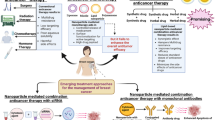
Abstract
Breast cancer (BC) is the most frequently diagnosed malignancy in women worldwide. Almost 70–80% of cases of BC are curable at the early non-metastatic stage. BC is a heterogeneous disease with different molecular subtypes. Around 70% of breast tumors exhibit estrogen-receptor (ER) expression and endocrine therapy is used for the treatment of these patients. However, there are high chances of recurrence in the endocrine therapy regimen. Though chemotherapy and radiation therapy have substantially improved survival rates and treatment outcomes in BC patients, there is an increased possibility of the development of resistance and dose-limiting toxicities. Conventional treatment approaches often suffer from low bioavailability, adverse effects due to the non-specific action of chemotherapeutics, and low antitumor efficacy. Nanomedicine has emerged as a conspicuous strategy for delivering anticancer therapeutics in BC management. It has revolutionized the area of cancer therapy by increasing the bioavailability of the therapeutics and improving their anticancer efficacy with reduced toxicities on healthy tissues. In this article, we have highlighted various mechanisms and pathways involved in the progression of ER-positive BC. Further, different nanocarriers delivering drugs, genes, and natural therapeutic agents for surmounting BC are the spotlights of this article.
Graphical Abstract











Similar content being viewed by others
Data availability
Not applicable.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022.
Tong CW, Wu M, Cho W, To KK. Recent advances in the treatment of breast cancer. Front Oncol. 2018;8:227.
Siegle R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29.
Ma Q, Gao Y, Xu P, Li K, Xu X, Gao J, et al. Atorvastatin inhibits breast cancer cells by downregulating PTEN/AKT pathway via promoting ras homolog family member B (RhoB). Biomed Res Int. 2019;2019.
Millikan RC, Newman B, Tse C-K, Moorman PG, Conway K, Smith LV, et al. Epidemiology of basal-like breast cancer. Breast Cancer Res Treat. 2008;109(1):123–39.
Malhotra GK, Zhao X, Band H, Band V. Histological, molecular and functional subtypes of breast cancers. Cancer Biol Ther. 2010;10(10):955–60.
Lumachi F, Brunello A, Maruzzo M, Basso U, Mm BS. Treatment of estrogen receptor-positive breast cancer. Curr Med Chem. 2013;20(5):596–604.
Gil EMC. Targeting the PI3K/AKT/mTOR pathway in estrogen receptor-positive breast cancer. Cancer Treat Rev. 2014;40(7):862–71.
Andrahennadi S, Sami A, Manna M, Pauls M, Ahmed S. Current landscape of targeted therapy in hormone receptor-positive and HER2-negative breast cancer. Curr Oncol. 2021;28(3):1803–22.
Cardoso F, Senkus E, Costa A, Papadopoulos E, Aapro M, André F, et al. 4th ESO–ESMO international consensus guidelines for advanced breast cancer (ABC 4). Ann Oncol. 2018;29(8):1634–57.
Khairnar P, Handa M, Shukla R. Nanocrystals: an approachable delivery system for anticancer therapeutics. Curr Drug Metab. 2022.
Scott AM, Wolchok JD, Old LJ. Antibody therapy of cancer. Nat Rev Cancer. 2012;12(4):278–87.
Allen TM, Cullis PR. Drug delivery systems: entering the mainstream. Science. 2004;303(5665):1818–22.
Singh PK, Chibh S, Dube T, Chauhan VS, Panda JJ. Arginine-α, β-dehydrophenylalanine dipeptide nanoparticles for pH-responsive drug delivery. Pharm Res. 2018;35(2):1–11.
Patil RM, Deshpande PP, Aalhate M, Gananadhamu S, Singh PK. An update on sophisticated and advanced analytical tools for surface characterization of nanoparticles. Surf Interface. 2022:102165.
Singh A, Handa M, Ruwali M, Flora S, Shukla R, Kesharwani P. Nanocarrier mediated autophagy: an emerging trend for cancer therapy. Process Biochem. 2021;109:198–206.
Siersbæk R, Kumar S, Carroll JS. Signaling pathways and steroid receptors modulating estrogen receptor α function in breast cancer. Genes Dev. 2018;32(17–18):1141–54.
Yaşar P, Ayaz G, User SD, Güpür G, Muyan M. Molecular mechanism of estrogen–estrogen receptor signaling. Reprod Med Biol. 2017;16(1):4–20.
Osborne CK, Schiff R, Fuqua SA, Shou J. Estrogen receptor: current understanding of its activation and modulation. Clin Cancer Res. 2001;7(12):4338s-s4342.
Kundu S, Ali MA, Handin N, Padhan N, Larsson J, Karoutsou M, et al. Linking FOXO3, NCOA3, and TCF7L2 to Ras pathway phenotypes through a genome-wide forward genetic screen in human colorectal cancer cells. Genome Med. 2018;10(1):1–13.
Cleator SJ, Ahamed E, Coombes RC, Palmieri C. A 2009 Update on the treatment of patients with hormone receptor—positive breast cancer. Clin Breast Cancer. 2009;9:S6–17.
Sorlie T, Tibshirani R, Parker J, Hastie T, Marron J, Nobel A, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci USA. 2003;100(14):8418–23.
Cardoso F, Costa A, Senkus E, Aapro M, André F, Barrios C, et al. 3rd ESO–ESMO international consensus guidelines for advanced breast cancer (ABC 3). Breast. 2017;31:244–59.
Ring A, Dowsett M. Mechanisms of tamoxifen resistance. Endocr Relat Cancer. 2004;11(4):643–58.
Nelson LR, Bulun SE. Estrogen production and action. J Am Acad Dermatol. 2001;45(3):S116–24.
Evans CT, Ledesma DB, Schulz TZ, Simpson ER, Mendelson CR. Isolation and characterization of a complementary DNA specific for human aromatase-system cytochrome P-450 mRNA. Proc Natl Acad Sci. 1986;83(17):6387–91.
Altundag K, Ibrahim NK. Aromatase inhibitors in breast cancer: an overview. Oncologist. 2006;11(6):553–62.
Pistelli M, Della Mora A, Ballatore Z, Berardi R. Aromatase inhibitors in premenopausal women with breast cancer: the state of the art and future prospects. Curr Oncol. 2018;25(2):168–75.
Castaneda CA, Cortes-Funes H, Gomez HL, Ciruelos EM. The phosphatidyl inositol 3-kinase/AKT signaling pathway in breast cancer. Cancer Metastasis Rev. 2010;29(4):751–9.
Lee JJ, Loh K, Yap Y-S. PI3K/Akt/mTOR inhibitors in breast cancer. Cancer Biol Med. 2015;12(4):342.
Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296(5573):1655–7.
Baselga J. Targeting the phosphoinositide-3 (PI3) kinase pathway in breast cancer. Oncologist. 2011;16:12–9.
Zhao L, Vogt PK. Class I PI3K in oncogenic cellular transformation. Oncogene. 2008;27(41):5486–96.
Kenerson HL, Aicher LD, True LD, Yeung RS. Activated mammalian target of rapamycin pathway in the pathogenesis of tuberous sclerosis complex renal tumors. Can Res. 2002;62(20):5645–50.
Dowling RJ, Topisirovic I, Fonseca BD, Sonenberg N. Dissecting the role of mTOR: lessons from mTOR inhibitors. Biochim Biophys Acta (BBA)-Proteins Proteom. 2010;1804(3):433–9.
Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12(1):21–35.
Yamnik RL, Digilova A, Davis DC, Brodt ZN, Murphy CJ, Holz MK. S6 kinase 1 regulates estrogen receptor α in control of breast cancer cell proliferation. J Biol Chem. 2009;284(10):6361–9.
Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307(5712):1098–101.
Miller TW, Balko JM, Arteaga CL. Phosphatidylinositol 3-kinase and antiestrogen resistance in breast cancer. J Clin Oncol. 2011;29(33):4452.
O’leary B, Finn RS, Turner NC. Treating cancer with selective CDK4/6 inhibitors. Nat Rev Clin Oncol. 2016;13(7):417–30.
Matutino A, Amaro C, Verma S. CDK4/6 inhibitors in breast cancer: beyond hormone receptor-positive HER2-negative disease. Ther Adv in Med Oncol. 2018;10:1758835918818346.
Aggelis V, Johnston SR. Advances in endocrine-based therapies for estrogen receptor-positive metastatic breast cancer. Drugs. 2019;79(17):1849–66.
Rugo H, Finn R, Diéras V, Ettl J, Lipatov O, Joy A, et al. Palbociclib plus letrozole as first-line therapy in estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer with extended follow-up. Breast Cancer Res Treat. 2019;174(3):719–29.
Talluri SV, Kuppusamy G, Karri VVSR, Tummala S, Madhunapantula SV. Lipid-based nanocarriers for breast cancer treatment–comprehensive review. Drug Deliv. 2016;23(4):1291–305.
Mahajan S, Aalhate M, Guru SK, Singh PK. Nanomedicine as a magic bullet for combating lymphoma. J Control Release. 2022;347:211–36.
Cleator S, Parton M, Dowsett M. The biology of neoadjuvant chemotherapy for breast cancer. Endocr Relat Cancer. 2002;9(3):183–95.
Charfare H, Limongelli S, Purushotham A. Neoadjuvant chemotherapy in breast cancer. Br J Surg. 2005;92(1):14–23.
Koch U, Krause M, Baumann M. Cancer stem cells at the crossroads of current cancer therapy failures—radiation oncology perspective. Seminars in cancer biology: Elsevier; 2010. p. 116–24.
Mayo CS, Urie MM, Fitzgerald TJ. Hybrid IMRT plans—concurrently treating conventional and IMRT beams for improved breast irradiation and reduced planning time. Int J Rad Oncol Biol Phys. 2005;61(3):922–32.
Polisena C. Nutrition concerns with the radiation therapy patient. The Clinical Guide to Oncology Nutrition. 2000:70–8.
Aebi S, Davidson T, Gruber G, Cardoso F. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2011;22:vi12–24.
Aapro MS. Adjuvant therapy of primary breast cancer: a review of key findings from the 7th international conference, St. Gallen, February 2001. Oncologist. 2001;6(4):376–85.
Lumachi F, Luisetto G, MM Basso S, Basso U, Brunello A, Camozzi V. Endocrine therapy of breast cancer. Curr Med Chem. 2011;18(4):513–22.
El Sayed R, El Jamal L, El Iskandarani S, Kort J, Abdel Salam M, Assi H. Endocrine and targeted therapy for hormone-receptor-positive, HER2-negative advanced breast cancer: Insights to sequencing treatment and overcoming resistance based on clinical trials. Front Oncol. 2019;9:510.
Gu G, Dustin D, Fuqua SA. Targeted therapy for breast cancer and molecular mechanisms of resistance to treatment. Curr Opin Pharmacol. 2016;31:97–103.
Afzal M, Alharbi KS, Alruwaili NK, Al-Abassi FA, Al-Malki AAL, Kazmi I, et al. Nanomedicine in treatment of breast cancer–a challenge to conventional therapy. Seminars in cancer biology: Elsevier; 2021. p. 279–92.
Jiang Y, Jiang Z, Wang M, Ma L. Current understandings and clinical translation of nanomedicines for breast cancer therapy. Adv Drug Deliv Rev. 2022;180:114034.
Maji I, Mahajan S, Sriram A, Medtiya P, Vasave R, Khatri DK, et al. Solid self emulsifying drug delivery system: superior mode for oral delivery of hydrophobic cargos. J Control Release. 2021;337:646–60.
Alasvand N, Urbanska AM, Rahmati M, Saeidifar M, Gungor-Ozkerim PS, Sefat F, et al. Therapeutic nanoparticles for targeted delivery of anticancer drugs. Multifunctional systems for combined delivery, biosensing and diagnostics. 2017:245–59.
Maeda H, Nakamura H, Fang J. The EPR effect for macromolecular drug delivery to solid tumors: Improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo. Adv Drug Deliv Rev. 2013;65(1):71–9.
Perrault SD, Chan WC. In vivo assembly of nanoparticle components to improve targeted cancer imaging. Proc Natl Acad Sci. 2010;107(25):11194–9.
Ernsting MJ, Murakami M, Roy A, Li S-D. Factors controlling the pharmacokinetics, biodistribution and intratumoral penetration of nanoparticles. J Control Release. 2013;172(3):782–94.
Stylianopoulos T. EPR-effect: utilizing size-dependent nanoparticle delivery to solid tumors. Ther Deliv. 2013;4(4):421–3.
Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46(12 Part 1):6387–92.
Byrne JD, Betancourt T, Brannon-Peppas L. Active targeting schemes for nanoparticle systems in cancer therapeutics. Adv Drug Deliv Rev. 2008;60(15):1615–26.
Kumar S, Li C. Targeting of vasculature in cancer and other angiogenic diseases. Trends Immunol. 2001;22(3):129.
Ellis LM, Hicklin DJ. VEGF-targeted therapy: mechanisms of anti-tumour activity. Nat Rev Cancer. 2008;8(8):579–91.
Kremer C, Breier G, Risau W, Plate KH. Up-regulation of flk-1/vascular endothelial growth factor receptor 2 by its ligand in a cerebral slice culture system. Can Res. 1997;57(17):3852–9.
Plate KH, Breier G, Weich HA, Risau W. Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo. Nature. 1992;359(6398):845–8.
Backer MV, Gaynutdinov TI, Patel V, Bandyopadhyaya AK, Thirumamagal B, Tjarks W, et al. Vascular endothelial growth factor selectively targets boronated dendrimers to tumor vasculature. Mol Cancer Ther. 2005;4(9):1423–9.
Chen J, Wu H, Han D, Xie C. Using anti-VEGF McAb and magnetic nanoparticles as double-targeting vector for the radioimmunotherapy of liver cancer. Cancer Lett. 2006;231(2):169–75.
Shi S, Yang K, Hong H, Chen F, Valdovinos HF, Goel S, et al. VEGFR targeting leads to significantly enhanced tumor uptake of nanographene oxide in vivo. Biomaterials. 2015;39:39–46.
Goel S, Chen F, Hong H, Valdovinos HF, Hernandez R, Shi S, et al. VEGF121-conjugated mesoporous silica nanoparticle: a tumor targeted drug delivery system. ACS Appl Mater Interfaces. 2014;6(23):21677–85.
Wu Y, Hooper AT, Zhong Z, Witte L, Bohlen P, Rafii S, et al. The vascular endothelial growth factor receptor (VEGFR-1) supports growth and survival of human breast carcinoma. Int J Cancer. 2006;119(7):1519–29.
Smith NR, Baker D, James NH, Ratcliffe K, Jenkins M, Ashton SE, et al. Vascular endothelial growth factor receptors VEGFR-2 and VEGFR-3 are localized primarily to the vasculature in human primary solid cancers. Clin Cancer Res. 2010;16(14):3548–61.
Jain S, Deore SV, Ghadi R, Chaudhari D, Kuche K, Katiyar SS. Tumor microenvironment responsive VEGF-antibody functionalized pH sensitive liposomes of docetaxel for augmented breast cancer therapy. Mater Sci Eng C. 2021;121:111832.
Danhier F, Le Breton A, Préat V. RGD-based strategies to target alpha (v) beta (3) integrin in cancer therapy and diagnosis. Mol Pharm. 2012;9(11):2961–73.
Li L, Wartchow CA, Danthi SN, Shen Z, Dechene N, Pease J, et al. A novel antiangiogenesis therapy using an integrin antagonist or anti–Flk-1 antibody coated 90Y-labeled nanoparticles. Int J Radiat Oncol Biol Phys. 2004;58(4):1215–27.
Felding-Habermann B, O’Toole TE, Smith JW, Fransvea E, Ruggeri ZM, Ginsberg MH, et al. Integrin activation controls metastasis in human breast cancer. Proc Natl Acad Sci. 2001;98(4):1853–8.
Dai W, Yang F, Ma L, Fan Y, He B, He Q, et al. Combined mTOR inhibitor rapamycin and doxorubicin-loaded cyclic octapeptide modified liposomes for targeting integrin α3 in triple-negative breast cancer. Biomaterials. 2014;35(20):5347–58.
Sorolla A, Sorolla MA, Wang E, Cena V. Peptides, proteins and nanotechnology: a promising synergy for breast cancer targeting and treatment. Expert Opin Drug Deliv. 2020;17(11):1597–613.
Schlesinger M, Bendas G. Vascular cell adhesion molecule-1 (VCAM-1)—an increasing insight into its role in tumorigenicity and metastasis. Int J Cancer. 2015;136(11):2504–14.
Cao H, Zhang Z, Zhao S, He X, Yu H, Yin Q, et al. Hydrophobic interaction mediating self-assembled nanoparticles of succinobucol suppress lung metastasis of breast cancer by inhibition of VCAM-1 expression. J Control Release. 2015;205:162–71.
Scallon BJ, Snyder LA, Anderson GM, Chen Q, Yan L, Weiner LM, et al. A review of antibody therapeutics and antibody-related technologies for oncology. J Immunother. 2006;29(4):351–64.
Skliris GP, Leygue E, Watson PH, Murphy LC. Estrogen receptor alpha negative breast cancer patients: estrogen receptor beta as a therapeutic target. J Steroid Biochem Mol Biol. 2008;109(1–2):1–10.
Shanle EK, Xu W. Selectively targeting estrogen receptors for cancer treatment. Adv Drug Deliv Rev. 2010;62(13):1265–76.
Wang Y, Wang Z, Qian Y, Fan L, Yue C, Jia F, et al. Synergetic estrogen receptor-targeting liposome nanocarriers with anti-phagocytic properties for enhanced tumor theranostics. J Mater Chem B. 2019;7(7):1056–63.
Pun SH, Tack F, Bellocq NC, Cheng J, Grubbs BH, Jensen GS, et al. Targeted delivery of RNA-cleaving DNA enzyme (DNAzyme) to tumor tissue by transferrin-modified, cyclodextrin-based particles. Cancer Biol Ther. 2004;3(7):641–50.
Anabousi S, Bakowsky U, Schneider M, Huwer H, Lehr C-M, Ehrhardt C. In vitro assessment of transferrin-conjugated liposomes as drug delivery systems for inhalation therapy of lung cancer. Eur J Pharm Sci. 2006;29(5):367–74.
Vandewalle B, Granier A, Peyrat J, Bonneterre J, Lefebvre J. Transferrin receptors in cultured breast cancer cells. J Cancer Res Clin Oncol. 1985;110(1):71–6.
Singh M, Mugler K, Hailoo DW, Burke S, Nemesure B, Torkko K, et al. Differential expression of transferrin receptor (TfR) in a spectrum of normal to malignant breast tissues: implications for in situ and invasive carcinoma. Appl Immunohistochem Mol Morphol. 2011;19(5):417–23.
Tonik SE, Shindelman JE, Sussman HH. Transferrin receptor is inversely correlated with estrogen receptor in breast cancer. Breast Cancer Res Treat. 1986;7(2):71–6.
Li J-L, Wang L, Liu X-Y, Zhang Z-P, Guo H-C, Liu W-M, et al. In vitro cancer cell imaging and therapy using transferrin-conjugated gold nanoparticles. Cancer Lett. 2009;274(2):319–26.
Zheng Y, Yu B, Weecharangsan W, Piao L, Darby M, Mao Y, et al. Transferrin-conjugated lipid-coated PLGA nanoparticles for targeted delivery of aromatase inhibitor 7α-APTADD to breast cancer cells. Int J Pharm. 2010;390(2):234–41.
Mahani M, Pourrahmani-Sarbanani M, Yoosefian M, Divsar F, Mousavi SM, Nomani A. Doxorubicin delivery to breast cancer cells with transferrin-targeted carbon quantum dots: an in vitro and in silico study. J Drug Deliv Sci Technol. 2021;62:102342.
He GZ, Lin WJ. Peptide-functionalized nanoparticles-encapsulated cyclin-dependent kinases inhibitor seliciclib in transferrin receptor overexpressed cancer cells. Nanomaterials. 2021;11(3):772.
Xu Q, Zhu M, Yang T, Xu F, Liu Y, Chen Y. Quantitative assessment of human serum transferrin receptor in breast cancer patients pre-and post-chemotherapy using peptide immunoaffinity enrichment coupled with targeted proteomics. Clin Chim Acta. 2015;448:118–23.
Inoue S, Golseiz H, Patil R, Ding H, Rudenkyy S, Holler E, et al. Direct tumor targeting using nanobioconjugate with a combination of monoclonal antibodies for breast cancer treatment. AACR; 2009.
Xu L, Huang C-C, Huang W, Tang W-H, Rait A, Yin YZ, et al. Systemic tumor-targeted gene delivery by anti-transferrin receptor scFv-immunoliposomes 1 this work was supported in part by National Cancer Institute Grant R01 CA45158 (to EC), National Cancer Institute Small Business Technology Transfer Phase I Grant R41 CA80449 (to EC), and a grant from SynerGene Therapeutics, Inc. 1. Mol Cancer Ther. 2002;1(5):337–46.
Harford JB, Kim S-S, Pirollo KF, Rait A, Chang EH. SGT-53: a novel nanomedicine capable of augmenting cancer immunotherapy. Immune Aspects of Biopharmaceuticals and Nanomedicines. Routledge; 2018. p. 929–70.
Salomon DS, Brandt R, Ciardiello F, Normanno N. Epidermal growth factor-related peptides and their receptors in human malignancies. Crit Rev Oncol Hematol. 1995;19(3):183–232.
Wang Y, Wang Y, Chen G, Li Y, Xu W, Gong S. Quantum-dot-based theranostic micelles conjugated with an anti-EGFR nanobody for triple-negative breast cancer therapy. ACS Appl Mater Interfaces. 2017;9(36):30297–305.
Low PS, Antony B. Folate receptor-targeted drugs for cancer and inflammatory diseases. Adv Drug Deliv Rev. 2004;56(8):1055–8.
Stella B, Arpicco S, Peracchia MT, Desmaële D, Hoebeke J, Renoir M, et al. Design of folic acid-conjugated nanoparticles for drug targeting. J Pharm Sci. 2000;89(11):1452–64.
Das M, Mohanty C, Sahoo SK. Ligand-based targeted therapy for cancer tissue. Expert Opin Drug Deliv. 2009;6(3):285–304.
Sharma S, Pukale S, Sahel DK, Singh P, Mittal A, Chitkara D. Folate targeted hybrid lipo-polymeric nanoplexes containing docetaxel and miRNA-34a for breast cancer treatment. Mater Sci Eng C. 2021;128:112305.
Andisheh F, Oroojalian F, Shakour N, Ramezani M, Shamsara J, Khodaverdi E, et al. Docetaxel encapsulation in nanoscale assembly micelles of folate-PEG-docetaxel conjugates for targeted fighting against metastatic breast cancer in vitro and in vivo. Int J Pharm. 2021;605:120822.
Al-Othman N, Alhendi A, Ihbaisha M, Barahmeh M, Alqaraleh M, Al-Momany BZ. Role of CD44 in breast cancer. Breast Dis. 2020;39(1):1–13.
Orian-Rousseau V, Chen L, Sleeman JP, Herrlich P, Ponta H. CD44 is required for two consecutive steps in HGF/c-Met signaling. Genes Dev. 2002;16(23):3074–86.
Yang C, He Y, Zhang H, Liu Y, Wang W, Du Y, et al. Selective killing of breast cancer cells expressing activated CD44 using CD44 ligand-coated nanoparticles in vitro and in vivo. Oncotarget. 2015;6(17):15283.
Louderbough JM, Schroeder JA. Understanding the dual nature of CD44 in breast cancer progression. Mol Cancer Res. 2011;9(12):1573–86.
Li J, Li M, Tian L, Qiu Y, Yu Q, Wang X, et al. Facile strategy by hyaluronic acid functional carbon dot-doxorubicin nanoparticles for CD44 targeted drug delivery and enhanced breast cancer therapy. Int J Pharm. 2020;578:119122.
Semkina AS, Abakumov MA, Skorikov AS, Abakumova TO, Melnikov PA, Grinenko NF, et al. Multimodal doxorubicin loaded magnetic nanoparticles for VEGF targeted theranostics of breast cancer. Nanomedicine. 2018;14(5):1733–42.
Wicki A, Rochlitz C, Orleth A, Ritschard R, Albrecht I, Herrmann R, et al. Targeting tumor-associated endothelial cells: anti-VEGFR2 immunoliposomes mediate tumor vessel disruption and inhibit tumor growth. Clin Cancer Res. 2012;18(2):454–64.
Zhong P, Gu X, Cheng R, Deng C, Meng F, Zhong Z. αvβ3 integrin-targeted micellar mertansine prodrug effectively inhibits triple-negative breast cancer in vivo. Int J Nanomed. 2017;12:7913.
Yan H, You Y, Li X, Liu L, Guo F, Zhang Q, et al. Preparation of RGD peptide/folate acid double-targeted mesoporous silica nanoparticles and its application in human breast cancer MCF-7 cells. Front Pharmacol. 2020;11:898.
Yadav AS, Radharani NNV, Gorain M, Bulbule A, Shetti D, Roy G, et al. RGD functionalized chitosan nanoparticle mediated targeted delivery of raloxifene selectively suppresses angiogenesis and tumor growth in breast cancer. Nanoscale. 2020;12(19):10664–84.
Cao H, Dan Z, He X, Zhang Z, Yu H, Yin Q, et al. Liposomes coated with isolated macrophage membrane can target lung metastasis of breast cancer. ACS Nano. 2016;10(8):7738–48.
Nazli C, Demirer GS, Yar Y, Acar HY, Kizilel S. Targeted delivery of doxorubicin into tumor cells via MMP-sensitive PEG hydrogel-coated magnetic iron oxide nanoparticles (MIONPs). Colloids Surf B Biointerfaces. 2014;122:674–83.
Zhou K, Zhu Y, Chen X, Li L, Xu W. Redox-and MMP-2-sensitive drug delivery nanoparticles based on gelatin and albumin for tumor targeted delivery of paclitaxel. Mater Sci Eng C. 2020;114:111006.
Pan J, Li P-J, Wang Y, Chang L, Wan D, Wang H. Active targeted drug delivery of MMP-2 sensitive polymeric nanoparticles. Chem Commun. 2018;54(79):11092–5.
Cui Y-N, Xu Q-X, Davoodi P, Wang D-P, Wang C-H. Enhanced intracellular delivery and controlled drug release of magnetic PLGA nanoparticles modified with transferrin. Acta Pharmacol Sin. 2017;38(6):943–53.
Sheng Y, Xu J, You Y, Xu F, Chen Y. Acid-sensitive peptide-conjugated doxorubicin mediates the lysosomal pathway of apoptosis and reverses drug resistance in breast cancer. Mol Pharm. 2015;12(7):2217–28.
Vinothini K, Rajendran NK, Ramu A, Elumalai N, Rajan M. Folate receptor targeted delivery of paclitaxel to breast cancer cells via folic acid conjugated graphene oxide grafted methyl acrylate nanocarrier. Biomed Pharmacother. 2019;110:906–17.
Shao W, Paul A, Zhao B, Lee C, Rodes L, Prakash S. Carbon nanotube lipid drug approach for targeted delivery of a chemotherapy drug in a human breast cancer xenograft animal model. Biomaterials. 2013;34(38):10109–19.
Yassemi A, Kashanian S, Zhaleh H. Folic acid receptor-targeted solid lipid nanoparticles to enhance cytotoxicity of letrozole through induction of caspase-3 dependent-apoptosis for breast cancer treatment. Pharm Dev Technol. 2020;25(4):397–407.
Shi J, Wang B, Wang L, Lu T, Fu Y, Zhang H, et al. Fullerene (C60)-based tumor-targeting nanoparticles with “off-on” state for enhanced treatment of cancer. J Control Release. 2016;235:245–58.
Wang W, Zhang X, Li Z, Pan D, Zhu H, Gu Z, et al. Dendronized hyaluronic acid-docetaxel conjugate as a stimuli-responsive nano-agent for breast cancer therapy. Carbohyd Polym. 2021;267:118160.
Ravar F, Saadat E, Gholami M, Dehghankelishadi P, Mahdavi M, Azami S, et al. Hyaluronic acid-coated liposomes for targeted delivery of paclitaxel, in-vitro characterization and in-vivo evaluation. J Control Release. 2016;229:10–22.
Torchilin V. Liposomes as targetable drug carriers. Crit Rev Ther Drug Carrier Syst. 1985;2(1):65–115.
Shah S, Dhawan V, Holm R, Nagarsenker MS, Perrie Y. Liposomes: advancements and innovation in the manufacturing process. Adv Drug Deliv Rev. 2020.
Henderson IC, Canellos GP. Cancer of the breast: the past decade. N Engl J Med. 1980;302(1):17–30.
Paliwal SR, Paliwal R, Mishra N, Mehta A, Vyas S. A novel cancer targeting approach based on estrone anchored stealth liposome for site-specific breast cancer therapy. Curr Cancer Drug Targets. 2010;10(3):343–53.
Yu J, Lee H, Kim J, Kong W, Kim Y, Cui Z, et al. Bio-distribution and anti-tumor efficacy of PEG/PLA nano particles loaded doxorubicin. J Drug Target. 2007;15(4):279–84.
Park JW. Liposome-based drug delivery in breast cancer treatment. Breast Cancer Res. 2002;4(3):1–5.
Tang H, Chen J, Wang L, Li Q, Yang Y, Lv Z, et al. Co-delivery of epirubicin and paclitaxel using an estrone-targeted PEGylated liposomal nanoparticle for breast cancer. Int J Pharm. 2020;573:118806.
Dorjsuren B, Chaurasiya B, Ye Z, Liu Y, Li W, Wang C, et al. Cetuximab-coated thermo-sensitive liposomes loaded with magnetic nanoparticles and doxorubicin for targeted EGFR-expressing breast cancer combined therapy. Int J Nanomed. 2020;15:8201.
Jain AS, Goel PN, Shah SM, Dhawan VV, Nikam Y, Gude RP, et al. Tamoxifen guided liposomes for targeting encapsulated anticancer agent to estrogen receptor positive breast cancer cells: in vitro and in vivo evaluation. Biomed Pharmacother. 2014;68(4):429–38.
Cosco D, Paolino D, Cilurzo F, Casale F, Fresta M. Gemcitabine and tamoxifen-loaded liposomes as multidrug carriers for the treatment of breast cancer diseases. Int J Pharm. 2012;422(1–2):229–37.
Ding Y, Cui W, Sun D, Wang G-L, Hei Y, Meng S, et al. In vivo study of doxorubicin-loaded cell-penetrating peptide-modified pH-sensitive liposomes: biocompatibility, bio-distribution, and pharmacodynamics in BALB/c nude mice bearing human breast tumors. Drug Des Dev Ther. 2017;11:3105.
Salkho NM, Paul V, Kawak P, Vitor RF, Martins AM, Al Sayah M, et al. Ultrasonically controlled estrone-modified liposomes for estrogen-positive breast cancer therapy. Art Cells Nanomed Biotechnol. 2018;46(sup2):462–72.
Xu G, Tang H, Chen J, Zhu M, Xie Y, Li Y, et al. Estrone-targeted liposomes for mitoxantrone delivery via estrogen receptor: in vivo targeting efficacy, antitumor activity, acute toxicity and pharmacokinetics. Eur J Pharm Sci. 2021;161:105780.
Jose A, Ninave KM, Karnam S, Venuganti VVK. Temperature-sensitive liposomes for co-delivery of tamoxifen and imatinib for synergistic breast cancer treatment. J Liposome Res. 2019;29(2):153–62.
Lingayat VJ, Zarekar NS, Shendge RS. Solid lipid nanoparticles: a review. Nanosci Nanotechnol Res. 2017;2:67–72.
Mehnert W, Mäder K. Solid lipid nanoparticles: production, characterization and applications. Adv Drug Deliv Rev. 2012;64:83–101.
Cai S, Yang Q, Bagby TR, Forrest ML. Lymphatic drug delivery using engineered liposomes and solid lipid nanoparticles. Adv Drug Deliv Rev. 2011;63(10–11):901–8.
Mukherjee S, Ray S, Thakur R. Solid lipid nanoparticles: a modern formulation approach in drug delivery system. Indian J Pharm Sci. 2009;71(4):349.
Zheng G, Zheng M, Yang B, Fu H, Li Y. Improving breast cancer therapy using doxorubicin loaded solid lipid nanoparticles: synthesis of a novel arginine-glycine-aspartic tripeptide conjugated, pH sensitive lipid and evaluation of the nanomedicine in vitro and in vivo. Biomed Pharmacother. 2019;116:109006.
Eskiler GG, Cecener G, Dikmen G, Egeli U, Tunca B. Solid lipid nanoparticles: reversal of tamoxifen resistance in breast cancer. Eur J Pharm Sci. 2018;120:73–88.
Fontana G, Maniscalco L, Schillaci D, Cavallaro G, Giammona G. Solid lipid nanoparticles containing tamoxifen characterization and in vitro antitumoral activity. Drug Delivery. 2005;12(6):385–92.
Wang W, Chen T, Xu H, Ren B, Cheng X, Qi R, et al. Curcumin-loaded solid lipid nanoparticles enhanced anticancer efficiency in breast cancer. Molecules. 2018;23(7):1578.
Baek J-S, Kim J-H, Park J-S, Cho C-W. Modification of paclitaxel-loaded solid lipid nanoparticles with 2-hydroxypropyl-β-cyclodextrin enhances absorption and reduces nephrotoxicity associated with intravenous injection. Int J Nanomed. 2015;10:5397.
Baek J-S, So J-W, Shin S-C, Cho C-W. Solid lipid nanoparticles of paclitaxel strengthened by hydroxypropyl-β-cyclodextrin as an oral delivery system. Int J Mol Med. 2012;30(4):953–9.
Yamazaki M, Ito T. Deformation and instability of membrane structure of phospholipid vesicles caused by osmophobic association: mechanical stress model for the mechanism of poly (ethylene glycol)-induced membrane fusion. Biochemistry. 1990;29(5):1309–14.
Bhagwat GS, Athawale RB, Gude RP, Md S, Alhakamy NA, Fahmy UA, et al. Formulation and development of transferrin targeted solid lipid nanoparticles for breast cancer therapy. Front Pharmacol. 2020;11.
Yuan Q, Han J, Cong W, Ge Y, Ma D, Dai Z, et al. Docetaxel-loaded solid lipid nanoparticles suppress breast cancer cells growth with reduced myelosuppression toxicity. Int J Nanomed. 2014;9:4829.
Lu B, Xiong S-B, Yang H, Yin X-D, Chao R-B. Solid lipid nanoparticles of mitoxantrone for local injection against breast cancer and its lymph node metastases. Eur J Pharm Sci. 2006;28(1–2):86–95.
Wang W, Zhang L, Chen T, Guo W, Bao X, Wang D, et al. Anticancer effects of resveratrol-loaded solid lipid nanoparticles on human breast cancer cells. Molecules. 2017;22(11):1814.
Müller RH, Mäder K, Gohla S. Solid lipid nanoparticles (SLN) for controlled drug delivery–a review of the state of the art. Eur J Pharm Biopharm. 2000;50(1):161–77.
Beloqui A, Solinís MÁ, Rodríguez-Gascón A, Almeida AJ, Préat V. Nanostructured lipid carriers: promising drug delivery systems for future clinics. Nanomedicine. 2016;12(1):143–61.
Liu Q, Li J, Pu G, Zhang F, Liu H, Zhang Y. Co-delivery of baicalein and doxorubicin by hyaluronic acid decorated nanostructured lipid carriers for breast cancer therapy. Drug Delivery. 2016;23(4):1364–8.
Sartaj A, Annu, Alam M, Biswas L, Yar MS, Mir SR, et al. Combinatorial delivery of Ribociclib and green tea extract mediated nanostructured lipid carrier for oral delivery for the treatment of breast cancer synchronising in silico, in vitro, and in vivo studies. J Drug Target. 2022:1–22.
Singh A, Neupane YR, Mangla B, Kohli K. Nanostructured lipid carriers for oral bioavailability enhancement of exemestane: formulation design, in vitro, ex vivo, and in vivo studies. J Pharm Sci. 2019;108(10):3382–95.
Jain S, Patil SR, Swarnakar NK, Agrawal AK. Oral delivery of doxorubicin using novel polyelectrolyte-stabilized liposomes (layersomes). Mol Pharm. 2012;9(9):2626–35.
Jain AK, Swarnakar NK, Godugu C, Singh RP, Jain S. The effect of the oral administration of polymeric nanoparticles on the efficacy and toxicity of tamoxifen. Biomaterials. 2011;32(2):503–15.
Kebebe D, Wu Y, Zhang B, Yang J, Liu Y, Li X, et al. Dimeric c (RGD) peptide conjugated nanostructured lipid carriers for efficient delivery of Gambogic acid to breast cancer. Int J Nanomed. 2019;14:6179.
Di H, Wu H, Gao Y, Li W, Zou D, Dong C. Doxorubicin-and cisplatin-loaded nanostructured lipid carriers for breast cancer combination chemotherapy. Drug Dev Ind Pharm. 2016;42(12):2038–43.
Liu D, Liu Z, Wang L, Zhang C, Zhang N. Nanostructured lipid carriers as novel carrier for parenteral delivery of docetaxel. Colloids Surf B. 2011;85(2):262–9.
Zhang Q, Zhao J, Hu H, Yan Y, Hu X, Zhou K, et al. Construction and in vitro and in vivo evaluation of folic acid-modified nanostructured lipid carriers loaded with paclitaxel and chlorin e6. Int J Pharm. 2019;569:118595.
Sabzichi M, Mohammadian J, Khosroushahi AY, Bazzaz R, Hamishehkar H. Folate-targeted nanostructured lipid carriers (NLCs) enhance (letrozol) efficacy in MCF-7 breast cancer cells. Asian Pac J Cancer Prev. 2016;17(12):5185.
Sabzichi M, Mohammadian J, Mohammadi M, Jahanfar F, Movassagh Pour AA, Hamishehkar H, et al. Vitamin D-loaded nanostructured lipid carrier (NLC): a new strategy for enhancing efficacy of doxorubicin in breast cancer treatment. Nutr Cancer. 2017;69(6):840–8.
Castro KCD, Costa JM, Campos MGN. Drug-loaded polymeric nanoparticles: a review. Int J Polym Mater Polym Biomater. 2020:1–13.
Torchilin VP. Multifunctional nanocarriers. Adv Drug Deliv Rev. 2006;58(14):1532–55.
Rezaei L, Safavi MS, Shojaosadati SA. Protein nanocarriers for targeted drug delivery. Characterization and biology of nanomaterials for drug delivery. 2019:199–218.
Elzoghby AO, Samy WM, Elgindy NA. Albumin-based nanoparticles as potential controlled release drug delivery systems. J Control Release. 2012;157(2):168–82.
Singh PK, Srivastava AK, Dev A, Kaundal B, Choudhury SR, Karmakar S. 1, 3β-Glucan anchored, paclitaxel loaded chitosan nanocarrier endows enhanced hemocompatibility with efficient anti-glioblastoma stem cells therapy. Carbohyd Polym. 2018;180:365–75.
Kumari A, Yadav SK, Yadav SC. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf B. 2010;75(1):1–18.
Bhardwaj RK, Glaeser H, Becquemont L, Klotz U, Gupta SK, Fromm MF. Piperine, a major constituent of black pepper, inhibits human P-glycoprotein and CYP3A4. J Pharmacol Exp Ther. 2002;302(2):645–50.
Bezerra D, Castro F, Alves A, Pessoa C, Moraes M, Silveira E, et al. In vivo growth-inhibition of Sarcoma 180 by piplartine and piperine, two alkaloid amides from Piper. Braz J Med Biol Res. 2006;39:801–7.
Katiyar SS, Muntimadugu E, Rafeeqi TA, Domb AJ, Khan W. Co-delivery of rapamycin-and piperine-loaded polymeric nanoparticles for breast cancer treatment. Drug Deliv. 2016;23(7):2608–16.
Kanade R, Boche M, Pokharkar V. Self-Assembling raloxifene loaded mixed micelles: formulation optimization, in vitro cytotoxicity and in vivo pharmacokinetics. AAPS PharmSciTech. 2018;19(3):1105–15.
Alyafee YA, Alaamery M, Bawazeer S, Almutairi MS, Alghamdi B, Alomran N, et al. Preparation of anastrozole loaded PEG-PLA nanoparticles: evaluation of apoptotic response of breast cancer cell lines. Int J Nanomed. 2018;13:199.
Pugazhendhi A, Edison TNJI, Karuppusamy I, Kathirvel B. Inorganic nanoparticles: a potential cancer therapy for human welfare. Int J Pharm. 2018;539(1–2):104–11.
Lidke DS, Nagy P, Heintzmann R, Arndt-Jovin DJ, Post JN, Grecco HE, et al. Quantum dot ligands provide new insights into erbB/HER receptor–mediated signal transduction. Nat Biotechnol. 2004;22(2):198–203.
Núñez C, Estévez SV, del Pilar CM. Inorganic nanoparticles in diagnosis and treatment of breast cancer. J Biol Inorg Chem. 2018;23(3):331–45.
Farrokhi F, Karami Z, Esmaeili-Mahani S, Heydari A. Delivery of DNAzyme targeting c-Myc gene using β-cyclodextrin polymer nanocarrier for therapeutic application in human breast cancer cell line. J Drug Delivery Sci Technol. 2018;47:477–84.
Kievit FM, Stephen ZR, Veiseh O, Arami H, Wang T, Lai VP, et al. Targeting of primary breast cancers and metastases in a transgenic mouse model using rationally designed multifunctional SPIONs. ACS Nano. 2012;6(3):2591–601.
Chen T-J, Cheng T-H, Chen C-Y, Hsu SC, Cheng T-L, Liu G-C, et al. Targeted Herceptin–dextran iron oxide nanoparticles for noninvasive imaging of HER2/neu receptors using MRI. J Biol Inorg Chem. 2009;14(2):253–60.
Liu J, Liang H, Li M, Luo Z, Zhang J, Guo X, et al. Tumor acidity activating multifunctional nanoplatform for NIR-mediated multiple enhanced photodynamic and photothermal tumor therapy. Biomaterials. 2018;157:107–24.
Liu D, Zhang Q, Wang J, Fan L, Zhu W, Cai D. Hyaluronic acid-coated single-walled carbon nanotubes loaded with doxorubicin for the treatment of breast cancer. Pharmazie. 2019;74(2):83–90.
Kumar M, Sharma G, Misra C, Kumar R, Singh B, Katare OP, et al. N-Desmethyl tamoxifen and quercetin-loaded multiwalled CNTs: a synergistic approach to overcome MDR in cancer cells. Mater Sci Eng, C. 2018;89:274–82.
Misra C, Kumar M, Sharma G, Kumar R, Singh B, Katare OP, et al. Glycinated fullerenes for tamoxifen intracellular delivery with improved anticancer activity and pharmacokinetics. Nanomedicine. 2017;12(9):1011–23.
Dreaden EC, Mwakwari SC, Sodji QH, Oyelere AK, El-Sayed MA. Tamoxifen− poly (ethylene glycol)− thiol gold nanoparticle conjugates: enhanced potency and selective delivery for breast cancer treatment. Bioconjug Chem. 2009;20(12):2247–53.
Moreira AF, Dias DR, Correia IJ. Stimuli-responsive mesoporous silica nanoparticles for cancer therapy: a review. Microporous Mesoporous Mater. 2016;236:141–57.
Feng Y, Panwar N, Tng DJH, Tjin SC, Wang K, Yong K-T. The application of mesoporous silica nanoparticle family in cancer theranostics. Coord Chem Rev. 2016;319:86–109.
Tsai C-P, Chen C-Y, Hung Y, Chang F-H, Mou C-Y. Monoclonal antibody-functionalized mesoporous silica nanoparticles (MSN) for selective targeting breast cancer cells. J Mater Chem. 2009;19(32):5737–43.
Latorre A, Posch C, Garcimartín Y, Celli A, Sanlorenzo M, Vujic I, et al. DNA and aptamer stabilized gold nanoparticles for targeted delivery of anticancer therapeutics. Nanoscale. 2014;6(13):7436–42.
Sadhukhan P, Kundu M, Chatterjee S, Ghosh N, Manna P, Das J, et al. Targeted delivery of quercetin via pH-responsive zinc oxide nanoparticles for breast cancer therapy. Mater Sci Eng C. 2019;100:129–40.
Ghosh S, More P, Derle A, Kitture R, Kale T, Gorain M, et al. Diosgenin functionalized iron oxide nanoparticles as novel nanomaterial against breast cancer. J Nanosci Nanotechnol. 2015;15(12):9464–72.
Oskoueian A, Amin Matori K, Bayat S, Oskoueian E, Ostovan F, Toozandehjani M. Fabrication, characterization, and functionalization of single-walled carbon nanotube conjugated with tamoxifen and its anticancer potential against human breast cancer cells. J Nanomater. 2018;2018.
Lee JY, Kim JH, Bae KH, Oh MH, Kim Y, Kim JS, et al. Low-density lipoprotein-mimicking nanoparticles for tumor-targeted theranostic applications. Small. 2015;11(2):222–31.
Rejeeth C, Kannan S. p53 gene therapy of human breast carcinoma: using a transferrin-modified silica nanoparticles. Breast Cancer. 2016;23(1):101–10.
Liang Y, Gao W, Peng X, Deng X, Sun C, Wu H, et al. Near infrared light responsive hybrid nanoparticles for synergistic therapy. Biomaterials. 2016;100:76–90.
He Q, Gao Y, Zhang L, Zhang Z, Gao F, Ji X, et al. A pH-responsive mesoporous silica nanoparticles-based multi-drug delivery system for overcoming multi-drug resistance. Biomaterials. 2011;32(30):7711–20.
Dunbar CE, High KA, Joung JK, Kohn DB, Ozawa K, Sadelain M. Gene therapy comes of age. Science. 2018;359(6372).
Mandal H, Katiyar SS, Swami R, Kushwah V, Katare PB, Meka AK, et al. ε-Poly-l-lysine/plasmid DNA nanoplexes for efficient gene delivery in vivo. Int J Pharm. 2018;542(1–2):142–52.
Yin H, Kanasty RL, Eltoukhy AA, Vegas AJ, Dorkin JR, Anderson DG. Non-viral vectors for gene-based therapy. Nat Rev Genet. 2014;15(8):541–55.
Kuche K, Pandey PK, Patharkar A, Maheshwari R, Tekade RK. Hyaluronic acid as an emerging technology platform for silencing RNA delivery. Biomaterials and Bionanotechnology. Elsevier; 2019. p. 415–58.
Behr JP. Synthetic gene-transfer vectors. Acc Chem Res. 1993;26(5):274–8.
Zhang M, Xu R, Xia X, Yang Y, Gu J, Qin G, et al. Polycation-functionalized nanoporous silicon particles for gene silencing on breast cancer cells. Biomaterials. 2014;35(1):423–31.
Xu G, Chhangawala S, Cocco E, Razavi P, Cai Y, Otto JE, et al. ARID1A determines luminal identity and therapeutic response in estrogen-receptor-positive breast cancer. Nat Genet. 2020;52(2):198–207.
Xu X, Liu C, Wang Y, Koivisto O, Zhou J, Shu Y, et al. Nanotechnology-based delivery of CRISPR/Cas9 for cancer treatment. Adv Drug Deliv Rev. 2021;176:113891.
Zuris JA, Thompson DB, Shu Y, Guilinger JP, Bessen JL, Hu JH, et al. Cationic lipid-mediated delivery of proteins enables efficient protein-based genome editing in vitro and in vivo. Nat Biotechnol. 2015;33(1):73–80.
Zhu H, Zhang L, Tong S, Lee CM, Deshmukh H, Bao G. Spatial control of in vivo CRISPR–Cas9 genome editing via nanomagnets. Nat Biomed Eng. 2019;3(2):126–36.
Gao S, Tian H, Guo Y, Li Y, Guo Z, Zhu X, et al. miRNA oligonucleotide and sponge for miRNA-21 inhibition mediated by PEI-PLL in breast cancer therapy. Acta Biomater. 2015;25:184–93.
Li X, Chen Y, Wang M, Ma Y, Xia W, Gu H. A mesoporous silica nanoparticle–PEI–fusogenic peptide system for siRNA delivery in cancer therapy. Biomaterials. 2013;34(4):1391–401.
Ni Q, Zhang F, Zhang Y, Zhu G, Wang Z, Teng Z, et al. In situ shRNA synthesis on DNA–polylactide nanoparticles to treat multidrug resistant breast cancer. Adv Mater. 2018;30(10):1705737.
Chen M, Wang L, Wang F, Li F, Xia W, Gu H, et al. Quick synthesis of a novel combinatorial delivery system of siRNA and doxorubicin for a synergistic anticancer effect. Int J Nanomed. 2019;14:3557.
Liang Z, Du L, Zhang E, Zhao Y, Wang W, Ma P, et al. Targeted-delivery of siRNA via a polypeptide-modified liposome for the treatment of gp96 over-expressed breast cancer. Mater Sci Eng C. 2021;121:111847.
Mbatha LS, Maiyo F, Daniels A, Singh M. Dendrimer-coated gold nanoparticles for efficient folate-targeted mRNA delivery in vitro. Pharmaceutics. 2021;13(6):900.
Alshaer W, Hillaireau H, Vergnaud J, Mura S, Deloménie C, Sauvage F, et al. Aptamer-guided siRNA-loaded nanomedicines for systemic gene silencing in CD-44 expressing murine triple-negative breast cancer model. J Control Release. 2018;271:98–106.
Devulapally R, Sekar TV, Paulmurugan R. Formulation of anti-miR-21 and 4-hydroxytamoxifen co-loaded biodegradable polymer nanoparticles and their antiproliferative effect on breast cancer cells. Mol Pharm. 2015;12(6):2080–92.
Hu F, Chen W, Zhao M, Yuan H, Du Y. Effective antitumor gene therapy delivered by polyethylenimine-conjugated stearic acid-g-chitosan oligosaccharide micelles. Gene Ther. 2013;20(6):597–606.
Hong RL, Tseng YL. Phase I and pharmacokinetic study of a stable, polyethylene-glycolated liposomal doxorubicin in patients with solid tumors: the relation between pharmacokinetic property and toxicity. Cancer. 2001;91(9):1826–33.
Fujiwara Y, Mukai H, Saeki T, Ro J, Lin Y-C, Nagai SE, et al. A multi-national, randomised, open-label, parallel, phase III non-inferiority study comparing NK105 and paclitaxel in metastatic or recurrent breast cancer patients. Br J Cancer. 2019;120(5):475–80.
Gradishar WJ, Tjulandin S, Davidson N, Shaw H, Desai N, Bhar P, et al. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil–based paclitaxel in women with breast cancer. J Clin Oncol. 2005;23(31):7794–803.
Gonzalez-Angulo AM, Meric-Bernstam F, Chawla S, Falchook G, Hong D, Akcakanat A, et al. Weekly nab-Rapamycin in patients with advanced nonhematologic malignancies: final results of a phase I trial. Clin Cancer Res. 2013;19(19):5474–84.
Tan YL, Ho HK. Navigating albumin-based nanoparticles through various drug delivery routes. Drug Discovery Today. 2018;23(5):1108–14.
Mita MM, Natale RB, Wolin EM, Laabs B, Dinh H, Wieland S, et al. Pharmacokinetic study of aldoxorubicin in patients with solid tumors. Invest New Drugs. 2015;33(2):341–8.
Unger C, Häring B, Medinger M, Drevs J, Steinbild S, Kratz F, et al. Phase I and pharmacokinetic study of the (6-maleimidocaproyl) hydrazone derivative of doxorubicin. Clin Cancer Res. 2007;13(16):4858–66.
Libutti SK, Paciotti GF, Byrnes AA, Alexander HR, Gannon WE, Walker M, et al. Phase I and pharmacokinetic studies of CYT-6091, a novel PEGylated colloidal gold-rhTNF nanomedicine. Clin Cancer Res. 2010;16(24):6139–49.
Tagami T, Ozeki T. Recent trends in clinical trials related to carrier-based drugs. J Pharm Sci. 2017;106(9):2219–26.
Deeken JF, Slack R, Weiss GJ, Ramanathan RK, Pishvaian MJ, Hwang J, et al. A phase I study of liposomal-encapsulated docetaxel (LE-DT) in patients with advanced solid tumor malignancies. Cancer Chemother Pharmacol. 2013;71(3):627–33.
Pushpalatha R, Selvamuthukumar S, Kilimozhi D. Nanocarrier mediated combination drug delivery for chemotherapy–a review. J Drug Delivery Sci Technol. 2017;39:362–71.
van der Meel R, Lammers T, Hennink WE. Cancer nanomedicines: oversold or underappreciated? Expert Opin Drug Deliv. 2017;14(1):1–5.
Nahleh ZA, Barlow WE, Hayes DF, Schott AF, Gralow JR, Perez EA, et al. Abstract P3–11–16: S0800: Nab-paclitaxel, doxorubicin, cyclophosphamide, and pegfilgrastim with or without bevacizumab in treating women with inflammatory or locally advanced breast cancer (NCI CDR0000636131). AACR; 2015.
Koca E, Niravath PA, Ensor J, Patel TA, Li X, Hemati P, et al. ANETT: PhAse II trial of NEoadjuvant TAK-228 plus Tamoxifen in patients with hormone receptor-positive breast cancer. Breast Cancer Res Treat. 2021:1–7.
Svenson S. What nanomedicine in the clinic right now really forms nanoparticles? Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2014;6(2):125–35.
Brandsma D, Kerklaan BM, Diéras V, Altintas S, Anders C, Ballester MA, et al. Phase 1/2a study of glutathione pegylated liposomal doxorubicin (2b3–101) in patients with brain metastases (BM) from solid tumors or recurrent high grade gliomas (HGG). Ann Oncol. 2014;25:iv157.
Anselmo AC, Mitragotri S. A review of clinical translation of inorganic nanoparticles. AAPS J. 2015;17(5):1041–54.
Shah N, Mohammad AS, Saralkar P, Sprowls SA, Vickers SD, John D, et al. Investigational chemotherapy and novel pharmacokinetic mechanisms for the treatment of breast cancer brain metastases. Pharmacol Res. 2018;132:47–68.
Acknowledgements
The authors would like to acknowledge the Department of Pharmaceuticals (DoP), Ministry of Chemicals and Fertilizers, Govt. of India.
Author information
Authors and Affiliations
Contributions
Mayur Aalhate and Srushti Mahajan: Literature survey and writing and draft preparation of the manuscript. Hoshiyar Singh: Proof reading and revision. Santosh Kumar Guru and Pankaj Kumar Singh: Reviewing and editing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
No animal or human studies were carried out by the authors for this article.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Conventional anticancer strategies suffer due to low tissue accumulation, poor bioavailability, and high drug resistance.
• Breast cancer relapse is a result of tumor heterogeneity and the complexity of pathways.
• Nanomedicine has the potential to combat unmet clinical needs in cancer treatments.
• Lipids, polymers, and inorganic material-based formulations can effectively target breast cancer and deliver therapeutic genes and drugs.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Aalhate, M., Mahajan, S., Singh, H. et al. Nanomedicine in therapeutic warfront against estrogen receptor–positive breast cancer. Drug Deliv. and Transl. Res. 13, 1621–1653 (2023). https://doi.org/10.1007/s13346-023-01299-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-023-01299-7