: https://www.pnas.org/doi/10.1073/pnas.2301067120.
Zamora ME, Omo-Lamai S, Patel MN, Wu J, Arguiri E, Muzykantov VR, et al. Combination of physicochemical tropism and affinity moiety targeting of lipid nanoparticles enhances organ targeting. Nano Lett. 2024;24(16):4774–84. Available from: https://doi.org/10.1021/acs.nanolett.3c05031.
Kim Y, Choi J, Kim EH, Park W, Jang H, Jang Y, et al. Design of PD-L1-targeted lipid nanoparticles to turn on PTEN for efficient cancer therapy. Adv Sci Weinh Baden-Wurtt Ger. 2024;e2309917. Available from: https://doi.org/10.1002/advs.202309917.
Stewart MP, Lorenz A, Dahlman J, Sahay G. Challenges in carrier‐mediated intracellular delivery: moving beyond endosomal barriers. WIREs Nanomedicine Nanobiotechnology. 2016;8(3):465–78. Available from: https://wires.onlinelibrary.wiley.com/doi/10.1002/wnan.1377.
Chatterjee S, Kon E, Sharma P, Peer D. Endosomal escape: A bottleneck for LNP-mediated therapeutics. Proc Natl Acad Sci. 2024 Mar 12;121(11):e2307800120. Available from: https://pnas.org/doi/10.1073/pnas.2307800120.
Vermeulen LMP, Brans T, Samal SK, Dubruel P, Demeester J, De Smedt SC, et al. Endosomal Size and Membrane Leakiness Influence Proton Sponge-Based Rupture of Endosomal Vesicles. ACS Nano. 2018;12(3):2332–45.
Tang X, Zhang J, Sui D, Yang Q, Wang T, Xu Z, et al. Simultaneous dendritic cells targeting and effective endosomal escape enhance sialic acid-modified mRNA vaccine efficacy and reduce side effects. J Controlled Release. 2023;364:529–45. Available from: https://www.sciencedirect.com/science/article/pii/S0168365923007253.
Kon E, Ad-El N, Hazan-Halevy I, Stotsky-Oterin L, Peer D. Targeting cancer with mRNA–lipid nanoparticles: key considerations and future prospects. Nat Rev Clin Oncol. 2023;20(11):739–54. Available from: https://www.nature.com/articles/s41571-023-00811-9.
Patel S, Ashwanikumar N, Robinson E, DuRoss A, Sun C, Murphy-Benenato KE, et al. Boosting intracellular delivery of lipid nanoparticle-encapsulated mRNA. Nano Lett. 2017;17(9):5711–8. Available from: https://pubs.acs.org/doi/10.1021/acs.nanolett.7b02664.
Sahay G, Querbes W, Alabi C, Eltoukhy A, Sarkar S, Zurenko C, et al. Efficiency of siRNA delivery by lipid nanoparticles is limited by endocytic recycling. Nat Biotechnol. 2013;31(7):653–8. Available from: https://www.nature.com/articles/nbt.2614.
Paramasivam P, Franke C, Stöter M, Höijer A, Bartesaghi S, Sabirsh A, et al. Endosomal escape of delivered mRNA from endosomal recycling tubules visualized at the nanoscale. J Cell Biol. 2022;221(2).
Khorkova O, Stahl J, Joji A, Volmar CH, Wahlestedt C. Amplifying gene expression with RNA-targeted therapeutics. Nat Rev Drug Discov. 2023;22(7):539–61. Available from: https://doi.org/10.1038/s41573-023-00704-7.
Liu C, Shi Q, Huang X, Koo S, Kong N, Tao W. mRNA-based cancer therapeutics. Nat Rev Cancer. 2023;23(8):526–43. Available from: https://doi.org/10.1038/s41568-023-00586-2.
Schaible P, Bethge W, Lengerke C, Haraszti RA. RNA Therapeutics for Improving CAR T-cell Safety and Efficacy. Cancer Res. 2023;83(3):354–62. Available from: https://doi.org/10.1158/0008-5472.CAN-22-2155.
Barbier AJ, Jiang AY, Zhang P, Wooster R, Anderson DG. The clinical progress of mRNA vaccines and immunotherapies. Nat Biotechnol. 2022;40(6):840–54. Available from: https://doi.org/10.1038/s41587-022-01294-2.
Blass E, Ott PA. Advances in the development of personalized neoantigen-based therapeutic cancer vaccines. Nat Rev Clin Oncol. 2021;18(4):215–29. Available from: https://doi.org/10.1038/s41571-020-00460-2.
Kim YK. RNA therapy: rich history, various applications and unlimited future prospects. Exp Mol Med. 2022;54(4):455–65. Available from: https://www.nature.com/articles/s12276-022-00757-5.
Gutbrod MJ, Martienssen RA. Conserved chromosomal functions of RNA interference. Nat Rev Genet. 2020;21(5):311–31. Available from: https://doi.org/10.1038/s41576-019-0203-6.
Gao H, Cheng R, A. Santos H. Nanoparticle-mediated siRNA delivery systems for cancer therapy. View. 2021;2(3):20200111. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1002/VIW.20200111.
Romano G, Acunzo M, Nana-Sinkam P. microRNAs as Novel Therapeutics in Cancer. Cancers. 2021;13(7):1526. Available from: https://www.mdpi.com/2072-6694/13/7/1526.
Afrin H, Geetha Bai R, Kumar R, Ahmad SS, Agarwal SK, Nurunnabi M. Oral delivery of RNAi for cancer therapy. Cancer Metastasis Rev. 2023;42(3):699–724. Available from: https://link.springer.com/10.1007/s10555-023-10099-x.
Huang X, Li J, Li G, Ni B, Liang Z, Chen H, et al. Cation-free siRNA-cored nanocapsules for tumor-targeted RNAi therapy. Acta Biomater. 2023;161:226–37. Available from: https://www.sciencedirect.com/science/article/pii/S1742706123001290.
Ward DM, Shodeinde AB, Peppas NA. Innovations in biomaterial design toward successful RNA interference therapy for cancer treatment. Adv Healthc Mater. 2021;10(13):2100350. Available from: https://doi.org/10.1002/adhm.202100350.
Jeong M, Lee Y, Park J, Jung H, Lee H. Lipid nanoparticles (LNPs) for in vivo RNA delivery and their breakthrough technology for future applications. Adv Drug Deliv Rev. 2023;200:114990. Available from: https://www.sciencedirect.com/science/article/pii/S0169409X23003058.
Azimzadeh M, Rahaie M, Nasirizadeh N, Ashtari K, Naderi-Manesh H. An electrochemical nanobiosensor for plasma miRNA-155, based on graphene oxide and gold nanorod, for early detection of breast cancer. Biosens Bioelectron. 2016;77:99–106. Available from: https://www.sciencedirect.com/science/article/pii/S0956566315304152.
Nemeth K, Bayraktar R, Ferracin M, Calin GA. Non-coding RNAs in disease: from mechanisms to therapeutics. Nat Rev Genet. 2024;25(3):211–32. Available from: https://doi.org/10.1038/s41576-023-00662-1.
Shekhar R, Kumari S, Vergish S, Tripathi P. The crosstalk between miRNAs and signaling pathways in human cancers: Potential therapeutic implications. Int Rev Cell and Mol Bio. 2024. Available from: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85183046391&doi=10.1016%2fbs.ircmb.2023.12.001&partnerID=40&md5=ea77bca5b1ea440febbb3365840bf94f.
Rui X, Zhao H, Xiao X, Wang L, Mo L, Yao Y. Microrna-34a suppresses breast cancer cell proliferation and invasion by targeting notch1. Vol. 16, Experimental and Therapeutic Medicine. 2018. p. 4387–92. Available from: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85055929837&doi=10.3892%2fetm.2018.6744&partnerID=40&md5=14c89e262f1646616d3e9c57366cd371.
Li ZH, Weng X, Xiong QY, Tu JH, Xiao A, Qiu W, et al. miR-34a expression in human breast cancer is associated with drug resistance. Vol. 8, Oncotarget. 2017. p. 106270–82. Available from: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85036637734&doi=10.18632%2foncotarget.22286&partnerID=40&md5=6d3d19d8f8707b7cfb0555cd813a446e.
Navarro-Manzano E, Luengo-Gil G, González-Conejero R, García-Garre E, García-Martínez E, García-Torralba E, et al. Prognostic and predictive effects of tumor and plasma miR-200c-3p in locally advanced and metastatic breast cancer. Vol. 14, Cancers. 2022. Available from: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85129789741&doi=10.3390%2fcancers14102390&partnerID=40&md5=e45807d762fd19c809b0b9542c9e5c36.
Zhang X, Ma G, Liu J, Zhang Y. MicroRNA-182 promotes proliferation and metastasis by targeting FOXF2 in triple-negative breast cancer. Vol. 14, Oncology Letters. 2017. p. 4805–11. Available from: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85028926049&doi=10.3892%2fol.2017.6778&partnerID=40&md5=22748e7156fd5116ca2eb1221df53fd2.
Lafuente-Gómez N, Wang S, Fontana F, Dhanjani M, García-Soriano D, Correia A, et al. Synergistic immunomodulatory effect in macrophages mediated by magnetic nanoparticles modified with miRNAs. Nanoscale. 2022;14(31):11129–38. Available from: https://doi.org/10.1039/D2NR01767A.
Liu J, Meng T, Ming Y, Wen L, Cheng B, Liu N, et al. MicroRNA-200c delivered by solid lipid nanoparticles enhances the effect of paclitaxel on breast cancer stem cell. Int J Nanomedicine. 2016;Volume 11:6713–25. Available from: https://www.dovepress.com/microrna-200c-delivered-by-solid-lipid-nanoparticles-enhances-the-effe-peer-reviewed-article-IJN.
Hayward SL, Francis DM, Kholmatov P, Kidambi S. Targeted Delivery of MicroRNA125a-5p by Engineered Lipid Nanoparticles for the Treatment of HER2 Positive Metastatic Breast Cancer. J Biomed Nanotechnol. 2016;12(3):554–68.
Dinami R, Pompili L, Petti E, Porru M, D’Angelo C, Di Vito S, et al. MiR‐182‐3p targets TRF2 and impairs tumor growth of triple‐negative breast cancer. EMBO Mol Med. 2023;15(1):e16033. Available from: https://www.embopress.org/doi/full/10.15252/emmm.202216033.
Nevskaya KV, Pershina AG, Hmelevskaya ES, Efimova LV, Ibragimova MK, Dolgasheva DS, et al. Prevention of metastasis by suppression of stemness genes using a combination of microRNAs. J Med Chem. 2024; Available from: https://doi.org/10.1021/acs.jmedchem.3c02199.
Valimukhametova AR, Lee BH, Topkiran UC, Gries K, Gonzalez-Rodriguez R, Coffer JL, et al. Cancer therapeutic siRNA delivery and imaging by nitrogen- and neodymium-doped graphene quantum dots. ACS Biomater Sci Eng. 2023;9(6):3425–34. Available from: https://doi.org/10.1021/acsbiomaterials.3c00369.
Wolff JA, Malone RW, Williams P, Chong W, Acsadi G, Jani A, et al. Direct gene transfer into mouse muscle in vivo. Science. 1990;247(4949 Pt 1):1465–8.
Conry RM, LoBuglio AF, Wright M, Sumerel L, Pike MJ, Johanning F, et al. Characterization of a messenger RNA polynucleotide vaccine vector. Cancer Res. 1995;55(7):1397–400.
Weinstein S, Toker IA, Emmanuel R, Ramishetti S, Hazan-Halevy I, Rosenblum D, et al. Harnessing RNAi-based nanomedicines for therapeutic gene silencing in B-cell malignancies. Proc Natl Acad Sci USA. 2016;113(1):E16-22.
Guimarães PPG, Figueroa-Espada CG, Riley RS, Gong N, Xue L, Sewastianik T, et al. In vivo bone marrow microenvironment siRNA delivery using lipid–polymer nanoparticles for multiple myeloma therapy. Proc Natl Acad Sci. 2023;120(25):e2215711120. Available from: https://www.pnas.org/doi/10.1073/pnas.2215711120.
Tarab-Ravski D, Hazan-Halevy I, Goldsmith M, Stotsky-Oterin L, Breier D, Naidu GS, et al. Delivery of therapeutic RNA to the bone marrow in multiple myeloma using CD38-targeted lipid nanoparticles. Adv Sci. 2023;10(21):2301377. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1002/advs.202301377.
Chatterjee S, Naidu GS, Hazan-Halevy I, Grobe H, Ezra A, Sharma P, et al. Therapeutic gene silencing of CKAP5 leads to lethality in genetically unstable cancer cells. Sci Adv. 2023;9(14):eade4800. Available from: https://www.science.org/doi/abs/10.1126/sciadv.ade4800.
Anthiya S, Öztürk SC, Yanik H, Tavukcuoglu E, Şahin A, Datta D, et al. Targeted siRNA lipid nanoparticles for the treatment of KRAS-mutant tumors. J Controlled Release. 2023;357:67–83. Available from: https://www.sciencedirect.com/science/article/pii/S0168365923001839.
Moukhtari SHE, Garbayo E, Amundarain A, Pascual-Gil S, Carrasco-León A, Prosper F, et al. Lipid nanoparticles for siRNA delivery in cancer treatment. J Controlled Release. 2023;361:130–46. Available from: https://www.sciencedirect.com/science/article/pii/S0168365923004789.
Kang SW, Kang OJ, Lee J young, Kim H, Jung H, Kim H, et al. Evaluation of the anti-cancer efficacy of lipid nanoparticles containing siRNA against HPV16 E6/E7 combined with cisplatin in a xenograft model of cervical cancer. PLOS ONE. 2024;19(2):e0298815. Available from: https://doi.org/10.1371/journal.pone.0298815.
Spankuch-Schmitt B, Bereiter-Hahn J, Kaufmann M, Strebhardt K. Effect of RNA Silencing of Polo-Like Kinase-1 (PLK1) on Apoptosis and spindle formation in human cancer cells. JNCI J Natl Cancer Inst. 2002;94(24):1863–77. Available from: https://academic.oup.com/jnci/article-lookup/doi/10.1093/jnci/94.24.1863.
Novel Synthesized Ionizable Lipid for LNP-mediated P2X7siRNA Inhibit Migration and Induce Apoptosis of Breast Cancer Cells. 2023. Available from: https://www.researchsquare.com.
Butowska K, Han X, Gong N, El-Mayta R, Haley RM, Xue L, et al. Doxorubicin-conjugated siRNA lipid nanoparticles for combination cancer therapy. Acta Pharm Sin B. 2023;13(4):1429–37. Available from: https://www.sciencedirect.com/science/article/pii/S2211383522003239.
Mirzaei S, Paskeh MDA, Entezari M, Bidooki SH, Ghaleh VJ, Rezaei S, et al. siRNA and targeted delivery systems in breast cancer therapy. Clin Transl Oncol Off Publ Fed Span Oncol Soc Natl Cancer Inst Mex. 2023;25(5):1167–88.
Li D, Liu C, Li Y, Tenchov R, Sasso JM, Zhang D, et al. Messenger RNA-based therapeutics and vaccines: What’s beyond COVID-19? ACS Pharmacol Transl Sci. 2023 Jul 14;6(7):943–69. Available from: https://doi.org/10.1021/acsptsci.3c00047.
Jahanafrooz Z, Baradaran B, Mosafer J, Hashemzaei M, Rezaei T, Mokhtarzadeh A, et al. Comparison of DNA and mRNA vaccines against cancer. Drug Discov Today. 2020;25(3):552–60.
Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines — a new era in vaccinology. Nat Rev Drug Discov. 2018;17(4):261–79. Available from: https://www.nature.com/articles/nrd.2017.243.
Karikó K, Muramatsu H, Ludwig J, Weissman D. Generating the optimal mRNA for therapy: HPLC purification eliminates immune activation and improves translation of nucleoside-modified, protein-encoding mRNA. Nucleic Acids Res. 2011;39(21):e142. Available from: https://doi.org/10.1093/nar/gkr695.
Guan S, Rosenecker J. Nanotechnologies in delivery of mRNA therapeutics using nonviral vector-based delivery systems. Gene Ther. 2017;24(3):133–43. Available from: https://www.nature.com/articles/gt20175.
Li B, Zhang X, Dong Y. Nanoscale platforms for messenger RNA delivery. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2019;11(2): e1530.
El-Mayta R, Padilla MS, Billingsley MM, Han X, Mitchell MJ. Testing the in vitro and in vivo efficiency of mRNA-Lipid nanoparticles formulated by microfluidic mixing. J Vis Exp. 2024;191(e64810). Available from: https://app.jove.com/t/64810/testing-vitro-vivo-efficiency-mrna-lipid-nanoparticles-formulated.
Zhang C, Zhang X, Zhao W, Zeng C, Li W, Li B, et al. Chemotherapy drugs derived nanoparticles encapsulating mRNA encoding tumor suppressor proteins to treat triple-negative breast cancer. Nano Res. 2019;12(4):855–61.
Nakashima I, Saito S, Akahoshi E, Yagyu S, Sugano-Ishihara M, Nakazawa Y. Non-viral inducible caspase 9 mRNA delivery using lipid nanoparticles against breast cancer: An in vitro study. Biochem Biophys Res Commun. 2022;635:144–53. Available from: https://www.sciencedirect.com/science/article/pii/S0006291X22013675.
Wang Y, Li S, Hu M, Yang Y, McCabe E, Zhang L, et al. Universal STING mimic boosts antitumour immunity via preferential activation of tumour control signalling pathways. Nat Nanotechnol. 2024; Available from: https://doi.org/10.1038/s41565-024-01624-2.
Wang Y, Peng Y, Zi G, Chen J, Peng B. Co-delivery of Cas9 mRNA and guide RNAs for editing of LGMN gene represses breast cancer cell metastasis. Sci Rep. 2024;14(1):8095. Available from: https://doi.org/10.1038/s41598-024-58765-6.
Okamoto A, Asai T, Hirai Y, Shimizu K, Koide H, Minamino T, et al. Systemic administration of siRNA with Anti-HB-EGF antibody-modified lipid nanoparticles for the treatment of triple-negative breast cancer. Mol Pharm. 2018;15(4):1495–504. Available from: https://doi.org/10.1021/acs.molpharmaceut.7b01055.
Bromma K, Rieck K, Kulkarni J, O’Sullivan C, Sung W, Cullis P, et al. Use of a lipid nanoparticle system as a Trojan horse in delivery of gold nanoparticles to human breast cancer cells for improved outcomes in radiation therapy. Cancer Nanotechnol. 2019;10(1):1. Available from: https://doi.org/10.1186/s12645-019-0046-z.
Dong Z, Huang Z, Li S, Wang Y, Yao Y, Yang X, et al. Nanoparticles (NPs)-mediated systemic mRNA delivery to reverse trastuzumab resistance for effective breast cancer therapy. Acta Pharm Sin B. 2023;13(3):955–66. Available from: https://www.sciencedirect.com/science/article/pii/S2211383522004154.
Lin X, Wang Y, Fang K, Guo Z, Lin N, Li L. The application of nanoparticles in theranostic systems targeting breast cancer stem cells: current progress and future challenges. Stem Cell Res Ther. 2023;14(1):356. Available from: https://doi.org/10.1186/s13287-023-03584-1.
Fattore L, Cafaro G, Di Martile M, Campani V, Sacconi A, Liguoro D, et al. Oncosuppressive miRNAs loaded in lipid nanoparticles potentiate targeted therapies in BRAF-mutant melanoma by inhibiting core escape pathways of resistance. Oncogene. 2023;42(4):293–307. Available from: https://doi.org/10.1038/s41388-022-02547-9.
Kampel L, Goldsmith M, Ramishetti S, Veiga N, Rosenblum D, Gutkin A, et al. Therapeutic inhibitory RNA in head and neck cancer via functional targeted lipid nanoparticles. J Controlled Release. 2021;337:378–89. Available from: https://www.sciencedirect.com/science/article/pii/S0168365921003771.
Zong Y, Lin Y, Wei T, Cheng Q. Lipid nanoparticle (LNP) enables mRNA delivery for cancer therapy. Adv Mater. 2023;35(51):2303261. Available from: https://doi.org/10.1002/adma.202303261.
Han EL, Padilla MS, Palanki R, Kim D, Mrksich K, Li JJ, et al. Predictive high-throughput platform for dual screening of mRNA lipid nanoparticle blood–brain barrier transfection and crossing. Nano Lett. 2024;24(5):1477–86. Available from: https://doi.org/10.1021/acs.nanolett.3c03509.
Acknowledgements
Dr. L. Serpico acknowledges financial support from European Union’s Horizon Europe research and innovation program under the Marie Sklodowska-Curie Actions. Grant agreement No 101103122. Prof. Santos acknowledges the UMCG Research Funds and The ArTCell project from the Horizon Europe programme under grant agreement no. 101130715.
Funding
This work was supported by European Union’s Horizon Europe and the University Medical Center Groningen.
Author information
Authors and Affiliations
Contributions
This work benefited from equal contributions by the authors.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors agree to publish this article in Drug Delivery and Translational Research.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Serpico, L., Zhu, Y., Maia, R.F. et al. Lipid nanoparticles-based RNA therapies for breast cancer treatment. Drug Deliv. and Transl. Res. (2024). https://doi.org/10.1007/s13346-024-01638-2
Accepted:
Published:
DOI: https://doi.org/10.1007/s13346-024-01638-2