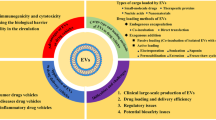
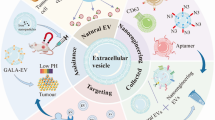
Abstract
Nanoparticle drug delivery systems (NDDSs) are promising platforms for efficient delivery of drugs. In the past decades, many nanomedicines have received clinical approval and completed translation. With the rapid advance of nanobiotechnology, natural vectors are emerging as novel strategies to carry and delivery nanoparticles and drugs for biomedical applications. Among diverse types of cells, macrophage is of great interest for their essential roles in inflammatory and immune responses. Macrophage-derived vesicles (MVs), including exosomes, microvesicles, and those from reconstructed membranes, may inherit the chemotactic migration ability and high biocompatibility. The unique properties of MVs make them competing candidates as novel drug delivery systems for precision nanomedicine. In this review, the advantages and disadvantages of existing NDDSs and MV-based drug delivery systems (MVDDSs) were compared. Then, we summarized the potential applications of MVDDSs and discuss future perspectives. The development of MVDDS may provide avenues for the treatment of diseases involving an inflammatory process.
Graphical abstract



Similar content being viewed by others
Availability of data and materials
Not applicable.
Abbreviations
- AA-PEG:
-
Aminoethylanisamide-polyethylene glycol
- BDNF:
-
Brain-derived neurotrophic factor
- BNCT:
-
Boron neutron capture therapy
- CCR2:
-
C-C chemokine receptor type 2
- DEX:
-
Dexamethasone sodium phosphate
- DOX:
-
Adriamycin
- EV:
-
Extracellular vesicles
- MM:
-
Macrophage membrane
- MPS:
-
Mononuclear phagocytic system
- MVDDS:
-
Macrophage vesicle-based drug delivery system
- NP:
-
Nanoparticle
- PLGA:
-
Poly(lactic-co-glycolic acid)
- PTX:
-
Paclitaxel
- RGE:
-
Neuropilin-1-targeted peptide
- RMM:
-
Reconstructed macrophage membrane
- RVG29:
-
Rabies virus glycoprotein
- ROS:
-
Reactive oxygen species
- SPION:
-
Superparamagnetic iron oxide nanoparticles
- TLR4:
-
Toll-like receptor 4
- TPP:
-
Triphenylphosphine cation
References
Florea A-M, Busselberg D. Cisplatin as an anti-tumor drug: cellular mechanisms of activity, drug resistance and induced side effects. Cancers. 2011;3(1): 1351–71.
de Jong WH, Borm PJA. Drug delivery and nanoparticles: applications and hazards. Int J Nanomed. 2008;3(2):133–49.
Dai Y, Xu C, Sun X, et al. Nanoparticle design strategies for enhanced anticancer therapy by exploiting the tumour microenvironment. Chem Soc Rev. 2017;46(12):3830–52.
Kalyane D, Raval N, Maheshwari R, et al. Employment of enhanced permeability and retention effect (EPR): nanoparticle-based precision tools for targeting of therapeutic and diagnostic agent in cancer. Mater Sci Eng C-Mater Biol Appl. 2019;98:1252–76.
Oberli MA, Reichmuth AM, Dorkin JR, et al. Lipid nanoparticle assisted mRNA delivery for potent cancer immunotherapy. Nano Lett. 2017;17(3):1326–35.
McNamara K, Tofail SAM. Nanoparticles in biomedical applications. Adv Phys-X. 2017;2(1):54–88.
Peer D, Karp JM, Hong S, et al. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2(12):751–60.
Fang J, Nakamura H, Maeda H. The EPR effect: unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv Drug Deliv Rev. 2011;63(3):136–51.
Anselmo AC, Mitragotri S. Nanoparticles in the clinic: an update. Bioeng Transl Med. 2019;4(3).
Caracciolo G. Clinically approved liposomal nanomedicines: lessons learned from the biomolecular corona. Nanoscale. 2018;10(9):4167–72.
Bulbake U, Doppalapudi S, Kommineni N, et al. Liposomal formulations in clinical use: an updated review. Pharmaceutics. 2017;9(2).
Janeway CA, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216.
Fang RH, Kroll AV, Gao W, et al. Cell membrane coating nanotechnology. Adv Mater. 2018;30(23).
Chen Z, Wen D, Gu Z. Cargo-encapsulated cells for drug delivery. Science China-Life Sciences. 2020;63(4):599–601.
Samuelsson B. Leukotrienes: mediators of immediate hypersensitivity reactions and inflammation. Science (New York, NY). 1983;220(4597):568–75.
Ley K, Laudanna C, Cybulsky MI, et al. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7(9):678–89.
Schaffner T, Keller HU, Hess MW, et al. Macrophage functions in antimicrobial defense. Klin Wochenschr. 1982;60(14):720–6.
Anderson NR, Minutolo NG, Gill S, et al. Macrophage-based approaches for cancer immunotherapy. Can Res. 2021;81(5):1201–8.
Vitale I, Manic G, Coussens LM, et al. Macrophages and metabolism in the tumor microenvironment. Cell Metab. 2019;30(1):36–50.
Clark RA, Stone RD, Leung DY, et al. Role of macrophages in would healing. Surgical forum. 1976;27(62):16–8.
Smigiel KS, Parks WC. Macrophages, wound healing, and fibrosis: recent insights. Curr Rheumatol Rep. 2018;20(4).
Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8(12):958–69.
Goulielmaki E, Ioannidou A, Tsekrekou M, et al. Tissue-infiltrating macrophages mediate an exosome-based metabolic reprogramming upon DNA damage. Nat Commun. 2020;11(1).
Lan J, Sun L, Xu F, et al. M2 macrophage-derived exosomes promote cell migration and invasion in colon cancer. Can Res. 2019;79(1):146–58.
Nguyen M-A, Karunakaran D, Geoffrion M, et al. Extracellular vesicles secreted by atherogenic macrophages transfer MicroRNA to inhibit cell migration. Arterioscler Thromb Vas Biol. 2018, 38(1): 49–63.
Zhu X, Shen H, Yin X, et al. Macrophages derived exosomes deliver miR-223 to epithelial ovarian cancer cells to elicit a chemoresistant phenotype. J Exp Clin Cancer Res. 2019;38.
Shao J, Li S, Liu Y, et al. Extracellular vesicles participate in macrophage-involved immune responses under liver diseases. Life Sci. 2020;240.
Vlassov AV, Magdaleno S, Setterquist R, et al. Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. BBA-Gen Subjects. 2012;1820(7):940–8.
Xu R, Greening DW, Zhu H-J, et al. Extracellular vesicle isolation and characterization: toward clinical application. J Clin Investig. 2016;126(4): 1152–62.
Kalluri R, Lebleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478): 640-+.
Hessvik NP, Llorente A. Current knowledge on exosome biogenesis and release. Cell Mol Life Sci. 2018;75(2):193–208.
Wu P, Zhang B, Ocansey DKW, et al. Extracellular vesicles: a bright star of nanomedicine. Biomaterials. 2021;269: 120467.
Bang C, Thum T. Exosomes: New players in cell-cell communication. Int J Biochem Cell Biol. 2012;44(11):2060–4.
Kanchanapally R, Deshmukh SK, Chavva SR, et al. Drug-loaded exosomal preparations from different cell types exhibit distinctive loading capability, yield, and antitumor efficacies: a comparative analysis. Int J Nanomed. 2019;14:531–41.
Khan I, Saeed K, Khan I. Nanoparticles: Properties, applications and toxicities. Arab J Chem. 2019;12(7):908–31.
Patra JK, Das G, Fraceto LF, et al. Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnol. 2018;16.
Shi J, Kantoff PW, Wooster R, et al. Cancer nanomedicine: progress, challenges and opportunities. Nat Rev Cancer. 2017;17(1):20–37.
Zhang L, Beatty A, Lu L, et al. Microfluidic-assisted polymer-protein assembly to fabricate homogeneous functionalnanoparticles. Mater Sci Eng C-Mater Biol Appl. 2020;111.
Strand MS, Krasnick BA, Pan H, et al. Precision delivery of RAS-inhibiting siRNA to KRAS driven cancer via peptide-based nanoparticles. Oncotarget. 2019;10(46):4761–75.
Seo Y-E, Suh H-W, Bahal R, et al. Nanoparticle-mediated intratumoral inhibition of miR-21 for improved survival in glioblastoma. Biomaterials. 2019;201: 87–98.
Petersen GH, Alzghari SK, Chee W, et al. Meta-analysis of clinical and preclinical studies comparing the anticancer efficacy of liposomal versus conventional non-liposomal doxorubicin. J Control Release. 2016;232:255–64.
Mitchell MJ, Billingsley MM, Haley RM, et al. Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discovery. 2021;20(2):101–24.
Anselmo AC, Mitragotri S. Impact of particle elasticity on particle-based drug delivery systems. Adv Drug Deliv Rev. 2017;108:51–67.
Mohammed L, Gomaa HG, Ragab D, et al. Magnetic nanoparticles for environmental and biomedical applications: a review. Particuology. 2017;30:1–14.
Dalzon B, Guidetti M, Testemale D, et al. Utility of macrophages in an antitumor strategy based on the vectorization of iron oxide nanoparticles. Nanoscale. 2019;11(19):9341–52.
Yang H, Shao R, Huang H, et al. Engineering macrophages to phagocytose cancer cells by blocking the CD47/SIRPα axis. Cancer Med. 2019;8(9):4245–53.
Liu X, Li H, Chen Y, et al. Mixed-charge nanoparticles for long circulation, low reticuloendothelial system clearance, and high tumor accumulation. Adv Healthcare Mater. 2014;3(9):1439–47.
Owens DE, Peppas NA. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int J Pharm. 2006;307(1):93–102.
Suk JS, Xu QG, Kim N, et al. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv Drug Deliv Rev. 2016;99:28–51.
Abuchowski A, McCoy JR, Palczuk NC, et al. Effect of covalent attachment of polyethylene glycol on immunogenicity and circulating life of bovine liver catalase. J Biol Chem. 1977;252(11):3582–6.
Elahi N, Kamali M, Baghersad MH. Recent biomedical applications of gold nanoparticles: a review. Talanta. 2018;184:537–56.
El-Hammadi MM, Small-Howard AL, Fernandez-Arevalo M, et al. Development of enhanced drug delivery vehicles for three cannabis-based terpenes using poly(lactic-co-glycolic acid) based nanoparticles. Ind Crops Prod. 2021;164.
Rafiei P, Haddadi A. Docetaxel-loaded PLGA and PLGA-PEG nanoparticles for intravenous application: pharmacokinetics and biodistribution profile. Int J Nanomed. 2017;12:935–47.
Lu J, Liu X, Liao Y-P, et al. Breast cancer chemo-immunotherapy through liposomal delivery of an immunogenic cell death stimulus plus interference in the IDO-1 pathway. Acs Nano. 2018;12(11): 11041–61.
Ishida T, Maeda R, Ichihara M, et al. Accelerated clearance of PEGylated liposomes in rats after repeated injections. J Control Release. 2003;88(1):35–42.
Lubich C, Allacher P, de la Rosa M, et al. The mystery of antibodies against polyethylene glycol (PEG) — what do we know? Pharm Res. 2016;33(9):2239–49.
Batrakova EV, Kim MS. Using exosomes, naturally-equipped nanocarriers, for drug delivery. J Control Release. 2015;219:396–405.
Patel HM. Serum opsonins and liposomes: their interaction and opsonophagocytosis. Crit Rev Ther Drug Carrier Syst. 1992;9(1):39–90.
Liu R, An Y, Jia W, et al. Macrophage-mimic shape changeable nanomedicine retained in tumor for multimodal therapy of breast cancer. J Control Release. 2020;321:589–601.
Zhang Y, Cai K, Li C, et al. Macrophage-membrane-coated nanoparticles for tumor-targeted chemotherapy. Nano Lett. 2018;18(3):1908–15.
Basel MT, Shrestha TB, Bossmann SH, et al. Cells as delivery vehicles for cancer therapeutics. Ther Deliv. 2014;5(5):555–67.
Li Z, Yu X-F, Chu PK. Recent advances in cell-mediated nanomaterial delivery systems for photothermal therapy. J Mater Chem B. 2018;6(9): 1296–311.
Lee CH, Choi EY. Macrophages and inflammation. J Rheum Dis. 2018;25(1): 11–8.
Sylvestre M, Crane CA, Pun SH. Progress on modulating tumor-associated macrophages with biomaterials. Adv Mater. 2020;32(13).
Wan S-W, Wu-Hsieh BA, Lin Y-S, et al. The monocyte-macrophage-mast cell axis in dengue pathogenesis. J Biomed Sci, 2018;25.
Ross R. Mechanisms of disease — atherosclerosis — an inflammatory disease. N Engl J Med. 1999;340(2):115–26.
Italiani P, Boraschi D. From monocytes to M1/M2 macrophages: phenotypical vs. functional differentiation. Front Immun. 2014;5.
Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496(7446):445–55.
Thery C, Witwer KW, Aikawa E, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracellular Vesicles. 2018;7(1).
D'souza-Schorey C, Schorey JS. Regulation and mechanisms of extracellular vesicle biogenesis and secretion [M]//STAHL P, RAPOSO G. Extracellular Vesicles and Mechanisms of Cell-Cell Communication. 2018: 125–33.
van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19(4):213–28.
Madsen SJ, Hirschberg H. Macrophages as delivery vehicles for anticancer agents. Ther Deliv. 2019;10(3):189–201.
Guo L, Zhang Y, Wei R, et al. Proinflammatory macrophage-derived microvesicles exhibit tumor tropism dependent on CCL2/CCR2 signaling axis and promote drug delivery via SNARE-mediated membrane fusion. Theranostics. 2020;10(15):6581–98.
Pang L, Zhu Y, Qin J, et al. Primary M1 macrophages as multifunctional carrier combined with PLGA nanoparticle delivering anticancer drug for efficient glioma therapy. Drug Delivery. 2018;25(1):1922–31.
Zhang H, Dong S, Li Z, et al. Biointerface engineering nanoplatforms for cancer-targeted drug delivery. Asian J Pharm Sci. 2020;15(4):397–415.
Xia Y, Rao L, Yao H, et al. Engineering macrophages for cancer immunotherapy and drug delivery. Adv Mater. 2020;32(40).
Arteaga-Blanco L, Bou-Habib D. The role of extracellular vesicles from human macrophages on host-pathogen interaction. Int J Mol Sci. 2021;22(19).
Marchetti B, Leggio L, L'episcopo F, et al. Glia-derived extracellular vesicles in Parkinson's disease. J Clin Med. 2020;9(6).
Giri P, Schorey J. Exosomes derived from M. bovis BCG infected macrophages activate antigen-specific CD4+ and CD8+ T cells in vitro and in vivo. Plos One. 2008;3(6): e2461.
Robbins P, Morelli A. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol. 2014;14(3):195–208.
Cheng L, Wang Y, Huang L. Exosomes from M1-polarized macrophages potentiate the cancer vaccine by creating a pro-inflammatory microenvironment in the lymph node. Mol Ther. 2017;25(7):1665–75.
Kim MS, Haney MJ, Zhao Y, et al. Engineering macrophage-derived exosomes for targeted paclitaxel delivery to pulmonary metastases: in vitro and in vivo evaluations. Nanomedicine-Nanotechnology Biology and Medicine. 2018;14(1):195–204.
Tang T-T, Lv L-L, Wang B, et al. Employing macrophage-derived microvesicle for kidney-targeted delivery of dexamethasone: an efficient therapeutic strategy against renal inflammation and fibrosis. Theranostics. 2019;9(16): 4740–55.
Tang T-T, Lv L-L, Cao J-Y, et al. Employing macrophage-derived microvesicle for kidney-targeted delivery of dexamethasone: an efficient therapeutic strategy against renal inflammation and fibrosis. Nephrol Dial Transplant. 2019;34.
Silva AKA, Luciani N, Gazeau F, et al. Combining magnetic nanoparticles with cell derived microvesicles for drug loading and targeting. Nanomed Nanotech Biol Med. 2015;11(3):645–55.
Sweeney MD, Sagare AP, Zlokovic BV. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat Rev Neurol. 2018;14(3):133–50.
Dong X. Current strategies for brain drug delivery. Theranostics. 2018;8(6):1481–93.
Xuan M, Shao J, Dai L, et al. Macrophage cell membrane camouflaged mesoporous silica nanocapsules for in vivo cancer therapy. Adv Healthcare Mater. 2015;4(11):1645–52.
Yuan D, Zhao Y, Banks WA, et al. Macrophage exosomes as natural nanocarriers for protein delivery to inflamed brain. Biomaterials. 2017;142:1–12.
Clayton A, Harris CL, Court J, et al. Antigen-presenting cell exosomes are protected from complement-mediated lysis by expression of CD55 and CD59. Eur J Immunol. 2003;33(2):522–31.
Qu Y, Franchi L, Nunez G, et al. Nonclassical IL-1 beta secretion stimulated by P2X7 receptors is dependent on inflammasome activation and correlated with exosome release in murine macrophages. J Immunol. 2007;179(3):1913–25.
Kamerkar S, Lebleu VS, Sugimoto H, et al. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature. 2017;546(7659): 498-+.
Yang W, Wang L, Mettenbrink E, et al. Nanoparticle toxicology. Annu Rev Pharmacol Toxicol. 2021;61:269–89.
Rao L, He Z, Meng Q-F, et al. Effective cancer targeting and imaging using macrophage membrane-camouflaged upconversion nanoparticles. J Biomed Mater Res Part A. 2017;105(2): 521–30.
Ji B, Cai H, Yang Y, et al. Hybrid membrane camouflaged copper sulfide nanoparticles for photothermal-chemotherapy of hepatocellular carcinoma. Acta Biomater. 2020;111:363–72.
Wang P, Wang H, Huang Q, et al. Exosomes from M1-polarized macrophages enhance paclitaxel antitumor activity by activating macrophages-mediated inflammation. Theranostics. 2019;9(6):1714–27.
Doyle LM, Wang MZ. Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells. 2019;8(7).
Moore C, Kosgodage U, Lange S, et al. The emerging role of exosome and microvesicle- (EMV-) based cancer therapeutics and immunotherapy. Int J Cancer. 2017;141(3):428–36.
Haraszti RA, Miller R, Stoppato M, et al. Exosomes produced from 3D cultures of MSCs by tangential flow filtration show higher yield and improved activity. Mol Ther. 2018;26(12):2838–47.
Christie C, Madsen SJ, Peng Q, et al. Macrophages as nanoparticle delivery vectors for photothermal therapy of brain tumors. Ther Deliv. 2015;6(3):371–84.
Charoenviriyakul C, Takahashi Y, Morishita M, et al. Cell type-specific and common characteristics of exosomes derived from mouse cell lines: yield, physicochemical properties, and pharmacokinetics. European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences. 2017;96:316–22.
Théry C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2(8):569–79.
Parada N, Romero-Trujillo A, Georges N, et al. Camouflage strategies for therapeutic exosomes evasion from phagocytosis. J Adv Res. 2021;31:61–74.
Jia G, Han Y, An Y, et al. NRP-1 targeted and cargo-loaded exosomes facilitate simultaneous imaging and therapy of glioma in vitro and in vivo. Biomaterials. 2018;178:302–16.
Wang S, Li F, Ye T, et al. Macrophage-tumor chimeric exosomes accumulate in lymph node and tumor to activate the immune response and the tumor microenvironment. Sci Transl Med. 2021;13(615): eabb6981.
Zhang M, Jin K, Gao L, et al. Methods and technologies for exosome isolation and characterization. Small Methods. 2018;2(9):1800021.
Li P, Kaslan M, Lee SH, et al. Progress in exosome isolation techniques. Theranostics. 2017;7(3):789–804.
Li YJ, Wu JY, Liu J, et al. Artificial exosomes for translational nanomedicine. J Nanobiotechnology. 2021;19(1):242.
Parodi A, Quattrocchi N, van de Ven AL, et al. Synthetic nanoparticles functionalized with biomimetic leukocyte membranes possess cell-like functions. Nat Nanotechnol. 2013;8(1):61–8.
He Z, Zhang Y, Feng N. Cell membrane-coated nanosized active targeted drug delivery systems homing to tumor cells: a review. Mater Sci Eng C-Mater Biol Appl. 2020;106.
Vijayan V, Uthaman S, Park I-K. Cell membrane-camouflaged nanoparticles: a promising biomimetic strategy for cancer theragnostics. Polymers. 2018;10(9).
Jeppesen DK, Fenix AM, Franklin JL, et al. Reassessment of exosome composition. Cell. 2019;177(2): 428-+.
Barenholz Y. Doxil (R) - The first FDA-approved nano-drug: lessons learned. J Control Release. 2012;160(2):117–34.
Si J, Shao S, Shen Y, et al. Macrophages as active nanocarriers for targeted early and adjuvant cancer chemotherapy. Small. 2016;12(37):5108–19.
Oun R, Moussa YE, Wheate NJ. The side effects of platinum-based chemotherapy drugs: a review for chemists (vol 47, pg 6645, 2018). Dalton Trans. 2018;47(23): 7848-.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. Ca-a Cancer J Clinic. 2020;70(1):7–30.
Dent R, Trudeau M, Pritchard KI, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13(15):4429–34.
Li S, Wu Y, Ding F, et al. Engineering macrophage-derived exosomes for targeted chemotherapy of triple-negative breast cancer. Nanoscale. 2020;12(19):10854–62.
Qiu Y, Ren K, Zhao W, et al. A “dual-guide” bioinspired drug delivery strategy of a macrophage-based carrier against postoperative triple-negative breast cancer recurrence. Journal of controlled release : official journal of the Controlled Release Society. 2020;329:191–204.
Gong C, Yu X, You B, et al. Macrophage-cancer hybrid membrane-coated nanoparticles for targeting lung metastasis in breast cancer therapy. J Nanobiotechnol. 2020;18(1).
Xiong F, Ling X, Chen X, et al. Pursuing specific chemotherapy of orthotopic breast cancer with lung metastasis from docking nanoparticles driven by bioinspired exosomes. Nano Lett. 2019;19(5):3256–66.
Li P, Gao M, Hu Z, et al. Synergistic ferroptosis and macrophage re-polarization using engineering exosome-mimic M1 nanovesicles for cancer metastasis suppression. Chem Eng J. 2021;409.
Haney MJ, Zhao Y, Jin YS, et al. Macrophage-derived extracellular vesicles as drug delivery systems for triple negative breast cancer (TNBC) therapy. J Neuroimmune Pharmacol. 2020;15(3):487–500.
Rayamajhi S, Nguyen TDT, Marasini R, et al. Macrophage-derived exosome-mimetic hybrid vesicles for tumor targeted drug delivery. Acta Biomater. 2019;94:482–94.
Cao H, Wang H, He X, et al. Bioengineered macrophages can responsively transform into nanovesicles to target lung metastasis. Nano Lett. 2018;18(8):4762–70.
Kim MS, Haney MJ, Zhao Y, et al. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomed Nanotechnol Biol Med. 2016;12(3):655–64.
Zhang X, Liu L, Tang M, et al. The effects of umbilical cord-derived macrophage exosomes loaded with cisplatin on the growth and drug resistance of ovarian cancer cells. Drug Dev Ind Pharm. 2020;46(7):1150–62.
Leonard F, Curtis LT, Yesantharao P, et al. Enhanced performance of macrophage-encapsulated nanoparticle albumin-bound-paclitaxel in hypo-perfused cancer lesions. Nanoscale. 2016;8(25):12544–52.
Li J, Li N, Wang J. M1 macrophage-derived exosome-encapsulated cisplatin can enhance its anti-lung cancer effect. Minerva medica. 2020.
Deng X, Shao Z, Zhao Y. Solutions to the drawbacks of photothermal and photodynamic cancer therapy. Adv Sci. 2021;8(3).
Li Y, Li X, Zhou F, et al. Nanotechnology-based photoimmunological therapies for cancer. Cancer Lett. 2019;442:429–38.
Huang X, Jain PK, El-Sayed IH, et al. Plasmonic photothermal therapy (PPTT) using gold nanoparticles. Lasers Med Sci. 2008;23(3):217–28.
Cheng L, Wang C, Feng L, et al. Functional nanomaterials for phototherapies of cancer. Chem Rev. 2014;114(21):10869–939.
Riley RS, Day ES. Gold nanoparticle-mediated photothermal therapy: applications and opportunities for multimodal cancer treatment. Wiley Interdiscip Rev Nanomed Nanobiotech. 2017;9(4).
Wang Z, Chang Z, Lu M, et al. Janus silver/silica nanoplatforms for light-activated liver cancer chemo/photothermal therapy. ACS Appl Mater Interfaces. 2017;9(36):30306–17.
Hu Y, Hu H, Yan J, et al. Multifunctional porous iron oxide nanoagents for MRI and photothermal/chemo synergistic therapy. Bioconjug Chem. 2018;29(4):1283–90.
Meng Q-F, Rao L, Zan M, et al. Macrophage membrane-coated iron oxide nanoparticles for enhanced photothermal tumor therapy. Nanotech. 2018;29(13).
Xuan M, Shao J, Dai L, et al. Macrophage cell membrane camouflaged Au nanoshells for in vivo prolonged circulation life and enhanced cancer photothermal therapy. ACS Appl Mater Interfaces. 2016;8(15):9610–8.
Liu Y, Bhattarai P, Dai Z, et al. Photothermal therapy and photoacoustic imaging via nanotheranostics in fighting cancer. Chem Soc Rev. 2019;48(7):2053–108.
Yousaf T, Dervenoulas G, Politis M. Advances in MRI methodology [M]//POLITIS M. Imaging in Movement Disorders: Imaging Methodology and Applications in Parkinson's Disease. 2018: 31–76.
Rayamajhi S, Marasini R, Tuyen Duong Thanh N, et al. Strategic reconstruction of macrophage-derived extracellular vesicles as a magnetic resonance imaging contrast agent. Biomater Sci. 2020;8(10): 2887–904.
Sier VQ, De Vries MR, Van Der Vorst JR, et al. Cell-Based tracers as Trojan horses for image-guided surgery. Int J Mol Sci. 2021;22(2).
Guidotti G, Brambilla L, Rossi D. Cell-penetrating peptides: from basic research to clinics. Trends Pharmacol Sci. 2017;38(4):406–24.
Abbott NJ, Patabendige AAK, Dolman DEM, et al. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37(1):13–25.
Pardridge WM. The blood-brain barrier: bottleneck in brain drug development. NeuroRx : the journal of the American Society for Experimental NeuroTherapeutics. 2005;2(1):3–14.
Madsen SJ, Gach HM, Hong SJ, et al. Increased nanoparticle-loaded exogenous macrophage migration into the brain following PDT-induced blood-brain barrier disruption. Lasers Surg Med. 2013;45(8):524–32.
Lu M, Huang Y. Bioinspired exosome-like therapeutics and delivery nanoplatforms. Biomater. 2020;242.
Tian T, Zhang H-X, He C-P, et al. Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy. Biomater. 2018;150: 137–49.
Beeraka NM, Doreswamy SH, Sadhu SP, et al. The role of exosomes in stemness and neurodegenerative diseases-chemoresistant-cancer therapeutics and phytochemicals. Int J Mol Sci. 2020;21(18).
Huang S, Ge X, Yu J, et al. Increased miR-124-3p in microglial exosomes following traumatic brain injury inhibits neuronal inflammation and contributes to neurite outgrowth via their transfer into neurons. FASEB J. 2018;32(1):512–28.
Gupta A, Pulliam L. Exosomes as mediators of neuroinflammation. J Neuroinflammation. 2014;11.
Batrakova EV. Macrophage-derived extracellular vesicles target inflamed brain and deliver therapeutic proteins for treatment of neurodegenerative disorders. J Neuroimmune Pharmacol. 2019;14(2): 336-.
Poewe W, Seppi K, Tanner CM, et al. Parkinson disease. Nat Rev Dis Primers. 2017;3.
Lane CA, Hardy J, Schott JM. Alzheimer’s disease. Eur J Neurol. 2018;25(1):59–70.
Haney MJ, Klyachko NL, Zhaoa Y, et al. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J Control Release. 2015;207:18–30.
Han Y, Gao C, Wang H, et al. Macrophage membrane-coated nanocarriers Co-modified by RVG29 and TPP improve brain neuronal mitochondria-targeting and therapeutic efficacy in Alzheimer’s disease mice. Bioact Mater. 2021;6(2):529–42.
Yao J, Wang Z, Cheng Y, et al. M2 macrophage-derived exosomal microRNAs inhibit cell migration and invasion in gliomas through PI3K/AKT/mTOR signaling pathway. J Transl Med. 2021;19(1).
Li J, Kong J, Ma S, et al. Exosome-coated B-10 carbon dots for precise boron neutron capture therapy in a mouse model of glioma in situ. Adv Funct Mater. 2021.
Li F, Zhao L, Shi Y, et al. Edaravone-loaded macrophage-derived exosomes enhance neuroprotection in the rat permanent middle cerebral artery occlusion model of stroke. Mol Pharm. 2020;17(9):3192–201.
Tabas I, Bornfeldt KE. Macrophage phenotype and function in different stages of atherosclerosis. Circ Res. 2016;118(4):653–67.
Virani SS, Alonso A, Benjamin EJ, et al. Heart disease and stroke statistics—2020 update: a report from the American Heart Association. Circulation. 2020;141(9):E139–596.
Chinetti-Gbaguidi G, Colin S, Staels B. Macrophage subsets in atherosclerosis. Nat Rev Cardiol. 2015;12(1):10–7.
Cao H, Dan Z, He X, et al. Liposomes coated with isolated macrophage membrane can target lung metastasis of breast cancer. ACS Nano. 2016;10(8):7738–48.
Peng R, Ji H, Jin L, et al. Macrophage-based therapies for atherosclerosis management. J Immunol Res. 2020;2020.
Bouchareychas L, Phat D, Covarrubias S, et al. Macrophage exosomes resolve atherosclerosis by regulating hematopoiesis and inflammation via MicroRNA cargo. Cell Rep. 2020;32(2).
Wang Y, Zhang K, Li T, et al. Macrophage membrane functionalized biomimetic nanoparticles for targeted anti-atherosclerosis applications. Theranostics. 2021;11(1):164–80.
Gao C, Huang Q, Liu C, et al. Treatment of atherosclerosis by macrophage-biomimetic nanoparticles via targeted pharmacotherapy and sequestration of proinflammatory cytokines. Nat Commun. 2020;11(1).
Kapoor G, Saigal S, Elongavan A. Action and resistance mechanisms of antibiotics: a guide for clinicians. J Anaesthesiol Clin Pharmacol. 2017;33(3):300–5.
Aslam B, Wang W, Arshad MI, et al. Antibiotic resistance: a rundown of a global crisis. Infect Drug Resist. 2018;11:1645–58.
Wang C, Wang Y, Zhang L, et al. Pretreated macrophage-membrane-coated gold nanocages for precise drug delivery for treatment of bacterial infections. Adv Mater. 2018;30(46).
Qin M, Du G, Sun X. Biomimetic cell-derived nanocarriers for modulating immune responses. Biomater Sci. 2020;8(2):530–43.
Li J, Wang Y, Yang J, et al. Bacteria activated-macrophage membrane-coated tough nanocomposite hydrogel with targeted photothermal antibacterial ability for infected wound healing. Chem Eng J. 2021;420: 127638.
Fu J, Li Y, Zhang Y, et al. An engineered pseudo-macrophage for rapid treatment of bacteria-infected osteomyelitis via microwave-excited anti-infection and immunoregulation. Adv Mater. 2021;33(41):2102926.
Cypryk W, Lorey M, Puustinen A, et al. Proteomic and bioinformatic characterization of extracellular vesicles released from human macrophages upon influenza A virus infection. J Proteome Res. 2017;16(1):217–27.
Wu W, Wu D, Yan W, et al. Interferon-induced macrophage-derived exosomes mediate antiviral activity against hepatitis B virus through miR-574-5p. J Infect Dis. 2021;223(4):686–98.
Cai C, Koch B, Morikawa K, et al. Macrophage-derived extracellular vesicles induce long-lasting immunity against hepatitis C virus which is blunted by polyunsaturated fatty acids. Front Immunol. 2018;9:723.
Kouwaki T, Okamotto M, Tsukamoto H, et al. Extracellular vesicles deliver host and virus RNA and regulate innate immune response. Int J Mol Sci 2017;18(3).
Li R, He Y, Zhu Y, et al. Route to rheumatoid arthritis by macrophage-derived microvesicle-coated nanoparticles. Nano Lett. 2019;19(1):124–34.
He H, Ghosh S, Yang H. Nanomedicines for dysfunctional macrophage-associated diseases. J Control Release. 2017;247:106–26.
YAN F, ZHONG Z, WANG Y, et al. Exosome-based biomimetic nanoparticles targeted to inflamed joints for enhanced treatment of rheumatoid arthritis. J Nanobiotechnol. 2020;18(1).
Thamphiwatana S, Angsantikul P, Escajadillo T, et al. Macrophage-like nanoparticles concurrently absorbing endotoxins and proinflammatory cytokines for sepsis management. Proc Natl Acad Sci USA. 2017;114(43):11488–93.
Molinaro R, Pasto A, Corbo C, et al. Macrophage-derived nanovesicles exert intrinsic anti-inflammatory properties and prolong survival in sepsis through a direct interaction with macrophages. Nanoscale. 2019;11(28):13576–86.
Sun T, Kwong CHT, Gao C, et al. Amelioration of ulcerative colitis via inflammatory regulation by macrophage-biomimetic nanomedicine. Theranostics. 2020;10(22):10106–19.
Yurkin ST, Wang Z. Cell membrane-derived nanoparticles: emerging clinical opportunities for targeted drug delivery. Nanomedicine. 2017;12(16):2007–19.
Spiller KL, Koh TJ. Macrophage-based therapeutic strategies in regenerative medicine. Adv Drug Deliv Rev. 2017;122:74–83.
Ou Z, Zhong H, Zhang L, et al. Macrophage membrane-coated nanoparticles alleviate hepatic ischemia-reperfusion injury caused by orthotopic liver transplantation by neutralizing endotoxin. Int J Nanomed. 2020;15:4125–38.
Jin Y, Liu R, Xie J, et al. Interleukin-10 deficiency aggravates kidney inflammation and fibrosis in the unilateral ureteral obstruction mouse model. Lab Invest. 2013;93(7):801–11.
Tang T-T, Wang B, Wu M, et al. Extracellular vesicle-encapsulated IL-10 as novel nanotherapeutics against ischemic AKI. Sci Adv. 2020;6(33).
Kumar P, Bose PP. Macrophage ghost entrapped amphotericin B: a novel delivery strategy towards experimental visceral leishmaniasis. Drug Deliv Transl Res. 2019;9(1):249–59.
Nie W, Wu G, Zhang J, et al. Responsive exosome nano-bioconjugates for synergistic cancer therapy. Angewandte Chemie-International Edition. 2020;59(5):2018–22.
Funding
The work was supported by the Hunan Provincial Science and Technology Plan (No. 2016TP2002).
Author information
Authors and Affiliations
Contributions
X.-W.J. and C.-J.X. formulated the idea. X.-W.J. made the figure and wrote the manuscript. C.-J.X. made the table. L.-Y.J., C.-J.X., and W.-J.Y. critically revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xu, WJ., Cai, JX., Li, YJ. et al. Recent progress of macrophage vesicle-based drug delivery systems. Drug Deliv. and Transl. Res. 12, 2287–2302 (2022). https://doi.org/10.1007/s13346-021-01110-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-021-01110-5