Abstract
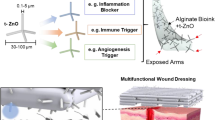
Nanocomposite alginate hydrogel containing Propranolol hydrochloride (PNL) cerosomes (CERs) was prepared as a repurposed remedy for topical skin Methicillin-Resistant Staphylococcus aureus (MRSA) infection. CERs were formed via an ethanol injection technique using different ceramides, Kolliphores® as a surfactant, and Didodecyldimethylammonium bromide (DDAB) as a positive charge inducer. CERs were optimized utilizing 13. 22 mixed-factorial design employing Design-Expert® software, the assessed responses were entrapment efficiency (EE%), particle size (PS), and zeta potential (ZP). The optimum CER, composed of 5 mg DDAB, ceramide VI, and Kolliphor® RH40 showed tubular vesicles with EE% of 92.91 ± 0.98%, PS of 388.75 ± 18.99 nm, PDI of 0.363 ± 0.01, and ZP of 30.36 ± 0.69 mV. Also, it remained stable for 90 days and manifested great mucoadhesive aspects. The optimum CER was incorporated into calcium alginate to prepare nanocomposite hydrogel. The ex-vivo evaluation illustrated that PNL was permeated in a more prolonged pattern from PNL-loaded CERs nanocomposite related to PNL-composite, optimum CER, and PNL solution. Confocal laser scanning microscopy revealed a perfect accumulation of fluorescein-labeled CERs in the skin. The in-silico investigation illustrated that the PNL was stable when mixed with other ingredients in the CERs and confirmed that PNL is a promising candidate for curing MRSA. Moreover, the PNL-loaded CERs nanocomposite revealed superiority over the PNL solution in inhibiting biofilm formation and eradication. The PNL-loaded CERs nanocomposite showed superiority over the PNL-composite for treating MRSA infection in the in-vivo mice model. Histopathological studies revealed the safety of the tested formulations. In conclusion, PNL-loaded CERs nanocomposite provided a promising, safe cure for MRSA bacterial skin infection.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Healthy skin acts as the body’s physical protective barrier from external factors such as bacteria, chemicals, and temperature. The main barrier function of the skin is related to the presence of the stratum corneum (SC) which is made up of corneocytes surrounded by lipid regions. These lipids are mainly composed of ceramides. When the skin becomes infected this might reduce the barrier function of SC resulting in increased permeability [1]. Eradication of the relevant pathogens demands efficient antibiotic treatment against the most likely microorganism [2].
Ceramides are known as the most lipophilic and the simplest kind of sphingolipids that give the SC layer its barrier function [3]. Various studies have shown that several skin conditions like psoriasis and topical infections are correlated to changes in ceramide content [4]. A few investigations reported that preparations comprising optimal ceramide concentration may help in the re-establishment of the SC layer and hence, enhance the protection property of the skin [5]. Earlier, it was demonstrated that sphingoid bases (a basic component of ceramides) may play a protective role in preventing skin colonization and infection with gram-positive organisms such as Staphylococcus aureus [6].
It is worth noting that the occurrence of multiple resistant Staphylococcus aureus skin infections has grown. These multi-resistant strains show frequent resistance to erythromycin and tetracycline, with promptly developing resistance to quinolones [7]. Bacterial resistance to antibiotics has lately grown and induced researchers to investigate diverse antimicrobial therapeutic procedures that are used to generate effective antibacterials such as drug repurposing.
Drug repurposing is a way to discover new uses for licensed and FDA-approved medicines. There are various advantages of using this method compared to developing new medications. Firstly, it has already been established that the repurposed medication is safe. Second, the time required for medication development might be decreased as the assessment of the pre-clinical studies will already have been completed. Finally, less finance is needed [8]. Several research papers are focused on developing repurposed antibacterials. For example, antipsychotics (such as Promazine) could interact with bacterial cell membranes and inhibit Methicillin-Resistant Staphylococcus aureus (MRSA), Klebsiella, and pneumoniae growth [9]. Further, antihyperlipidemic (simvastatin, pravastatin, rosuvastatin, and atorvastatin) could inhibit both Staphylococcus aureus, and Streptococcus pneumoniae (by reduction in cell viability, apoptosis promotion, bacterial membrane disruption, and protein synthesis inhibition) [10]. Moreover, a previous investigation confirmed that βeta-blockers such as propranolol hydrochloride (PNL) are effective as anti-bacterial against Staphylococcus aureus [11].
The efficiency of antimicrobials to eradicate bacteria from the skin’s surface depends on their efficiency on both the surface and the underlying SC. Almost all antimicrobials efficiently eliminate bacteria from the skin’s surface but not from the SC due to its tight junction [12]. Furthermore, the primary difficulty with topical medications is their ability to penetrate the skin’s deeper layers through SC to achieve efficient treatment. On the other hand, shifting from conventional therapy to high-tech-based nanomaterial therapy might be one of the most effective strategies for managing bacterial infections. Nanocarriers (NC) operate by inhibiting bacterial defense against drug resistance and the formation of biofilms or other crucial processes related to a bacterium’s potential for antipathy [13]. In addition, NC could penetrate the bacterial cell wall and membrane and interfere with essential molecular processes [14].
Numerous NCs have been developed recently, including polymeric lipid hybrid nanoparticles, nanoemulsions, and nanostructured lipid carriers, via diverse approaches and for various purposes [15]. Cerosomes (CERs) are tubular vesicles comprising ceramide prepared utilizing various surface-active agents and phospholipids (PC). When used topically they exhibit excellent permeability, increased bioavailability, and skin tolerability. The usage of surfactants in the preparation of CERs might produce stable non-aggregated lipidic vesicles [16]. It is worth noting that CERs have been reported by Abdelgawad et al. as successful carriers for the topical delivery of tazarotene to treat psoriasis [17]. Also, another investigation carried out by Albash et al. supported the efficacy of CERs topical delivery of fenticonazole nitrate for the management of Candida albicans infection [18].
Hydrogels are cross-linked hydrophilic polymers arranged in three-dimensional (3D) networks that can hold a lot of water while maintaining the polymer integrity and structure. Hydrogels were formulated utilizing biodegradable natural or synthetic polymers [19]. Alginate is utilized as a polymer for drug delivery applications because of its distinct features including non-toxicity, biocompatibility, non-immunogenicity, biodegradability, mucoadhesion, easy accessibility, and reasonable cost. Diverse drug delivery systems can be produced utilizing alginates, such as hydrogels, microparticles, and nanoparticles. Several methods can be employed to develop the carriers, depending on the intended usage and formulation properties. Because of its ease of use and non-toxic nature, ionotropic gelation is the most widely utilized technique for producing alginate-based particles, and hydrogels, among other systems [20].
Combining the advantages of hydrogel preparation technology and nanotechnology can help treat skin topical infections by adding new functionalities to the hydrogel structure. These functionalities improve drug therapeutic outcomes by controlling drug release and improving permeation [21].
As far as we know, no research has examined the potential of CERs-loaded alginate hydrogel to augment the deposition of Propranolol hydrochloride (PNL) as a repurposed drug in treating MRSA. Therefore, our study aimed to detect CERs’ potential in enhancing the topical retention of PNL and assess its safety. To attain this, various factors affecting CERs’ aspects were analyzed employing 13. 22 mixed factorial designs to select the optimum CER. DDAB amount (mg) (X1), ceramide type (X2), and Kolliphor® type (X3) were examined as factors, whereas entrapment efficiency percentage (EE%; Y1), particle size (PS; Y2), and zeta potential (ZP; Y3), were examined as responses. The shape, effect of storage, and mucoadhesive aspects of the optimum CER were evaluated. Ex-vivo permeation investigations were carried out for the PNL solution, the optimum CER, PNL-composite, and PNL-loaded CERs nanocomposite to assess their permeability. The accumulation of the fluoro-labeled optimum CER within skin layers was traced employing a confocal microscope. In addition, in-vitro antibacterial and biofilm eradication were carried out. Moreover, the in-silico investigation was performed for the constituents of the optimum CER to evaluate how they were stable throughout binding. Finally, in-vivo investigations were carried out to estimate the antibacterial and safety capability of the prepared formulations.
Materials
Propranolol hydrochloride (PNL) was purchased by El-Kahira Pharmaceutical Co. (Cairo, Egypt). Phospholipid from egg yolk (PC), fluorescein diacetate (FDA), Didodecyldimethylammonium Bromide (DDAB), and alginic acid sodium salt from brown algae (1000 to 1500 cP for 1% w/v in water at 25 ℃ viscosity) were purchased from Sigma Aldrich, USA. Kolliphors® RH 40 and EL were gained from Acros Organics, Belgium. Ceramides III and VI were supplied by Evonic Co. (Germany). Ethanol, and chloroform were purchased from El-Nasr Pharmaceutical Co., Cairo, Egypt.
Methods
Preparation of PNL-loaded CERs
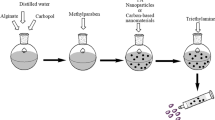
PNL-CERs were fabricated utilizing a modified ethanol injection approach [22]. In a mix of 1:1 ethanol/ chloroform (2mL), PC (100 mg), ceramides (III or VI), the surfactants (Kolliphors® RH40 or EL), and DDAB in various amounts were all dissolved. The organic mix was added to a 10 mL hot (60 ℃) aqueous phase that had previously included PNL (60 mg) pre-solved. After 30 min of stirring the formed mix on a magnetic stirrer (Model MSH-20D, Germany) at 800 rpm, the produced mix was kept in the refrigerator.
Characterization of PNL-loaded CERs
Determination of entrapment efficiency percentage (EE%)
1mL of the fabricated CERs was centrifuged at 4 °C for 1 h at 20,000 rpm utilizing a cooling centrifuge (Sigma 3 K 30, Germany). The supernatant (containing unentrapped PNL) was then diluted and PNL concentration was determined at λmax 289 nm [23] utilizing a UV-VIS spectrophotometer (Shimadzu UV1650 Spectrophotometer, Japan). EE% was calculated utilizing the subsequent equation [24]:
Determination of particle size (PS), polydispersity index (PDI), and Zeta potential (ZP)
PS, PDI, and ZP were measured for the formulated CERs employing Zetasizer 2000 (Malvern Instrument Ltd., UK). Following proper dilution (10-fold with de-ionized water), the measurement was carried out. Every specimen was evaluated in triplicate and the mean value was noted.
Determination of the amount of drug released after 6 h Q6h (%)
In-vitro release investigations were conducted utilizing the United States Pharmacopeia (USP) dissolution apparatus (Pharma Test, Germany) with a diffusion area of 3.14 cm2. The cellulose membrane was placed between the compartments of the donor and receptor with one end sealed with a cellulose membrane and the other end, instead of baskets, linked to the USP dissolution apparatus shaft. Precisely measured 1 ml of the optimum CER dispersions, and a PNL solution equivalent to 6 mg was added into the donor cells. The receptor compartment was loaded with 50 mL phosphate buffer (pH 5.5) and kept at 37 ± 1 °C [25]. At an appropriate interval, 1 mL of release media was removed, and the same quantity of fresh media was placed in the receiver cell. Specimens were withdrawn at 1, 2, 3, 4, 5, and 6 h and scanned utilizing a UV-spectrophotometer at λmax 289 nm.
Mixed factorial experimental design
The mixed factorial design was utilized to estimate the impact of different independent variables when fabricating CERs. The factors evaluated were: DDAB amount (mg) (X1), ceramide type (X2), and Kolliphor® type (X3). EE%, PS, and ZP were selected as responses (Table 1). Every parameter was submitted to an analysis of variance (ANOVA) testing employing Design-Expert® (Version 13, Stat-Ease., USA). Statistical significance was detected utilizing p-values < 0.05.
Selection of the optimum PNL-loaded CER
The choice of the optimum CER was based on the function of the desirability that permitted the studying of whole responses concurrently. The aim was to attain a solution with maximum ZP and EE% and minimum PS. The suggestion with the greatest desirability solution was opted as shown in (Table 1). The optimum CER was formulated, inspected, and compared with the predicted data to make sure that the model was performing accurately.
Transmission electron microscopy (TEM)
TEM (Joel JEM 1230, Japan) was used to determine the morphological features of the optimum CER. The dyed formulation was added to a copper-covered carbon grid and then left for drying till TEM investigations were conducted [26].
Differential scanning calorimetry (DSC)
The thermal evaluation of PNL, the optimum CER ingredients, the physical mixture, and the optimum CER was done by utilizing differential scanning calorimetry (DSC-60, Shimadzu Corp., Japan) standardized with indium. Almost 5 mg of specimens were put into an aluminum pan using a temperature ranging from 10 to 350 ℃ at a rate of 5 ℃/min under a nitrogen stream [27].
Muco-adhesion test
The mucoadhesive characteristics of the optimum CER were assessed utilizing porcine mucin (1% w/v) that was blended under stirring for five minutes with an equal quantity of the optimum CER in a dropwise pattern. The mix was permitted to equate at room temperature overnight then it was diluted 10-fold with de-ionized water before measuring its charge. Utilizing a zetasizer the charge of mucin particles in the presence and absence of mucoadhesive vesicles was assessed [28].
Impact of storage
The optimum CER stability was examined to monitor vesicles’ formation, PNL leakage, or any change. The optimum CER was stored for 3 months in a refrigerator and its stability was then tested by comparing the EE%, PS, ZP, PDI, and Q6h (%) of the stored optimum CER to the newly formulated one. Furthermore, the preparation was examined for any precipitation or aggregation. Statistical significance was conducted utilizing Student’s t-test employing SPSS® software [25].
Preparation of alginate hydrogel composite (PNL-composite and PNL-loaded CERs nanocomposite)
Alginate hydrogel-loaded with the optimum CER was formulated utilizing the approach described in the literature [29], with some minor adjustments. Briefly, under stirring at 1500 rpm and 70 ℃ for 15 min (Hot plate magnetic stirrer., Korea), 2% w/v of sodium alginate was added to the CERs to ensure the entire dissolving of sodium alginate and a consistent dispersion of the CERs throughout the fabricated hydrogel. A precisely determined volume was removed from the prepared alginate-gel and put into circular-base vessels. Later, the same amount of anhydrous CaCl2 aqueous solution (0.2 M) was inserted all at once into the vessels holding the alginate gel until the development of uniform dispersal; then the mix was kept at room temperature for 10 min to allow the cross-linking process [30]. Subsequently, the fabricated hydrogels were taken out and washed with distilled water three times with a constant volume of 20 mL to get rid of any excess non-crosslinked CaCl2 and CERs then kept in separate containers at room temperature. To prepare PNL-alginate hydrogel (PNL-composite) and non-medicated alginate hydrogel (blank), the same procedures were followed, but distilled water was used in place of the CERs dispersion for comparison in the in-vivo investigations.
Ex-vivo studies
Tissue collection
In this examination, rat skin was utilized because of its availability, small size, and low cost. First, animals were slaughtered, and the skin was eliminated. After that, an electrical clipper was utilized to remove hair from rat skin and the subcutaneous tissues and adhering fats were removed by cotton brushing. The removed skin specimens were equilibrated by immersing them in saline before starting the examination [31].
Ex-vivo permeation studies
This investigation aimed to examine the ability of 1 mL of PNL solution, optimum CER, PNL-composite, and PNL-loaded CERs nanocomposite gel (containing 6 mg PNL) to penetrate the rat’s skin. The specimens were added into cylindrical tubes featuring a diffusion area of 3.14 cm2 with one end covered with skin and the other end linked to the USP dissolution apparatus shaft (Pharma Test, Germany). The formulations were placed in 50 mL phosphate buffer (pH 5.5) at 37℃. Specimens were removed at 1, 2, 3, 4, 5, and 6 h and examined utilizing HPLC [32]. Using ANOVA test statistical significance was tested employing SPSS®. Post-hoc-analysis was carried out utilizing Tukey’s honestly significant difference (HSD) test. The significance was opted at P < 0.05.
Ex-vivo confocal laser scanning microscopy studies
To determine the passing of the optimum CER via the skin, the fluoro-labeled CERs were fabricated as described in the preparation section but with the inclusion of 10 mg FDA instead of PNL. Rat’s skin was placed as the ex-vivo investigation. FDA-loaded CERs were put on the dorsal skin surface and kept for 6 h. After that, longitudinal sections of skin were sliced utilizing a microtome (Cambridge, UK), and the skin tissues were then examined under an inverted microscope (Carl Zeiss, Germany) to determine the presence of fluorescence.
In-silico studies
Molecular docking of PNL against multiple MRSA biotargets
PNL was constructed and energy-minimized at MMFF94s partial charges and MMFF94s-modified forcefield as previously described using MOE software package (Quebec, Canada) [33,34,35]. MRSA’s biological targets involved within peptidoglycan synthesis; MurE (PDB: 4c12), MraY (AlphaFold: Q2FZ93), FemA (PDB: 1lrz), and PBP2a (PDB: 3zg0) were deposited and structurally prepared under 3D-protonation (pH 7.4, and 0.1 M salt solution /Volumn-Integral implicit solvent model), in addition, autocorrected for atom types, partial charges, and bond connectivity. Binding sites were defined by harboring the co-crystallized ligand, explored via the MOE Alpha Site Finder geometrical method, and finally optimized for including the essential residues that were previously stated within the context.
The docking was conducted using the induced docking protocol that allowed significant pocket residue elasticity. Ligand configurations were established On-the-Fly utilizing a bond rotation/ligand placement approach, within the determined active site, and guided utilizing triangular-matcher protocol [36]. The resultant configurations were ordered utilizing London_dG and the top 10 docked poses/ligands were optimized, and energy minimized allowing the protein’s residue sidechains to be tethered. Optimized poses were subsequently rescored utilizing Generalized Born-solvation-VI/Weighed Surface-Area_dG forcefield depending on explicit solvation electrostatics, current-loaded charges, exposure-weighted surface area, and Coulombic electrostatics via protein-ligand van der Waals scores [37, 38]. High docking scores, RMSD values below 2.0 Å cut-off, and/or significant interactions with stated essential-pocket-residues were regarded for selecting the best pose. PyMol2.0.6 (Schrödinger, USA) and MOE wizard/measurement apparatus were employed for visualization.
Molecular modeling simulation of the nano-formulation drug complex
Computational investigation under vacuum conditions was performed by applying the AutoDock package v1.2.0 (Scripps Research Institute, California, United States). PNL and formulation additives were 2D constructed using the isomeric SMILES deposited at PubChem database (PNL ID_4946), ceramide-VI ID_44625889, PC ID_65167, (DDAB) ID_18669, and Kolliphor® RH40 unit molecule ID_482024909. Constructed molecules were transformed into 3D structures and energy was reduced under AMBER/modified partial charges and forcefield. Molecular docking protocol was done utilizing Lamarckian Genetic Algorithm-driven conformational search under AMBER Forcefield [39].
Docking protocol proceeded through Triangular/Matching combined with the London ΔG-ranking scoring system as well as a refinement for the 10 top-scored poses via energy minimization under Generalized Born-solvation_VI/Weighted-SA/ΔG forcefield. The refinement forcefield scoring is generally based on van der Waals potentials, Coulomb’s interactions, loaded partial charges, polar solvation potentials, and weighed exposure SA [40]. Provided docked binding scores as binding energies, whereas choosing the best-docked pose was a combination of high docking score consideration and significant polar/hydrophobic interactions between corresponding formulation additives. PyMol v2.0.6 (Schrödinger, United States) was utilized to visualize the predicted docking results and analyze the inter-compound binding contacts. The docked drug/nano-formulation complex was set as a reference structure for all-atom molecular dynamics simulations using MOE software under the explicit Amber10: EHT forcefields [41, 42]. The 3D water cube-shaped box was used to solvate the drug-formulation complex under periodic boundary conditions with distances of 10 Å (Table 2) [43]. The system was minimized through NVT ensemble (300 K) for 50 ps, subsequently equilibrated under NPT ensemble (300 K; 1 atm. Pressure) for 50 ps. Explicit molecular dynamics simulations were then produced through 1000 ps under the NPT ensemble. Studying the MD trajectories was performed utilizing the MOE Database Calculator for plotting potential/kinetic energy versus time as well as the compound’s average binding-free energy towards the PC nano-formulation components. Time-evolution conformational changes for the drug nano-formulation were monitored across the extracted timeframes at 200 ps intervals.
In-vitro antibacterial activity
This investigation aimed to determine the antibacterial potency of PNL against MRSA USA300. Utilizing the broth microdilution approach following the guidelines of the Clinical and Laboratory Standards Institute, the minimum inhibitory concentration (MIC) was determined [44, 45].
Anti-biofilm activity of PNL
Biofilm inhibition assay
Utilizing flat-bottom 96-well plates, the biofilm inhibition assay was conducted as formerly mentioned [46]. Various sub-MIC concentrations (0.3125–0.0098 mg/mL) of PNL were examined. Untreated groups (no drug was included) were opted as controls. The experiment was repeated in triplicates. The % of biofilm inhibition was computed utilizing the subsequent equation [47]:
Biofilm eradication assay
This investigation aimed to evaluate the capability of PNL to detach and eradicate the already formed MRSA USA300 biofilm. The experiment was performed as described before [46]. Various sub-MIC concentrations (0.3125–0.0098 mg/mL) of PNL were formulated in fresh media and then inserted into the plates of the biofilm. For the biofilm control groups, nothing was applied to the plates (untreated biofilm, 100% reference value). The trial was conducted in triplicates. The % of biofilm eradication was computed utilizing the subsequent equation [47]:
In-vivo studies
In-vivo MRSA skin infection model
All trials and animal-procedures were accepted by the Research Ethics Committee of the Faculty of Pharmacy Cairo University (Approval# MI3394) following the “Guide for the Care and Use of Laboratory Animals” published by the Institute of Laboratory Animal Research (Washington, DC, USA). The MRSA-related skin infections model was performed as previously reported [45, 48, 49]. Twenty-eight BALB/C male mice aged 7 weeks old were kept in cages at ambient temperature with food and water. Before the assessment, the mice’s dorsal backs were shaved and then injected intradermally with 100 µL MRSA USA300 suspended in sterile saline (9 × 108 CFU). Mice were randomly classified into 4 groups (n = 7). After 48 h of infection and the development of an open wound, the first group served as the negative-control. The second group was handled with a blank formula to be used as vehicle-control. The third group was handled with the PNL-composite. The fourth group was topically handled with PNL-loaded CERs-nanocomposite. All groups were topically handled at the infection site utilizing 100 µL of the assigned medication one time a day for three days. The experiment ended 24 h after the last treatment and the animals were euthanized; the skin laceration was cleaned off, and then homogenized by 0.5 ml saline. Specimens were diluted 10 folds and then they were plated on mannitol salt agar and incubated at 37 °C to determine the aerobic viable count. Following incubation for 24 h, the plates were checked for colony-forming units (CFU) and the findings of the examined groups were analyzed and contrasted.
Histopathologic evaluation
Fifteen mice were classified into 5 groups, each containing 3 mice, and treated for one week: group I served as negative control, group II served as positive control, group III was handled with vehicle gel, group IV was handled with PNL-composite, and group V was handled with PNL-loaded CERs nanocomposite. After the autopsy, specimens were kept in 10% formalin and dried and sectioned at 4 μm utilizing a microtome (Cambridge, UK). The samples were subsequently deparaffinized, dyed, and histopathologically evaluated employing light microscopy [48].
Results and discussion
Optimization of CERs using mixed factorial design
Quality by design is a powerful statistical technique that validates the procedure’s effectiveness in terms of mathematical correlations. The desirability function is an efficient method for determining the optimum levels of the variables. The two-factor interaction (2FI) model was selected because it produced the highest prediction R2. Adequate precision is employed to assert that the model could be used for navigating the design space [50]. A ratio (signal-to-noise ratio) greater than 4 (for adequate precision) is favored which was observed in each of the responses (except PDI) as seen in Table 3. For design-analysis outcomes (Table 3), it was obvious that the predicted R2 values were in good harmony with the adjusted R2 in each of the responses.
Effect of formulation variables on the EE%
EE% of the formulated PNL-loaded CERs ranged from 65.71 ± 0.95% to 96.06 ± 0.97% (Table 4). The relatively great EE% values from the prepared CERs might be related to the presence of ceramide that enhances the nano-dispersions’ viscosity, impeding the drug diffusion, and leading to greater EE% values [26]. Statistical analysis of the data utilizing ANOVA showed that all the investigated variables had a significant impact on the EE% (Fig. 1(A–C)). Considering the DDAB amount (mg) (X1) (p < 0.0001) it was obvious that by augmenting the DDAB amount the EE% decreased significantly. This might be related to the DDABs’ improvement of PC solubility, which encourages the leakage of PNL from CERs. Conversely, the greater EE% values at low DDAB amounts might be attributed to the formation of tight bilayers around PNL and insufficient surface-active agent (SAA) amounts to solubilize PC bilayers [28].
Regarding ceramide type (X2) (p < 0.0001) it was found that the EE% increased by using ceramide VI compared to ceramide III. As ceramide VI consists of a saturated phytosphingosine structure acylated with hydroxy stearic acid long chain [51]. On the other hand, ceramide III is composed of a phytosphingosine backbone acylated with oleic acid in addition, it has one unsaturated bond in its fatty acid chain [5] the previously observed unsaturated double-bond in the carbon chain may affect it to twist, making CERs leakier since the packing of the CERs may not be tight [27].
For Kolliphor® type (X3) (p < 0.0001), it was found that Kolliphor® RH 40 produced higher EE% values compared to Kolliphor® EL. This might be ascribed to the presence of unsaturation sites in the alkyl chains of Kolliphor® EL that may enhance the permeability of the vesicle membrane, consequently diminishing EE% [52]. The existence of the unsaturated double-bond in the chain of carbon as previously stated might cause twisting that promotes leaking from CERs as the packing of the CERs might not be firm. These findings were consistent with Abdelbary et al. who found that bilosomes containing Kolliphor® RH 40 entrapped more terconazole than those containing Kolliphor EL [53]. On the other hand, Kolliphor® RH 40 has a higher molecular weight than Kolliphor® EL, previous literature stated that higher molecular weight SAA might aid in steric stabilization and keep the nanocarriers from aggregating or fusing. This may also result in a greater degree of trapping effectiveness [54].
Effect of formulation variables on PS
The size of the nanocarriers plays a prime role in improving the rate of dissolution as well as in their adhesion and interaction with biological cells [55]. PS of the fabricated PNL-loaded-CERs ranged from 250.34 ± 17.51 to 586.42 ± 14.46 nm (Table 4). Statistical analysis of the data utilizing ANOVA showed that all the investigated variables had a significant impact on the PS (Fig. 1 (D–F)). Considering the DDAB amount (mg) (X1) (p < 0.0001) it was apparent that by increasing the DDAB amount the PS decreased significantly. The prior findings were consistent with Abdellatif et al. [56] who discovered that the increase in cationic SAA produces steric repulsion, which prevents or decreases vesicle aggregation. Further, the increase in DDAB might diminish the interfacial tension between aqueous lipids, resulting in the formation of smaller vesicles, or due to surfactants’ lipid solubilization, resulting in a smaller PS.
Regarding ceramide type (X2) (p = 0.0076), the PS was greater in CERs contained VI compared to CERs contained III the previous results agreed with EE% results as higher PS CERs were found to be with CERs with higher EE% [57]. On the other hand, ceramide III and ceramide VI molecular weights are 582 and 600, respectively. Although there was a slight difference in the molecular weight of ceramides, it resulted in the development of larger CERs in the case of CERs produced by ceramide VI. As previously reported, the rise in the molecular weight might augment the viscosity of the nano-dispersion developing aggregations and augmenting the PS [58].
For Kolliphor® type (X3) (p < 0.0001), it was found that Kolliphor® RH 40 produced smaller PS values compared to Kolliphor® EL. Both SAAs are made up of hydrophilic and hydrophobic counterparts. The hydrophilic counterparts of both SAAs are accountable for preventing vesicle aggregation. They are made of polyethylene oxide (PEO) units with 40 PEO units for Kolliphor® RH 40 and only 35 PEO units for Kolliphor® EL [58]. Consequently, the employment of the former Kolliphor® which has a more stabilizing ability led to the formation of smaller PS, the previous findings agreed with previous literature [59].
Evaluation of PDI
It is commonly known that sample heterogeneity is indicated by PDI values close to 1, whereas values close to 0 indicate size homogeneity [60]. The observed values (Table 4) ranged from 0.356 ± 0.01 to 0.370 ± 0.01. These confirmed the evaluated samples’ relative homogeneity as the ethanol injection method usually produces a homogenous population [61].
Effect of formulation variables on ZP
The ZP values ranged from 1.87 ± 0.06 to 37.88 ± 1.08 mV (Table 4). Because of the negatively charged protein residues that are present on the surface of human skin, the skin is negatively charged [62]. Hence, the positive charge in the skin nano-formulations is preferred to augment electrostatic interaction between cationic formulations as presented in CERs and the skin surface, leading to greater retention of the drug in skin layers. The resulting data (Table 4) and (Fig. 2 (A-C)) reveal the impact of the investigated variables on the ZP of the CERs.
Considering the DDAB amount (mg) (X1) (p < 0.0001) it was evident that by increasing the DDAB amount the ZP increased significantly. The existence of the cationic surfactant (DDAB) affected the fabricated CERs-ZP values as it reduced the surface charge’s negativity. Further, the increase in ZP could be ascribed to the existence of DDAB molecules in high amounts at the interface of CERs, resulting in greater positive ZP values [63].
For Kolliphor® type (X3) (p < 0.0001), it was found that Kolliphor® RH 40 produced higher ZP values compared to Kolliphor® EL. Kolliphor® RH40 is more hydrophilic (HLB ∼ 15) than Kolliphor® EL (HLB ∼ 13), which helps the dispersion of the lipids in the aqueous phase [64]. A previous study stated as the HLB of the SAA increases it usually produces a more stable nanosystem with higher ZP values [64].
Determination of the optimum CER
The optimum CER from the experimental design was CER6 which met the previously established criteria (maximum ZP and EE% and minimum PS). It was selected utilizing Design Expert software® version 13. The observed and predicted values of CER6 revealed a high correlation (Table 3). Also, the high predictive capacity of the model was demonstrated by the bias percent values, which were lower than 10% for all the evaluated responses [65]. Subsequently, CER6 was subjected to extra studies.
In-vitro drug release
NCs have lately proven probable utility in the treatment of bacterial infections through their topical application that enables controlled and long-lasting release. Due to their small size and the possibility of modifying their surface charge, they can biologically interact with the body systems and hence, improve their in vivo therapeutic efficacy [65, 66]. From Fig. 3A, it was found that Q6h (%) for the optimum CER was 86.60 ± 5.5% and the release pattern of PNL from the optimum CER was at first rapid and then significantly (p = 0.0001) changed to sustained compared to the PNL solution where PNL was entirely released from the PNL solution after 2 h. The prior findings might be ascribed to the following reasons, firstly: PNL being an amphoteric drug, it has affinity toward the aqueous phase. Secondly: the presence of both PC and ceramide could offer a depot property that contributes to the continual release of PNL in a sustained manner from CERs for fighting against bacterial infection [67].
Transmission electron microscopy (TEM)
TEM investigation (Fig. 3B) revealed that ceramide addition to the composition of the CERs led to a dramatic change in the morphology of their membrane from spherical to tubular [17]. In the TEM image of CERs, the prevalent morphological form was elongated ceramide tubules, with vesicles appearing less frequently. This was also confirmed by Xu et al. [51] who found that adding ceramide to PC resulted in the elongation of the fabricated vesicles which was ascribed to the partitioning of ceramide VI into the PC bilayer associated with the interface rigidification. Also, the high packing parameter of ceramide (1.2) in comparison to that of the PC (0.7) made the PC bilayer curvature flattened upon hydration with an aqueous solution [51]. The irregular existence of tubules along with the spherical vesicles as shown in the optimum CER resulted in a distinctive bulbous feature of the tubules. It was confirmed by the authors who stated that ceramide VI tends to distribute in a non-uniform design in the bilayer, resulting in ceramide-rich areas with a flat shape and ceramide-poor areas with a spherical shape.
Differential scanning calorimetry (DSC)
From Fig. 4A, PNL showed an endothermic peak representing its melting point at 166.91℃ [25]. In addition, PC showed two endothermal peaks. The first peak (159.9 °C) was described as mild, showing hot movements of polarity parts of PC. The second peak manifested at 234.6 °C, which might be attributed to the transition from gel to liquid crystal state, the melting of the carbon-hydrogen chain in PC, and changes in isomers or the crystal [25]. Ceramide VI showed an exothermic peak at 100.3℃ [68]. Moreover, the thermogram of DDAB showed a sharp peak at 110℃ [69]. The thermogram of Kolliphor® RH40 showed no exothermal or endothermal peaks [70]. The DSC of the physical mixture of PNL with CERs constituents disclosed that the PNL peak was existent supporting its presence in the crystalline state. The thermogram of the optimum CER did not reveal the melting peak for PNL. This manifested that PNL was in an amorphous state, and it was completely entrapped into the CER [71]. In addition, the disappearance of a distinctive peak of PNL might also suggest a major interaction between the drug and the constituents of the CER structure which might be the reason for the high EE% of PNL. These interactions could account for the good vesicle shape, structure, and excellent stability.
Differential scanning calorimetry study (a) PNL, (b) PC, (c) ceramide VI, (d) DDAB, (e) Kolliphor RH40, (f) physical mixture, and (g) optimum CER (A), and Mucoadhesion test for the optimum CER (B). Abbreviations CER; Cerosome, DDAB; Didodecyldimethylammonium Bromide, and PNL; Propranolol Hydrochloride
Mucoadhesion test
To investigate the mucoadhesive properties of the investigated optimum CER, the mucin solution was mixed with the examined formulation. Results revealed a significant change in the ZP value of pure mucin solution from − 7.99 ± 1.20 to 10.11 ± 0.09 mV after being mixed with the tested formulation (Fig. 4B). This change might have resulted from the interaction of the negatively charged sialic-acid found in mucin with the positively-charged-amino groups of the utilized cationic SAA (DDAB). It is worth mentioning that DDAB is a quaternary ammonium SAA that possesses a central ammonium group with a permanent positive charge attached to double alkyl chains. In addition, DDAB has an amphiphilic character due to the presence of both a polar head and hydrophobic tail, respectively present in its structure. This ionic interaction indicated the mucoadhesive properties of CERs. These mucoadhesive features can prolong the duration that the applied formulation remains on the skin, hence augmenting the local concentration of the drug at the application site and enhancing the drug’s bioavailability.
Impact of storage
At the end of the storage interval (3 months in a refrigerator 4℃), there was no marked variation in the appearance of the optimum CER dispersion. The evaluated physical parameters of the stored CER in comparison to the fresh one with EE% of 91.99 ± 1.01%, PS of 390.00 ± 1.30 nm, PDI of 0.370 ± 0.001, ZP 31.00 ± 0.09 mV, and Q6h (%) of 67.00 ± 0.03% were statistically analyzed and displayed that there was no significant difference (P > 0.05) in the EE%, PS, PDI, and Q6h (%). (Fig. 5A) reveals that both fresh and stored CERs showed almost identical release profiles. This observation was proved by the computed value of the similarity factor (f2 = 85.20) indicating that the storage had no remarkable impact on the release of the PNL. The previous findings might be because of the presence of DDAB which potentially helps in the stability of vesicles by increasing the ZP [72].
Ex-vivo studies
Ex-vivo permeation
The application of nano-systems can deliver the drugs to the skin tissues in a very precise manner and enable their sustained release resulting in an extended activity and a possible reduction in adverse effects [73]. It is worth noting that Hirose, et al. suggested for topical MRSA infections, the systemic treatment was not often effective. Hence, topical treatment for MRSA with controlled release aspects is required [74]. From Fig. 5B, it is obvious that the amount of PNL-permeated from PNL-loaded CERs nanocomposite was significantly (P < 0.5) the lowest compared to PNL-composite (PNL-loaded-alginate hydrogel), the optimum CER and PNL solution (Table 5). The significantly lower permeability from PNL-loaded CERs nanocomposite could be explained due to the efficacy of alginate hydrogel to produce a sustainable drug delivery system by increasing the viscosity of the formed system [21]. Regarding the significant (P < 0.5) sustained release of PNL-loaded CERs nanocomposite over the whole formulae, this could be attributed to the successful PNL incorporation within the cross-linked-polymer, consequently for drug release to take place, the drug molecules should diffuse firstly through the CERs membrane and then the hydrogel network. Also, the presence of lipids (PC, and ceramide) might successfully encapsulate PNL and provide sustained release, protection, and controlled release profiles [75]. The prior reasons explained the significant sustained release from PNL-loaded CERs nanocomposite and optimum CER compared to PNL- composite and PNL solution. It is worth bearing in mind that the amphoteric aspect of PNL explains its higher permeability profile from PNL solution in comparison to the other formulations. The amount of drug deposited in the skin was in the following order: 13.28 ± 0.94, 26.25 ± 1.88, 42.97 ± 16.63, and 57.04 ± 1.79 µg/cm2, for PNL solution, the optimum CER, PNL-composite, and PNL-loaded CERs nanocomposite, respectively. The significant (P ≤ 0.5) highest deposition from the PNL-loaded CERs nanocomposite could be related to the hydrogel-loaded CERs that could form a depot from which the drug can be released.
Ex-vivo confocal laser scanning microscopy studies (ClSM)
The CLSM images showed improved cell internalization and greater drug penetration into the various skin layers which were distinctly displayed in (Fig. 6). The skin treated with the fluoro-labeled optimum CER revealed deep penetration of the dye into the various skin layers. These findings confirmed the accumulation of CERs into deeper skin layers that could be effective for the treatment of MRSA-related skin infections. The previous outcomes correlated with Yang et al. during their preparation of CERs for the delivery of methotrexate and nicotinamide for psoriasis management [76].
In silico studies
PNL with affinity towards multiple peptidoglycan-associated MRSA biotargets
The molecular aspects of PNL’s anti-MRSA activity were explored by estimating the compound’s binding affinity with several targets involved in MRSA’s peptidoglycan biosynthesis. Most of the marketed drugs commonly applied for managing MRSA are those designed to hamper its peptidoglycan biosynthesis, the crucial component of the bacterial cell wall [38]. Typically, peptidoglycans confer the bacterial cell wall’s flexibility and robustness and thus interfering with their biosynthesis would mediate bactericidal actions [77]. The presented study explored the potential of PNL to block four multiple steps across peptidoglycan biosynthesis. The first hampered step was mediated by bacterial MurE ligase being involved within the cytosolic biosynthesis of peptidoglycan’s starting units; UDP-N-acetylglucosamine for producing the UDP-N-acetylmuramyl-multipeptide product [78]. The second target stage was that mediated by the membrane-bound enzyme, MraY, where C55-PyroPhosphate-UDP-N-acetylmuramyl-pentapeptide (Lipid I) is formed through catalytic conjugation reaction at the bacterial cytoplasmic side [79]. The third stage involves interpeptide bridging through disaccharide pentapeptide modifications using a pentaglycine chain to be added to the lysine amino acid of the pentapeptide chain. The step is catalyzed by several peptidyl transferases, including FemA. The final stage involved the formation of linear peptidoglycans via the DD-transpeptidase catalytic activity of penicillin-binding proteins (e.g. PBP2a) following the transfer of the disaccharide pentapeptides to the cell membrane’s outer surface [80]. The development of multi-target drugs has been considered advantageous for circumventing the most prevalent mechanism of antibiotic resistance which is the target mutations [81, 82]. Out of an evolutionary concept, targeting multiple independent paths for inhibitions is unlikely to allow bacteria to develop resistance over time the thing that would circumvent the pipeline of antimicrobial drug discovery [83].
The molecular docking of PNL at MRSA’s MurE revealed preferential anchoring of the hypertensive compound at the binding domain of the co-crystallized product UDP-N-acetylmuramyl-tripeptide. Typically, the product bounds predominantly across the central domain of the ligase protein in proximity to the ATP-binding site (Fig. 7A). Several MRSA MurE key residues have been reported as important including Asp406, Ser456, and Glu460, for product/substrate binding and recognition [78], as well as affinity for promising inhibitors [84,85,86]. Both the negatively charged sidechains of Asp406 and Glu460 as well as the polar mainchain of Ser456 served as the electrostatic trap mediating the stability of UDP-N-acetylmuramyl-tripeptide at the binding site. Validation of the docking protocol was highlighted through redocking the co-crystallized ligand under the same adopted parameter, highlighting great superimposed alignment for the co-crystallized and redocked conformation (RMSD = 1.8 Å) (Fig. 7A). Furnishing RMSD below 2.0 Å for co-crystallized ligand relative to its reference conformation/orientation signifies that both the assumed parameters and algorithms were efficient for predicting relevant binding poses, highlighting respective biological significance [87].
Architecture of MRSA MurE and depicted molecular docking poses. (A) Upper panel; Cartoon 3D-representation of MRSA MurE (PDB; 4c12) ligase showing structural domains; I (Met1–Tyr98; green), II (Pro99–Val332; yellow), and III (Glu333–Asp493; orange) in complex with co-crystallized adenosine diphosphate (ADP), product UDP-N-acetylmuramyl-tripeptide (UNAM-tripeptide), and two magnesium ions (cyan) all as spheres. Letters N and C in bold denote amino- and carboxy terminals, respectively. Lower panel; Aligned redocked MurE product (UNAM-tripeptide; magenta) over its co-crystalline state (yellow). Predicted binding mode of (B) propranolol and (C) Antibacterial compound T26 as positive reference control. Only surrounding residues within a 5 Å radius as lines are shown and polar interactions are illustrated as black-dash lines. Predicted binding poses of PNL-PC docked complex. Three-dimensional representation of PNL (blue) loaded on (D) PC (magenta); (E) in combination with nano-formulation additives; ceramide VI (yellow), DDAB (yellow), and kolliphor-RH40 (green). Context-described hydrogen bonding and polar interactions are represented as black dashed lines. Abbreviations DDAB; Didodecyldimethylammonium Bromide, PC; Phospholipid, PNL; Propranolol Hydrochloride
Interestingly, PNL showed an extensive polar network with surrounding residues including the ionizable residues, Asp406 (3.1 Å; 121.2°) and Glu460 (2.4 Å; 128.9°), as well as polar Ser456 (2.9 Å; 132.4°) (Fig. 7B). Electrostatic-mediated stability of PNL was further predicted through hydrogen bond interaction between the ligand’s quaternary amine as hydrogen bond donor with nearby Tyr351 mainchain (2.0 Å; 161.8°) as well as ether linker oxygen with Thr152 sidechain (3.0 Å; 134.2°). On the other hand, the PNL aromatic structure was held through hydrophobic and π-mediated contacts (< 5.0 Å) with proximal aromatic residues (His181 and His205) and cationic ones (Arg383). Based on the depicted binding preferentiality, a high docking score was assigned for the anchored compound (–6.3 Kcal/mol). Notably, a thiazolidinylidene-based compound (T26) was adopted as a positive control as a MurE inhibitor with just a higher docking score (–7.2 Kcal/mol) than PNL. The reference compound was reported with high dual inhibition activities towards MurE and MurD from Staphylococcus aureus (IC50 = 17.0 µM and 6.4 µM, respectively) and Escherichia Coli (IC50 = 180.0 µM and 8.2 µM, respectively) based on radioactivity inhibition assays [88]. Reported studies highlighted the close similarity between MurE secondary structure originating from MRSA and Escherichia Coli microorganisms (RMSD 1.48 Å along > 450 Cα-atoms and Z-score 21.2) [78, 89]. Furthermore, T26 highlighted great antibacterial activity against MRSA and its wild-type strain with MIC of 9.0 µg/ml [88]. Docking of T26 at MRSA MurE highlighted dominant electrostatic potentiality guiding its anchoring at the substrate site with interactions with Thr46 (2.6 Å; 159.1°), Asp406 (2.6 Å; 118.4° and 2.8 Å; 115.0°), Asn407 (2.6 Å; 123.3°), and Glu460 (2.0 Å; 140.5°) residues (Fig. 7C). For more information regarding MRSA biotargets please see supplementary materials.
Molecular modeling simulation of the nano-formulation drug complex
The magnitude and nature of interaction for PNL with the adopted formulation additives were investigated through a molecular docking study. Throughout the in-silico study, the nature of PC-PNL interaction, in the absence of other formulation additives, was dominated by van der Waal hydrophobic potential. The PNL illustrated favored orientation with its aromatic scaffold towards the PC acyl arms with proximity towards the ester functionalities. On the contrary, the aliphatic arm (PNL amine side chain) depicted favored orientation via the ionizable amino group (pKa = 9.46) towards the negatively charged phosphate head of the PC molecule (Fig. 7D). This favored orientation-mediated triple polar interactions, two via the drug’s ionizable amino group towards the oxygen functionality of the PC (3.34 Å/128.81° and 2.60 Å/152.82°) and a singular one by the drug’s free hydroxyl group (2.29 Å/131.34°). Such depicted compound-PC binding mode and preferential orientation near the phosphate head are consistent with reported studies investigating the binding affinity of small molecules with drug-likeness properties including rosuvastatin, spironolactone, metformin hydrochloride, and levocetirizine dihydrochloride towards the PC molecules [90,91,92]. Despite the relevant binding interaction for the PNL-PC complex, further stabilization was required since humble docking binding energy was depicted (–3.15 Kcal.mol− 1).
Investigating the PNL-PC complex in the presence of the formulation additives depicted more stabilized and favored binding interactions (Fig. 7E). The complex stability was highlighted by utilizing the combined polar and hydrophobic interactions via the formulation additives, ceramide-VI, and DDAB. Both molecules predicted sandwich-like orientation around the PC-PNL complex with their polar heads mainly located near the PC’s phosphate group. The positively charged nitrogen of DDAB and polar NH group at ceramide-VI mediated polar interactions with the PC negatively-charged head (–OPO(O)OH). These predicted hydrogen bonds were considered relevant being at favored bond distances and angles; 2.28 Å/170.90° and 3.56 Å/124.27° for ceramide-VI and DDAB, respectively. On the other side, both DDAB and ceramide-VI depicted extended orientation via their aliphatic hydrophobic arms around the PC’s acyl chains and PNL aromatic scaffold (~ 3.75 Å). This was suggested as beneficial for satisfying the hydrophobic characteristics of the PC-PNL complex. Regarding the docked Kolliphor® RH40 unit, the polymeric molecule depicted favored orientation near the PC acyl arms owing to its linear hydrocarbon chains at repeated intervals. Nevertheless, the free hydroxyl groups at the Kolliphor® RH40 unit showed proximity towards the PNL’s aromatic ring suggesting relevant π-driven non-polar contacts (< 5.00 Å). It is worth noting that Kolliphor® RH40 almost totally shields the PNL’s aromatic scaffold in a way that is highly associated with dominant van der Waal non-polar interactions. Based on all depicted docking orientations, a more stabilized PC-PNL complex was observed following the introduction of the four formulation components mediating an extended network of both electrostatic and hydrophobic potentials. These docking interactions were highlighted with boosted docking energy for PNL (–6.65 Kcal.mol− 1) towards the nano-formulation complex. This could be the reason for the improved formulation parameters succeeding the addition of formulation additives serving as carrier agents to mediate the drug loading on the PC molecule for optimized solubilization of the drug.
Thermodynamic stability and dispersion behaviors of the PC-PNL nano-formulation complex in the final solvent of the formulation (100% water) were evaluated through molecular dynamics simulations (Fig. 8A). The whole system stability was highlighted as being relaxed and balanced throughout the monitored kinetic and potential energies across the entire time frame (Fig. 8B). The depicted minimal energy fluctuations with an attained plateau for most of the MD simulation run would confer adequate system stability and convergence. Conformational analysis across extracted time frames (200, 400, 600, 800, and 1000 ps) illustrated significant stability for the simulated PNL-PC nano-formulation complex (Fig. 8C). The PNL maintained its orientation at the PC’s acyl chains till the end of the simulation run (RMSD < 3.00 Å). Moreover, PNL depicted a large negative free-binding energy (average ΔG = − 89.26 ± 3.86 Kcal.mol− 1) towards the formulation components conferring adequate stability at the nano-formulation complex (Fig. 8D). Ceramide-VI and DDAB formulation additives maintained their enveloped orientation around both the PNL and PC molecules. The polymeric kolliphor® RH40 furnished relevant non-polar binding support for the PC acyl chains as well as the drug’s naphthyl core ring.
PNL-PC nano-formulation heterocomplex throughout all-atom molecular dynamics simulation within a 100% aqueous solvation system. (A) Solvated PNL-PC formulation complex within water cube and ionizable potassium and chloride atoms; (B) Plots for the system’s potential and kinetic energies and the drug’s binding-free energy versus the simulated time frames (ps). (C) Conformation alterations-time evolution of PNL-PC-formulation additive heterocomplex. Thermodynamic movement formulation components (sticks and differentially colored as previously described) were monitored over simulation trajectories captured at different snapshots ① 200 ps, ② 400 ps, ③ 3600 ps, ④ 800 ps, and ⑤ 1000 ps. (D) Overlaid PC-PNL nano-formulation heterocomplex across molecular simulation frames (left panel) and molecular surface 3D-representation of the inverted cone micellar configuration at 100% water solvation system (right panel). Molecular sticks and surface 3D representations were illustrated in colors previously assigned for the optimized formulation components. Abbreviations PC; Phospholipid, and PNL; Propranolol Hydrochloride
Finally, interesting findings were depicted for the PC’s spatial conformation in terms of its lipophilic elongated chains. Conserved polar contacts at the phosphate group made both PC’s hydrophobic acyl tails pull apart. Such thermodynamic behavior depicted an open-compass conformational structure for the PC’s extended tails causing increased volumes and higher solvent-accessible surface areas. On the contrary, smaller solvent-accessible surface areas were maintained throughout the imitation run as the PC complex maintained several robust and dense polar interactions at the phosphate polar head. This depicted packing fashion allowed the PNL-PC nano-formulation complex to acquire an inverted cone structure with maintained micellar configuration being formerly described with various tiny molecules [93, 94] (Fig. 8D).
In-vitro antibacterial activity
The PNL showed in-vitro antibacterial action against MRSA USA300 with MIC of 0.625 ± 0 mg/ml. PNL’s antimicrobial efficacy has been assessed against a broad spectrum of microorganisms, The minimum inhibitory concentrations (MICs) of PNL were determined to be 2 mg/mL for Pseudomonas aeruginosa and 1 mg/mL for Serratia marcescens [95]. The MIC of PNL was evaluated using the agar dilution method, yielding a value exceeding 120 µg/mL, implying its effectiveness against Staphylococcus aureus, Escherichia coli, and Pseudomonas aeruginosa, as evidenced by growth inhibition zones ranging from 8 to 28 mm [96]. Notably, no previous studies have reported the activity of PNL against Methicillin-resistant Staphylococcus aureus (MRSA).
Anti-biofilm activity of PNL
Because of the distinct antibacterial actions of nano-sustained-release materials, they are less likely to cause resistance; thus, they have gained more research attention in treating bacterial biofilm infections. Small-volume and high-surface-area nanomaterials, due to their ability to effectively penetrate biofilms and stick to the deep layers of the infected-tissues, are frequently used as carriers for slow-release antibiotics to produce sustained antibacterial effects [97]. The anti-biofilm activity of PNL was tested at concentrations lower than the established MIC (0.3125–0.0098 mg/ml). PNL significantly hindered the formation of MRSA USA300 biofilm and significantly eradicated, at all the examined concentrations, the previously formed MRSA USA300 biofilm (Two-way ANOVA, Bonferroni’s post-hoc test, P < 0.05) (Fig. 9). Interestingly, PNL recorded significantly higher biofilm eradication activity at all the examined concentrations (Two-way ANOVA, Bonferroni’s post-hoc test, P < 0.0001 (Fig. 9).
Anti-biofilm activity. Effect of different sub-minimum inhibitory concentrations (MIC) (0.3125–0.0098 mg/ml) of PNL on MRSA USA300 biofilm. Results are expressed as mean biofilm inhibition % ± standard error and mean biofilm eradication % ± standard error. **** indicate that the difference is significant at p < 0.0001 (two-way ANOVA, Bonferroni’s post-hoc test). Abbreviations PNL; Propranolol Hydrochloride
In-vivo studies
In-vivo MRSA skin infection model
The in-vivo antibacterial activity of PNL-composite and PNL-loaded CERs nanocomposite was evaluated against MRSA utilizing a murine-infection-model. Four groups of male BALB/C mice (n = 7) were intradermally inserted with MRSA USA300. After 48 h of infection, the wound appeared at the injection site. PNL-composite and PNL-loaded CERs nanocomposite significantly reduced the count of MRSA USA300 compared to the negative control group and vehicle control group (One-way ANOVA, Tukey’s post-hoc test, P < 0.0001) (Fig. 10). The in-vivo antibacterial activity of the PNL-loaded CERs nanocomposite was significantly greater than that of the PNL-composite (One-way ANOVA, Tukey’s post-hoc test, P < 0.0001). The count of bacteria recovered from the PNL-loaded CERs nanocomposite-treated group was 2.543 and 3.008 logs less than that of the vehicle control and negative control groups, respectively. The count of bacteria produced from the PNL-composite-treated group was 1.364 and 1.829 logs less than that of the vehicle control and negative control groups, respectively.
Efficacy of PNL-composite and PNL-loaded CERs nanocomposite in an in-vivo murine model of MRSA skin infection. Twenty-eight BALB/C mice were divided into four groups (n = 7). A: Photo image of the efficacy of different treatment groups on MRSA skin infection in the posterior backs of mice at the end of the experiment. B: Efficacy of different treatment groups on the bacterial load in murine model MRSA skin infection. Each data point in the figure represents a mouse. Results are expressed as mean ± standard error. **** indicate that the difference is significant at p < 0.0001 (One-way ANOVA, Tukey’s post-hoc test) Abbreviations PNL; Propranolol Hydrochloride
Histopathologic evaluation
The tissue investigation of the five groups (Fig. 11) (group I: negative control, group II: positive control, group III: vehicle control, group IV: PNL-composite, and group V: PNL-loaded CERs nanocomposite). Group I and III photomicrographs showed the normal histological structure of the epidermis and dermis. For group II, the photomicrograph showed an increase in the thickness of the epidermis with infiltration of the dermis by a high number of inflammatory cells. Regarding group IV, the photomicrograph showed infiltration of the dermis by a low number of inflammatory cells. Finally, group V photomicrograph showed the normal histological structure of the epidermis and dermis. Based on the observations made, it appears that the PNL-loaded CERs nanocomposite is safe and unlikely to induce skin irritation in clinical trials that agreed with the in-vivo investigations that did not demonstrate skin intolerability.
Light microscope photomicrographs showing histopathological sections (hematoxylin and eosin stained) of rat skin normal control (group I), positive control (group II), vehicle-treated group (III), rat skin treated with PNL-composite (group IV), and rat skin treated with PNL-loaded CERs nanocomposite (group V) with a magnification power of 16X to illustrate all skin layers (Left side) and magnification power of 40X (Right side). Abbreviations PNL; Propranolol Hydrochloride, CERs; Cerosomes, D; Dermis, E; Epidermis, F; Hair Follicles, K; keratin, and SF; Subcutaneous Fat
Conclusion
Propranolol hydrochloride (PNL) loaded cerosomes (CERs) were prepared using an ethanol injection technique, and the optimum CER revealed tubular vesicles with excellent EE%, PS, PDI, and ZP. Also, the optimum CER showed good stability for 90 days. Ex-vivo investigations proved the sustained release pattern of PNL-loaded CERs nanocomposite. Furthermore, CLSM images revealed excellent optimum CER deposition into the skin layers. The in-silico investigation confirmed good PNL stability after its incorporation with the other formulation additives. The in-vivo examinations confirmed the efficacy of PNL against MRSA. Histopathological examinations revealed that PNL-loaded CERs nanocomposite was safe. These promising outcomes proved the potential application of PNL-loaded CERs nanocomposite as an antibacterial agent against skin infections caused by MRSA.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Zakir F, Vaidya B, Goyal AK, Malik B, Vyas SP. Development, and characterization of oleic acid vesicles for the topical delivery of fluconazole. Drug Deliv. 2010;17(4):238–48.
Percival SL, Emanuel C, Cutting KF, Williams DW. Microbiology of the skin and the role of biofilms in infection. Int Wound J. 2012;9(1):14–32.
Khazanov E, Priev A, Shillemans JP, Barenholz Y. Physicochemical and biological characterization of ceramide-containing liposomes: paving the way to ceramide therapeutic application. Langmuir. 2008;24(13):6965–80.
Meckfessel MH, Brandt S. The structure, function, and importance of ceramides in the skin and their use as therapeutic agents in skin-care products. J Am Acad Dermatol. 2014;71(1):177–84.
Su R, Yang L, Wang Y, Yu S, Guo Y, Deng J, et al. Formulation, development, and optimization of a novel octyldodecanol-based nanoemulsion for transdermal delivery of ceramide IIIB. Int J Nanomed. 2017;12:5203–21.
Asano S, Ichikawa Y, Kumagai T, Kawashima M, Imokawa G. Microanalysis of an antimicrobial peptide, β-defensin-2, in the stratum corneum from patients with atopic dermatitis. Br J Dermatol. 2008;159(1):97–104.
Nishijima S, Kurokawa I. Antimicrobial resistance of Staphylococcus aureus isolated from skin infections. Int J Antimicrob Agents. 2002;19(3):241–3.
Pushpakom S, Iorio F, Eyers PA, Escott KJ, Hopper S, Wells A, et al. Drug repurposing: progress, challenges, and recommendations. Nat Rev Drug Discov. 2019;18(1):41–58.
Nehme H, Saulnier P, Ramadan AA, Cassisa V, Guillet C, Eveillard M, et al. Antibacterial activity of antipsychotic agents, their association with lipid nanocapsules, and their impact on the properties of the nanocarriers and antibacterial activity. PLoS ONE. 2018;13(1):e0189950.
Jampilek J. Drug repurposing to overcome microbial resistance. Drug Discov Today. 2022;27(7):2028–41.
Abruzzo A, Bigucci F, Cerchiara T, Cruciani F, Vitali B, Luppi B. Mucoadhesive chitosan/gelatin films for buccal delivery of propranolol hydrochloride. Carb Polym. 2012;87(1):581–8.
Hendley JO, Ashe KM. Effect of topical antimicrobial treatment on aerobic bacteria in the stratum corneum of human skin. Antimicrob Agents Chemother. 1991;35(4):627–31.
Mishra S, Gupta A, Upadhye V, Singh SC, Sinha RP. Therapeutic strategies against biofilm infections. Life (Basel). 2023;13(1):172.
Ozdal M, Gurkok S. Recent advances in nanoparticles as antibacterial agents. ADMET DMPK. 2022;10(2):115–29.
Song CK, Balakrishnan P, Shim C, Chung S, Chong S, Kim D. A novel vesicular carrier, transethosome, for enhanced skin delivery of voriconazole: characterization and in vitro / in vivo evaluation. Colloids Surf B Biointerfaces. 2012;92:299–304.
Albash R, Fahmy AM, Hamed MIA, Darwish KM, El-Dahmy RM. Spironolactone hyaluronic acid enriched cerosomes (HAECs) for the topical management of hirsutism: in silico studies, statistical optimization, ex vivo, and in vivo studies. Drug Deliv. 2021;28:2289–300.
Abdelgawad R, Nasr M, Moftah NH, Hamza MY. Phospholipid membrane tubulation using ceramide doping cerosomes: characterization and clinical application in psoriasis treatment. Eur J Pharm Sci. 2017;101:258–68.
Albash R, Yousry C, Al-Mahallawi AM, Alaa-Eldin AA. Utilization of PEGylated cerosomes for effective topical delivery of fenticonazole nitrate: in-vitro characterization, statistical optimization, and in-vivo assessment. Drug Deliv. 2021;28(1):1–9.
Tang Y, Wang X, Li Y, Lei M, Du Y, Kennedy JF, et al. Production and characterization of novel injectable chitosan/methylcellulose/salt blend hydrogels with potential application as tissue engineering scaffolds. Carbohydr Polym. 2010;82:833–41.
Spadari CDC, Lopes LB, Ishida K. Potential use of alginate-based carriers as an antifungal delivery system. Front Microbiol. 2017;8:97.
Eldeeb AE, Salah S, Amer MS, Elkasabgy NA. 3D nanocomposite alginate hydrogel loaded with pitavastatin nanovesicles as a functional wound dressing with controlled drug release; preparation, in-vitro, and in-vivo evaluation. J Drug Deliv Sci Technol. 2022;71:103292.
Kakkar S, Kaur IP. Spanlastics - a novel nanovesicular carrier system for ocular delivery. Int J Pharm. 2011; 413(1–2): 202– 10.
Kraisit P, Limmatvapirat S, Luangtana-Anan M, Sriamornsak P. Buccal administration of mucoadhesive blend films saturated with propranolol loaded nanoparticles. Asian J Pharm Sci. 2018;13(1):34–43.
Mosallam S, Ragaie MH, Moftah NH, Elshafeey AH, Abdelbary AA. Use of novasomes as a vesicular carrier for improving the topical delivery of terconazole: in vitro characterization, in vivo assessment, and exploratory clinical experimentation. Int J Nanomed. 2021;16:119–32.
Teaima MH, Eltabeeb MA, El-nabarawi MA, Abdellatif MM. Utilization of propranolol hydrochloride mucoadhesive invasomes as a locally acting evaluation. Drug Deliv. 2022;29(1):2549–60.
Albash R, El-Dahmy RM, Hamed MIA, Darwish KM, Alahdal AM, Kassem AB, et al. Repurposing levocetirizine hydrochloride loaded into cationic ceramide/phospholipid composite (CCPCs) for management of alopecia: central composite design optimization, in-silico, and in-vivo studies. Drug Deliv. 2022;29(1):2784–95.
Albash R, El-Nabarawi MA, Refai H, Abdelbary AA. Tailoring of PEGylated bilosomes for promoting the transdermal delivery of olmesartan medoxomil: in-vitro characterization, ex-vivo permeation, and in-vivo assessment. Int J Nanomed. 2019;14:6555–74.
Albash R, Abdellatif MM, Hassan M, Badawi NM. Tailoring terpesomes and leciplex for the effective ocular conveyance of moxifloxacin hydrochloride (comparative assessment): in-vitro, ex-vivo, and in-vivo evaluation. Int J Nanomed. 2021;16:5247–63.
Abasalizadeh F, Moghaddam SV, Alizadeh E, Kashani E, Mohammad S, Fazljou B, et al. Alginate-based hydrogels as drug delivery vehicles in cancer treatment and their applications in wound dressing and 3D bioprinting. J Biol Eng. 2020;14:1–22.
Farrell S, Vernengo J. A controlled drug-delivery experiment using alginate beads. Chem Eng Educ. 2012;46(2):97–109.
Aziz DE, Abdelbary AA, Elassasy AI. Investigating superiority of novel bilosomes over niosomes in the transdermal delivery of diacerein: in-vitro characterization, ex-vivo permeation and in-vivo skin deposition study. J Liposome Res. 2019;29(1):73–85.
Zahálka L, Matysová L, Šklubalová Z, Klovrzová S, Solich P. Simultaneous determination of propranolol hydrochloride and sodium benzoate in oral liquid preparations by HPLC. Chromatographia. 2013;76:1553–8.
Wadie MA, Kishk SM, Darwish KM, Mostafa SM, Elgawish MS. Simultaneous determination of losartan and rosuvastatin in rat plasma using liquid chromatography–a tandem mass spectrometric technique for application into pharmacokinetic and drug-drug interaction studies. Chromatographia. 2020;83:1477–94.
Malebari AM, Ibrahim TS, Salem IM, Salama I, Khayyat AN, Mostafa SM, et al. The anticancer activity for the bumetanide-based analogs via targeting the tumor-associated membrane-bound human carbonic anhydrase-IX enzyme. Pharmaceuticals. 2020;13(9):252.
El Raey MA, El-Hagrassi AM, Osman AF, Darwish KM, Emam M. Acalypha wilkesiana flowers: phenolic profiling, the cytotoxic activity of their biosynthesized silver nanoparticles and molecular docking study for its constituents as Topoisomerase-I inhibitors. Biocatal Agric Biotechnol. 2019;20:101243.
Kitchen DB, Decornez H, Furr JR, Bajorath J. Docking and scoring in virtual screening for drug discovery: methods and applications. Nat Rev Drug Discov. 2004;3(11):935–49.
Wojciechowski M, Lesyng B. Generalized born model: analysis, refinement, and applications to proteins. J Phys Chem B. 2004;108(47):18368–76.
Labute P. The generalized Born/volume integral implicit solvent model: estimation of the free energy of hydration using London dispersion instead of atomic surface area. J Comput Chem. 2008;29(10):1693–8.
O’Boyle NM, Banck M, James CA, Morley C, Vandermeersch T, Hutchison GR. Open babel: an open chemical toolbox. J Cheminform. 2011;1:1–14.
Eberhardt J, Santos-Martins D, Tillack AF, Forli S. AutoDock Vina 1.2.0: new docking methods, expanded force field, and python bindings. J Chem Inf Model. 2021;61(8):3891–8.
Xue Q, Liu X, Russell P, Li J, Pan W, Fu J, et al. Evaluation of the binding performance of flavonoids to estrogen receptor alpha by Autodock, Autodock Vina, and surflex-dock. Ecotoxicol Environ Saf. 2022;233:113323.
Elhady SS, Abdelhameed RFA, Malatani RT, Alahdal AM, Bogari HA, Almalki AJ, et al. Molecular docking and dynamics simulation study of hyrtios erectus isolated scalarane sesterterpenes as potential SARS-CoV-2 dual-target inhibitors. Biology (Basel). 2021;10(5):389.
Markidis S, Laure E. Solving software challenges for exascale: international conference on exascale applications and software, EASC 2014 Stockholm, Sweden, April 2–3, 2014, revised selected papers. Springer; 2015.
Salem MA, Mohamed OG, Mosalam EM, Elberri AI, Abdel-Bar HM, Hassan M, et al. Investigation of the phytochemical composition, antioxidant, antibacterial, anti-osteoarthritis, and wound healing activities of selected vegetable waste. Sci Rep. 2023;13(1):13034.
Humphries RM, Ambler J, Mitchell SL, Castanheira M, Dingle T, Hindler JA, et al. CLSI methods development and standardization working group best practices for evaluation of antimicrobial susceptibility tests. J Clin Microbiol. 2018;56(4):e01934–17.
Ali NB, El-Shiekh RA, Ashour RM, El-Gayed SH, Abdel-Sattar E, Hassan M. In-vitro and in-vivo antibiofilm activity of red onion scales: an agro-food waste. Molecules. 2023;28(1):355.
Salem MA, El-Shiekh RA, Hashem RA, Hassan M. In vivo antibacterial activity of star anise (Illicium verum hook.) Extract using murine MRSA skin infection model in relation to its metabolite profile. Infect Drug Resist. 2021;14:33–48.
El-Naggar MM, El-Nabarawi MA, Teaima MH, Hassan M, Hamed MIA, Elrashedy AA, et al. Integration of terpesomes loaded Levocetrizine dihydrochloride gel as a repurposed cure for Methicillin-Resistant Staphylococcus aureus (MRSA)-induced skin infection; D-optimal optimization, ex-vivo, in-silico, and in-vivo studies. Int J Pharm. 2023;633:122621.
El-Shiekh RA, Hassan M, Hashem RA, Abdel-Sattar E. Bioguided isolation of antibiofilm and antibacterial pregnane glycosides from caralluma quadrangula: disarming multidrug-resistant pathogens. Antibiotics. 2021;10(7):811.
Schier L, Lima D, Dennison M, Araujo M, Pércio S, Migliorine DW, et al. Adsorption modeling of Cr, Cd, and Cu on activated carbon of different origins by using fractional factorial design. Chem Eng J. 2011;166(3):881–9.
Xu P, Tan G, Zhou J, He J, Lawson LB, Mcpherson GL, et al. Undulating tubular liposomes through the incorporation of a synthetic skin ceramide into phospholipid bilayers. Langmuir. 2009;25(18):10422–5.
Nemr AA, El-mahrouk GM, Badie HA. Development and evaluation of surfactant-based elastic vesicular system for transdermal delivery of cilostazole: ex-vivo permeation and histopathological evaluation studies. J Liposome Res. 2021;32(2):159–71.
Abdelbary AA, Abd-elsalam WH, Al-mahallawi AM. Fabrication of novel ultradeformable bilosomes for enhanced ocular delivery of terconazole: in vitro characterization, ex vivo permeation and in vivo safety assessment. Int J Pharm. 2016; 513(1–2): 688– 96.
Anzengruber M, Nepustil LM, Kurtaj F, Tahir A, Skoll K, Sami H, Wirth M, Gabor F. A versatile brij-linker for one-step preparation of targeted nanoparticles. Pharmaceutics. 2023;15(5):1403.
Kumar M, Tiwari A, Mohammed S, Asdaq B, Nair AB, Bhatt S, et al. Itraconazole loaded nanostructured lipid carrier for topical ocular delivery: optimization and evaluation. Saudi J Biol Sci. 2022;29(1):1–10.
Abdellatif MM, Ahmed SM, El-nabarawi MA, Teaima M. Oral bioavailability enhancement of Vancomycin hydrochloride with cationic nanocarrier (Leciplex): optimization, in vitro, ex vivo, and in vivo studies. Sci Pharm. 2023;91:1–18.
Albash R, Abdelbary AA, Refai H, El-Nabarawi MA. Use of transethosomes for enhancing the transdermal delivery of olmesartan medoxomil: in vitro, ex vivo, and in vivo evaluation. Int J Nanomed. 2019;14:1953–68.
Yang Xyan, Li Y xia, Li M, Zhang L, Feng L, xia, Zhang N. Hyaluronic acid-coated nanostructured lipid carriers for targeting paclitaxel to cancer. Cancer Lett. 2013; 334(2): 338– 45.
Chong JY, Mulet X, Waddington LJ, et al. High-throughput discovery of novel steric stabilizers for cubic lyotropic liquid crystal nanoparticle dispersions. Langmuir. 2012;28(25):9223–32.
Fahmy AM, Hassan M, El- DA, Tayel SA, Al-mahallawi AM, Muhammad A, et al. Statistical optimization of hyaluronic acid enriched ultradeformable elastosomes for ocular delivery of voriconazole via Box-Behnken design: in vitro characterization and in vivo evaluation. Drug Deliv. 2021;28(1):77–86.
Gouda A, Sakr OS, Nasr M, Sammour O. Ethanol injection technique for liposomes formulation: an insight into the development, influencing factors, challenges, and applications. J Drug Deliv Sci Technol. 2020;1:102174.
Fang J, Leu Y, Chang C, Lin C. Lipid nano / submicron emulsions as vehicles for topical flurbiprofen delivery. Drug Deliv. 2004;11(2):97–105.
Asasutjarit R, Lorenzen S, Sirivichayakul S, Ruxrungtham K, Ruktanonchai U, Ritthidej GC. Effect of solid lipid nanoparticles formulation compositions on their size, zeta potential, and potential for in vitro pHIS-HIV-hugag transfection. Pharm Res. 2007;24(6):1098–107.
Partenhauser A, Lam HT, Rohrer J, Bernkop-schn A. Development, and in vitro characterization of an oral self-emulsifying delivery system for daptomycin. Eur J Pharm Sci. 2016;81:129–36.
Abdel-hafez SM, Hathout RM, Sammour OA. Tracking the transdermal penetration pathways of optimized curcumin-loaded chitosan nanoparticles via confocal laser scanning microscopy. Int J Biol Macromol. 2018;108:753–64.
Hasan N, Cao J, Lee J, Hlaing SP, Oshi MA, Naeem M, et al. Bacteria-targeted clindamycin loaded polymeric nanoparticles: effect of surface charge on nanoparticle adhesion to MRSA, antibacterial activity, and wound healing. Pharmaceutics. 2019;11(5):236.
Li Y, Lin J, Yang X, Li Y, Wu S, Huang Y, et al. Self-assembled nanoparticles based on amphiphilic anticancer drug– phospholipid complex for targeted drug delivery and intracellular dual-controlled release. ACS Appl Mater Interfaces. 2015;7(32):17573–81.
Roy S, Ho JC, Teo DL, Gupta S, Nallani M. Biomimetic stratum corneum liposome models: lamellar organization and permeability studies. Membranes. 2023;20(2):135.
Sagitova EA, Donfack P, Prokhorov KA, Nikolaeva GY, Gerasin VA, Merekalova ND, Materny A, Antipov EM, Pashinin PP. Raman spectroscopic characterization of the interlayer structure of Na+-montmorillonite clay modified by ditetradecyl dimethyl ammonium bromide. J Phys Chem B. 2009;113(21):7482–90.
Ahsan MN, Verma PR, Singh SK, Samridhi, Yashpal M. Formulation of rosuvastatin-loaded self-nanoemulsifying drug delivery system using Box-Behnken design. Part Sci Technol. 2014;32(1):46–60.
Elsayed I, Sayed S. Tailored nanostructured platforms for boosting transcorneal permeation: box– behnken statistical optimization, comprehensive in vitro, ex vivo and in vivo characterization. Int J Nanomed. 2023;12:7949–62.
Mokhtary P, Javan B, Sharbatkhari M, Soltani A, Erfani-Moghadam V. Cationic vesicles for efficient shRNA transfection in the MCF-7 breast cancer cell line. Int J Nanomed. 2018;13:7107–21.
Gupta M, Agrawal U, Vyas SP. Nanocarrier-based topical drug delivery for the treatment of skin diseases. Expert Opin Drug Deliv. 2012;9(7):783–804.
Hirose K, Marui A, Arai Y, Nomura T, Inoue S, Kaneda K, et al. Sustained-release Vancomycin sheet may help to prevent prosthetic graft methicillin-resistant Staphylococcus aureus infection. J Vasc Surg. 2006;44(2):377–82.
Noh GY, Suh JY, Park SN. Ceramide-based nanostructured lipid carriers for transdermal delivery of isoliquiritigenin: development, physicochemical characterization, and in vitro skin permeation studies. Korean J Chem Eng. 2017;34:400–6.
Yang X, Tang Y, Wang M, Wang Y, Wang W, Pang M, et al. Co-delivery of methotrexate and nicotinamide by cerosomes for topical psoriasis treatment with enhanced efficacy. Int J Pharm. 2021;605:120826.
Monteiro JM, Covas G, Rausch D, Filipe SR, Schneider T, Sahl HG, et al. The pentaglycine bridges of Staphylococcus aureus peptidoglycan are essential for cell integrity. Sci Rep. 2019;9(1):5010.
Ruane KM, Lloyd AJ, Fülöp V, Dowson CG, Barreteau H, Boniface A, et al. Specificity determinants for lysine incorporation in Staphylococcus aureus peptidoglycan as revealed by the structure of a MurE enzyme ternary complex. J Biol Chem. 2013;288(46):33439–48.
Hao H, Cheng G, Dai M, Wu Q, Yuan Z. Inhibitors targeting cell wall biosynthesis pathway of MRSA. Mol Biosyst. 2012;8(11):2828–38.
Bouhss A, Trunkfield AE, Bugg TDH, Mengin-Lecreulx D. The biosynthesis of peptidoglycan lipid-linked intermediates. FEMS Microbiol Rev. 2008;32(2):208–33.
Nikolaidis I, Favini-Stabile S, Dessen A. Resistance to antibiotics targeted to the bacterial cell wall. Protein Sci. 2014;23(3):243–59.
Stelitano G, Sammartino JC, Chiarelli LR. Multitargeting compounds: a promising strategy to overcome multi-drug resistant tuberculosis. Molecules. 2020;25(5):1239.
Igler C, Rolff J, Regoes R. Multi-step vs. single-step resistance evolution under different drugs, pharmacokinetics, and treatment regimens. Elife. 2021;10:e64116.
Billones JB, Bangalan MA. Structure-based discovery of inhibitors against mure in methicillin-resistant staphylococcus aureus. Orien J Chem. 2019;35(2):618–25.
Zouhir A, Jemli S, Omrani R, kthiri A, Jridi T, sebei K. In silico molecular analysis and docking of potent antimicrobial peptides against MurE enzyme of Methicillin-Resistant Staphylococcus Aureus. Int J Pept Res Ther. 2021;27:1253–63.
Oselusi SO, Fadaka AO, Wyckoff GJ, Egieyeh SA. Computational target-based screening of anti-MRSA natural products reveals potential multitarget mechanisms of action through peptidoglycan synthesis proteins. ACS Omega. 2022;7(42):37896–906.
Kontoyianni M, McClellan LM, Sokol GS. Evaluation of docking performance: comparative data on docking algorithms. J Med Chem. 2004;47(3):558–65.
Tomašić T, Šink R, Zidar N, Fic A, Contreras-Martel C, Dessen A, et al. Dual inhibitor of MurD and MurE ligases from Escherichia coli and Staphylococcus aureus. ACS Med Chem Lett. 2012;3(8):626–30.
Gordon E, Flouret B, Chantalat L, Van Heijenoort J, Mengin-Lecreulx D, Dideberg O. Crystal structure of UDP-N-acetylmuramoyl-L-alanyl-D-glutamate: meso-diaminopimelate ligase from Escherichia Coli. J Biol Chem. 2001;276(14):10999–1006.
Abd-Elsalam WH, El-Helaly SN, Ahmed MA, Al-mahallawi AM. Preparation of novel phospholipid-based sonocomplexes for improved intestinal permeability of rosuvastatin: in vitro characterization, dynamic simulation, Caco-2 cell line permeation, and in vivo assessment studies. Int J Pharm. 2018;548(1):375–84.
Farag MM, Abd El Malak NS, Yehia SA, Ahmed MA. Hyaluronic acid conjugated metformin-phospholipid sonocomplex: a biphasic complexation approach to correct hypoxic tumor microenvironment. Int J Nanomed. 2021;16:1005–19.
Albash R, Badawi NM, Hamed MIA, Ragaie MH, Mohammed SS, Elbesh RM, et al. Exploring the synergistic effect of bergamot essential oil with spironolactone loaded nano-phytosomes for treatment of acne vulgaris: in vitro optimization, in silico studies, and clinical evaluation. Pharmaceuticals. 2023;16(1):128.
Darden T, York D, Pedersen L. Particle mesh Ewald: an N·log(N) method for Ewald sums in large systems. J Chem Phys. 1993;98(12):10089–92.
Hess B, Bekker H, Berendsen HJC, Fraaije JGEM. LINCS: a linear constraint solver for molecular simulations. J Comput Chem. 1997;18:1463–72.
Alotaibi, H. F., Alotaibi, H., Darwish, K. M., Khafagy, E. S., Abu Lila, A. S., Ali, M. A.,... & Alshawwa, S. Z. The anti-virulence activities of the antihypertensive drug propranolol in light of its anti-quorum sensing effects against pseudomonas aeruginosa and serratia marcescens." Biomedicines. 2023;11:(12) 3161.
Abruzzo, A., Bigucci, F., Cerchiara, T., Cruciani, F., Vitali, B. and Luppi, B., Mucoadhesive chitosan/gelatin films for buccal delivery of propranolol hydrochloride. Carb Polym. 2012;87(1):581–588.
He S, Wen H, Yao N, Wang L, Huang J, Li Z. A sustained-release nanosystem with MRSA biofilm-dispersing and-eradicating abilities accelerates diabetic ulcer healing. Int J Nanomed. 2023;18:3951–72.
Acknowledgements
Not applicable.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This research received no funding.
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Conceptualization, M.E., M.E., M.H., and M.A.; Formal analysis, M.E., M.E., M.T., M.H., K.D., M.H., and M.A.; Investigation, M.E., M.E., M.T., M.H., K.D., M.H., and M.A.; Resources, M.E., M.H., M.H., R.H., and M.A.; Writing—original draft preparation, M.E.; Writing—review and editing, M.E., R.H., M.E., M.T., M.H., K.D., M.H., and M.A.; Supervision, M.A., M.T., and M.E. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All the ex-vivo and in-vivo tests were approved by REC-FOPCU (approval no. MI 3394) which followed the Guide for Care and Use of Laboratory Animals declared by the US National Institute of Health (NIH Publication No. 85–23, revised 2011).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Eltabeeb, M.A., Hamed, R.R., El-Nabarawi, M.A. et al. Nanocomposite alginate hydrogel loaded with propranolol hydrochloride kolliphor® based cerosomes as a repurposed platform for Methicillin-Resistant Staphylococcus aureus-(MRSA)-induced skin infection; in-vitro, ex-vivo, in-silico, and in-vivo evaluation. Drug Deliv. and Transl. Res. (2024). https://doi.org/10.1007/s13346-024-01611-z
Accepted:
Published:
DOI: https://doi.org/10.1007/s13346-024-01611-z