Abstract
Precision medicine has put forward the proposition of "precision targeting" for modern drug delivery systems. Inspired by techniques from biology, pharmaceutical sciences, and nanoengineering, numerous targeted drug delivery systems have been developed in recent decades. But the large-scale applications of these systems are limited due to unsatisfactory targeting efficiency, cytotoxicity, easy removability, and instability. As such, the natural endogenous cargo delivery vehicle—extracellular vesicles (EVs)—have sparked significant interest for its unique inherent targeting properties, biocompatibility, transmembrane ability, and circulatory stability. The membranes of EVs are enriched for receptors or ligands that interact with target cells, which endows them with inherent targeting mission. However, most of the natural therapeutic EVs face the fate of being cleared by macrophages, resulting in off-target. Therefore, the specificity of natural EVs delivery systems urgently needs to be further improved. In this review, we comprehensively summarize the inherent homing mechanisms of EVs and the effects of the donor cell source and administration route on targeting specificity. We then go over nanoengineering techniques that modify EVs for improving specific targeting, such as source cell alteration and modification of EVs surface. We also highlight the auxiliary strategies to enhance specificity by changing the external environment, such as magnetic and photothermal. Furthermore, contemporary issues such as the lack of a gold standard for assessing targeting efficiency are discussed. This review will provide new insights into the development of precision medicine delivery systems.
Graphical Abstract

Highlights
-
1.
Tracing the full range of factors affecting EVs targeting mission in terms of EV components, cell sources, and delivery methods.
-
2.
Strategies for EVs surface display and post-isolation methods facilitate the targeted delivery efficiency of the loading therapeutic cargo.
-
3.
EVs complemented by non-invasive strategies such as magnetic fields, ultrasound, and photothermal further enhance targeting efficacy.
Similar content being viewed by others
Introduction
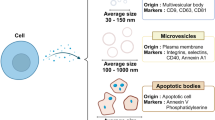
Precision medicine poses new challenges for modern drug delivery systems, the most important of which is targeted precision delivery [1]. Although researchers have spent decades developing targeted drug delivery systems, they still face challenges such as their side effects and failure to deliver precisely on target, being blocked by tissue barriers, being eliminated by the body as a foreigner [2,3,4]. Extracellular vesicles (EVs), 50–1000 nm nanovesicles that play roles in near and distant-range intercellular communication, are natural cargo carriers in our bodies [5,6,7]. Due to limited isolation techniques, EVs are currently divided into microvesicles, exosomes, and other EV subgroups [8], which differ in size, mode of production, and contents, and we focus on the EVs targeting drug delivery systems in this review. Researchers have used it as a novel drug delivery tool because of several properties: (1) excellent biocompatibility and low immunogenicity [9, 10]; (2) the internal vesicle structure provides drug-carrying space and protects the internal material from degradation in circulating tissue fluid [11]; (3) the external lipid bilayer allows it to cross various tissue barriers (99% of molecules are blocked) [12,13,14,15]; and (4) the proteins, lipids, and glycans on the membrane surface give it homing mission and provide natural sites for artificial modifications [16, 17]. As such, if the targeting specificity can be improved further, EVs are expected to be a milestone step for covering the current shortage in targeted precision drug delivery. It is imperative to explore its homing mission and develop solutions to improve its targeting ability.
The main equipment for EVs to accomplish targeted missions is structures enriched on the surface of EVs, such as receptor-ligand proteins. The surface enrichment of EVs with numerous receptors or ligands that have a natural affinity for receptors heavily expressed on the surface of target cells is the method by which they are targeted [18]. Therapeutic drugs or nucleic acid molecules are distributed to tissues or organs employing EVs as transporters when they are released into the extracellular matrix [19, 20]. Numerous studies have demonstrated that released EVs tend to convey specific molecules to the parental cells, which aids EV homing [21]. However, because not all EVs are directed at source cells, researchers need tailor their parental cell selection. Furthermore, different delivery methods can impact EVs distribution, and understanding these characteristics can help to reduce adverse effects and boost EVs bioavailability.
Unfortunately, the surface shape, cellular origin, and mode of administration targeting missions provided to EVs are insufficient to contend with the complicated in vivo microenvironment. Structural fragments such as proteins on the surface of EVs are expected to customize switches for their targeting capabilities. Nano-engineered EVs are those created by changing EVs’ parent cells or directly on EVs [22, 23]. For example, presenting nested peptides or antibody fragments on the surface of EVs to obtain targeted EVs [24, 25], as well as direct modifications to the EV surface, including click chemistry [26] and developing a membrane anchoring platform called "cloaking" to facilitate uptake by target cells [27]. Furthermore, by adding a second switch to EVs, it can be employed to increase EV accumulation in target organs using external auxiliary strategies, including magnetic and photothermal effects [28, 29]. These efforts usher in a new era of EVs-targeted drug delivery systems.
In this review, we provide a comprehensive description of the natural homing mechanism of EVs, starting from the enhanced permeability and retention (EPR) effects of EVs in targeting tumor cells and the specific targeting molecules on the surface of EVs. Furthermore, we discussed the effects of various cell sources and administration routes on EVs reaching the target cells. We highlighted and analyzed numerous methodologies for genetic alteration and direct modification of EVs in the realm of nanoengineered EVs targeting technology. The use of magnetic fields, ultrasound, photothermal and PH-sensing peptides as targeting aids to facilitate the effective accumulation of EVs at the site of injury. Finally, we describe some difficulties associated with EV-targeted drug delivery design systems.
Mission: what determine where the EVs to go?
The components of EVs determine their biodistribution
Tetraspanins are often viewed as surface markers of EVs and are involved in processes such as cell activation and adhesion [30, 31]. For example, tetraspanin-8 (Tspan8) is localized on the EV surface and forms a complex with integrin α4 or CD49d to target CD54-mediated endothelial and pancreatic cells [16, 32]. Another transmembrane protein focused on in the targeting process is integrins. Various combinations of α and β subunits result in different expression forms of integrins, which affect the specific uptake of EVs by target cells [33]. Their binding to target cells is an essential factor in EVs leading to tumor spread in most cases. Ligands highly expressed on the surface of receptor cells, such as a large amount of transferrin (Tf) on the surface of cancer cells, can bind to transferrin receptors (TfR) naturally present on the surface of EVs [34]. Furthermore, milk-derived EVs also express specific proteins on their surface that contribute to targeting uptake. This fleshes out the role of proteins in the specific uptake of EVs of multiple cellular origins. Milk-derived EVs naturally express soluble mucin 1 (MUC1) and lymphocyte function associated antigen-1 (LFA-1) as ligands for DC-SIGN (dendritic cell-specific ICAM-grabbing non-integrin) and ICAM-1 (Intercellular-adhesion molecule 1), respectively [35]. Based on this, Näslund et al. found that milk-derived EVs target monocyte-derived dendritic cells partly through the interaction between DC-SIGN and MUC1 [17]. Glycan modification of milk-derived EVs affects EV capture by human intestinal cells [36].
The phospholipid bilayer of EVs, composed of phosphatidylserine (PS), cholesterol and sphingolipids, is also responsible for the process of EVs targeting to specific cells. For example, PS can target immune cells by binding to the phosphatidylserine receptor (PSR) that causes immunosuppression, but more often, PS is recognized by macrophages and leads to EV clearance [37, 38]. Flannagan et al. demonstrated that the T-cell immunoglobulin mucin 4 (TIM4) can also specifically bind PS to be engulfed by macrophages [39]. Matsumoto et al. found a 66% reduction in phagocytosis of EVs by macrophages after cloaking the PS on the EV surface by annexin V (AnV) [40]. The researchers further explored and concluded that this recognition effect is mainly dependent on the negative charge on the PS surface [40]. Finally, glycans on the surface of EVs also bind to the chemokine (C–C motif) receptor 8 (CCR8) and the soluble ligand chemokine (C–C motif) ligand 18 (CCL18), targeting cancer cells [41]. Thus, the identification of specific molecules on the surface of EVs is the most critical part of the targeted therapy chain (Fig. 1).
Due to the removal of macrophages and the reticuloendothelial system (RES) [42], EVs are often off-target and fail to reach their destination. Therefore, reducing this off-target effect is essential to improve the bioavailability of targeted tissues. In addition to the series of molecules expressed above that promote EVs-specific uptake, molecules that reduce off-target effects. CD47 is an integrin protein widely found on the surface of EVs that reduces phagocytosis by macrophages and improves circulatory stability. Upon the release of EVs into the circulatory system, CD47 readily binds to signal regulatory protein-α (SIRPα) on the macrophage surface, releasing a "don’t eat me" signal [43]. Belhadj et al. proposed a new targeting strategy named "eat me/don’t eat me" [44]. Reduced EVs binding to macrophages means that more EVs can reach the target cells/tissues.
When focusing on tumor cells in receptor cells, we found that natural EVs have been extensively studied for targeting tumors, which is largely attributed to the EPR effect [45, 46]. Specifically, the small diameter of EVs allows them to be retained on the surface of tumor endothelial cells in close proximity to loosely arranged blood vessels (up to 500 nm) [47]. Follow-up experiments have demonstrated that the passive accumulation of EVs within solid tumors is not limited to this simple mechanism, but also depends on the role of immune cells within the tumor [48]. Zhang et al. designed a series of targeted drug delivery systems based on principles including the EPR effect, which included tumor stem cell-derived EVs, ultimately achieving preferential accumulation of drugs within the tumor. The EPR effect can be combined with active targeting strategies to achieve further targeting complementary effects [49]. However, the EPR effect is limited by patient-to-patient heterogeneity and is more appropriate for xenograft models [48, 50].
Cell sources point the way for EVs to go
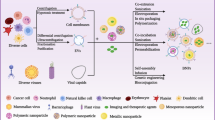
The above targeting effect is mainly determined by the molecules on the surface of EVs, and EVs inherit some proteins from parental cells when they are secreted [51], so the cell source is an important factor influencing targeting (Fig. 2). EVs prefer to be found in cells histogenetically close to them: tumor cells, schwann cells, and microglia aim to homologous tumor cells, peripheral nerve, and central nervous system, respectively [52,53,54]. Moreover, the lower pH of the injured tissue provides a favorable environment for EVs targeting [55]. The parental cells that produce EVs can thus be chosen based on the condition to be treated.
The way EVs target specific cells or tissues. Different donor cells and injection methods affect EVs targeting. Secreted EVs often have the characteristics of parent cells, so most EVs choose to home to cells or tissues that are histologically similar. Most EVs reach the liver via intravenous injection, the adipose tissue via intraperitoneal injection, specific sites such as tumors via local injection, and the intestine via oral administration. Select EVs donor cells and injection methods according to different needs. (Created with BioRender.com.)
Tumor-derived EVs
Due to the organ tropism of extracellular vesicles, most tumor-derived EVs will home to the tumor cells themselves. Based on this feature, EVs can be used as a powerful tool for targeting cancer therapy. Cancer cell membrane antigens E-cadherin is expressed on the surface of urine-derived EVs from prostate cancer patients, which allows EVs to preferentially target homologous tumors [56, 57]. Li et al. isolated tumor cell-derived EVs loading with the anticancer drug doxorubicin (Dox), and injected them into mice intravenously. They found that tumor cell-derived EVs localized to tumors more efficiently, while 64.86% of HeLa cell-produced EVs accumulated in the liver. Moreover, the parental cell tropism of cancer cell-derived EVs was twice as high as that of HeLa cells [52]. Human colorectal cancer cell-derived EVs loaded with anticancer drugs demonstrate concentration-dependent intra-tumor cell accumulation [58].
However, tumor-derived EVs also target other sites, such as immune cells, and endothelial cells. Molecules on tumor-derived EVs that achieve natural targeting include tumor antigens expressed on their surface, which are presented to immune cells as specific stimuli and mediate anti-tumor effects. Chen et al. demonstrated that B lymphoma cell-derived EVs containing heat shock proteins (HSP60 and HSP90) and major histocompatibility complex (MHC) molecules enabled them to act as antigen presentation vehicles, targeting T cells and exhibiting more potent immunostimulatory activity [59]. The presence of integrins inclines melanoma-derived EVs to be distributed in the brain and liver. Because of the metastatic nature of tumors, visual tracking of tumor-derived EVs reveals that they eventually preferentially tend to accumulate in organs such as the liver [60].
Immune cell-derived EVs
Dendritic cell (DC) -derived EVs express MHC on their surface and trigger immune effects [61]. It was shown that dendritic cell-derived EVs can home in on themselves, allowing MHC molecules to be expressed on the surface of dendritic cells and presented to T cells [62, 63]. Segura et al. further demonstrated that mature DC-derived vesicles can express MHC antigen complexes more efficiently compared with immature DCs [64]. Kowal et al. specifically reviewed the journey of DC-derived EVs to reach target cells [18].
LFA-1 is a part of integrins and is involved in cell adhesion and T-cell activation [65]. LFA-1 is present on the surface of EVs produced by macrophages. ICAM-1 is a ligand for LFA-1 acting in the immune process [66]. Yuan et al. exploited this specific binding to achieve targeting of macrophage-derived EVs to cerebrovascular endothelial cells, a process in which C-type lectin receptors also mediated the uptake of EV. Compared to controls, EVs accumulation in the brain was increased 3.6-fold [67]. In addition, T cell-derived EVs could target endothelial cells through the interaction between platelet-reactive protein-1 and cluster of differentiation 47 (CD47) [68]. EVs produced by stimulation of B cells using CD40 and interleukin-4 (IL-4) can be delivered to macrophages or hepatocytes as carriers of miRNA [69].
Mesenchymal stem cells-derived EVs
Mesenchymal stem cells (MSCs) have become an effective treatment for various diseases. After systemic injection, because of the large size of bone marrow mesenchymal stem cells (BMSCs), they quickly became trapped in the pulmonary vascular bed and typically less than 1% of BMSCs could be homed to the target site [70]. MSC-derived EVs offer new insights into alternative MSC-targeted therapies for a variety of diseases: such as type 2 diabetes due to insulin resistance [71], bronchopulmonary dysplasia [72], and neurodegenerative diseases [73]. MSC-derived EVs translocate to lymph nodes via surface glycans bound to lectins [74]. It has also been shown that MSC- derived EVs effectively accumulate in the liver (71% ± 4.0) with less accumulation found in the intestine (3% ± 1.5) [75]. It has also been demonstrated that MSC-derived EVs can inhibit STAT3 signaling and reduce pulmonary hypertension in a mouse model of hypoxic pulmonary hypertension, whereas fibroblast-derived EVs have no such effect [76]. Given the tumor tropism of MSC-derived EVs, researchers often encapsulate nucleic acids and drugs into vesicles to target and inhibit tumor growth. Naseri et al. used MSC-derived EVs loaded with LNA-anti-miR-142-3p to penetrate tumors to induce apoptosis [77]. Lou et al. used MSC-derived EVs-mediated miR-199a targeting to improve drug resistance in hepatocellular carcinoma [78].
Other sources-derived EVs
In addition to the abovementioned common EVs-producing cells, many other cells could be suitable sources for EVs-targeted therapy, including endothelial cells, blood, and milk. The lack of bone targeting strategies has been a bottleneck in the clinical management of degenerative bone lesions [79]. Endothelial cell-secreted EVs have a higher bone targeting capacity compared with other sources of EVs. Song et al. evaluated the bone-targeting ability of endothelial cells, bone marrow MSCs and osteoblast-derived EVs by observing the distribution of intravenously injected Dil-labeled EVs and found that only endothelium-derived EVs had fluorescent signals in bone [80]. The barrier permeability of EVs brings light to brain drug delivery, which is difficult to reach the target location due to the obstruction of the blood–brain barrier. Moreover, the abundant expression of transferrin receptors on the surface of blood-derived EVs can further facilitate the natural homing of EVs to the brain by binding to transferrin. Blood-derived EVs allow for a broader distribution of the drug in the brain, 15 times more than that of freely administered drugs [81,82,83]. Animal-derived EVs are approximately subject to safety concerns, so the use of milk as an EV carrier for targeting has started to become a focus of interest [84]. The surface glycoproteins of lactogenic EVs are important in specific recognition with target cells and EVs uptake [85]. Milk-derived EVs encapsulated with paclitaxel are stable, have low toxicity and exhibit significant targeting and tumor suppression [86].
Administration route determines EVs targeting fate
Different routes of administration lead to different rates of drug arrival at the target organ and eventual accumulation in different organs. For example, when nitroglycerin is used to treat angina pectoris, sublingual administration is often chosen to reach the treatment site more rapidly. The EVs serve as vehicles for drug delivery, and the way they are injected is also critical for targeted therapy. In this section, we will focus on the effect of the mode of administration on the destination of the EVs (Fig. 2).
Intravenous injection
The most common mode of administration is intravenous, considering that intravenous injections allow direct access to the body circulation. Wiklander et al. evaluated the effect of the injection route on EVs distribution and found that intravenous injection accumulated more in the liver compared to other methods (60% ± 3.9) [75]. The results of Brossa et al. showed that intravenous injection of EVs derived from human liver stem cells was the optimal route of administration and significantly inhibited the growth of subcutaneous tumors [87]. In addition to this, there are many examples of tail vein targeted therapies that can target the liver, lung, spleen, kidney and heart [88,89,90,91]. However, the use of such injections often presents significant challenges, including drug accumulation in non-targeted tissues and problems with short half-lives (from minutes to hours) and rapid clearance rates.
Intraperitoneal injection
Intraperitoneal injection is also an effective method for EVs to deliver therapeutic molecules to target cells. Heidari et al. injected mesenchymal stem cell-derived EVs into the intraperitoneal cavity for treating acute colitis caused by dextran sulfate sodium [92]. Nojehdehi et al. demonstrated that intraperitoneal injection of adipose MSC-derived EVs stabilized blood glucose and effectively modulated immune responses compared with controls [93]. Zhou et al. compared intravenous and intraperitoneal administration of EVs and found that intravenous administration made EVs more likely to accumulate in organs that clear drugs, such as the liver, compared with intraperitoneal injection of EVs that exhibited a broad distribution within adipose tissue [94].
Oral administration
With the development of milk as one of the promising sources of EVs, oral EVs have become a more convenient and safer way to administer drugs without the need for a medical professional. Oral administration of milk-derived EVs can be used to observe improvements in joint inflammation. The data conclude that EVs in the treatment group do affect T-cell immune gut microbiota directly or indirectly through inflammation and are important for autoimmune arthritis in IL-1Ra−/− mice and collagen-induced arthritic mice [95]. Nazmek et al. found that EVs modulating the gut microbiota with anti-OVA Ab FLC acquired antigen specificity and used EVs delivery of inhibitory miR-150 to target antigen-presenting cells to suppress delayed hypersensitivity reactions [96]. One researcher compared the distribution of EVs in vivo after intravenous and oral administration. The results showed that intravenous administration tended to be distributed in the liver and spleen, whereas oral administration tended to accumulate in the small intestine [97].
Local administration
In addition to the abovementioned injection methods, local delivery, particularly intratumoral delivery in cancer treatment, has received a lot of attention. This is because it allows the drug to concentrate on a specific site. Taking advantage of this feature, local injection of the drug facilitates its accumulation in the injured tissue. In the prostate cancer mouse model, intratumor-injected EVs were more bound to tumor tissue, and TNF-related apoptosis-inducing ligand (TRAIL+) EVs in cholesterol liposomes resulted in a significant reduction in tumor growth in mice (58%), which depended on the intratumoral EVs administration [98, 99]. Wang et al. observed in vivo the distribution of Dil-labeled extracellular vesicles by intratumor injection and tail vein injection, and showed that intratumor injection could more effectively increase local EVs concentration and inhibit tumor growth [100]. Despite the high delivery efficiency, this method is extremely invasive and often not easily accepted by patients. Moreover, there is a possibility of sudden drug release by local injection.
In summary, EVs are taken up by various routes, and specific routes of injection may result in different amounts of drug accumulating at different sites. Intravenous injection allows EVs to rapidly enter the body circulation and the procedure is simple, allowing the drug to reach and maintain therapeutic concentrations in a short period, but it is easy to off-target and is suitable for systemic diseases; intraperitoneal injection is suitable for metabolic diseases, and oral administration tends to target EVs to digestive system such as the small intestine, while local injection can solve the problem of low targeting efficiency, with rapid drug onset and few side effects, playing an irreplaceable role in tumor treatment. However, it sometimes leads to abnormal accumulation of EVs at a specific site. Therefore, the choice of drug delivery method should be based on the purpose of treatment and the site of targeting. Table 2 summarized the different sources of EVs targeted to various organs by suitable injection routes.
Upgraded: what strategies contribute to the targeting ability?
The specific benefits of unmodified EVs are targeted. Unfortunately, this weak specificity is not always sufficient to achieve the targeted emission of the therapeutic payload. Scholars have turned their attention to nanoengineering EVs for improved targeting. Several approaches led to significant advances in targeted EVs delivery, such as genetic engineering, click chemistry, aptamers, and several complementary targeting strategies.
Surface display
The natural targeting ability of EVs is based on the binding of EVs membrane proteins to receptors on the surface of the cell membrane, but this approach is limited by the need to find highly expressed receptors or ligands on the surface of the EV. Researchers have therefore devised a series of active targeting approaches to address this problem. Surface display means genetic modification of parental cells, and the classical approach is to transfect specific peptide fragments by fusing them to sequences encoding EVs transmembrane peptides, thereby allowing the protein of interest to attach to the vesicle surface. Transmembrane proteins on the surface of EVs, such as integrins, lactadherin (LA) and lysosome-associated membrane protein-2b (Lamp-2b), tetraspanins (CD9, CD63, and CD81), which can be combined with specific targeting ligands for enhancing their targeting ability. Lamp-2b, often found on dendritic cell-derived EVs, is a commonly used membrane protein that contributes to EVs homing [101]. Alvarez-Erviti et al. developed nanoengineering EVs by transfecting plasmids encoding the Lamp-2b constructs into the dendritic cells 4 days before EV purification, and then rabies viral glycoprotein (RVG) peptide was cloned into Lamp2b [24]. This strategy resulted in the generation of EVs exhibiting a higher tropism for the central nervous system. Liang et al. transfected dendritic cells with CAP-GFP-lamp2b plasmid to generate EVs targeting cartilage arthritis. They combined the superior tissue penetration capability of the EVs and the cell-specific targeting of a chondrocyte-affinity peptide (CAP) [25]. Cell-specific binding peptides can be fused to the extracellular domain of Lamp-2b at the N-terminus and be degraded by endosomal proteases during EVs biogenesis and secretion. To suppress peptide loss, a glycosylation motif (GNSTM) can be added to the N-terminus of peptide–Lamp-2b fusions [102].
In addition to Lamp-2b, other membrane proteins on the surface of EVs can serve as anchor points for targeting motifs, for example, cluster of differentiation 9 (CD9) can bind to human antigen R (HuR), an RNA binding protein [103]; the transmembrane protein platelet-derived growth factor receptor (PDGFR) can fuse with the GE11 peptides. Ohno et al. established a novel strategy to engineer the human embryonic kidney 293 (HEK293) cells to generate EVs expressing the GE11 peptide. EVs modified with GE11 peptide bind specifically to the epidermal growth factor receptor (EGFR), which is highly expressed on the surface of tumor cells and is a receptor target in the cancer drug delivery system [104]. The C1C2 structural domain can serve as a bridge between the PS and antigen linkage in EV membrane for targeting [105]. Thus, the design of a fusion of a targeting peptide such as Arg-Gly-Asp (RGD) peptide to the C1C2 structural domain would allow specific delivery of therapeutic EVs to the lesion area [106]. Table 1 demonstrates the use of various EV surface display protein-linked targeting peptides for treating of multiple diseases (Table 1).
Post-isolation methods
The above methods for modifying the parental cells of EVs suffer from low transfection efficiency, and researchers are currently working on further modifications of the secreted EVs to improve targeting efficiency. A nanoengineered EVs membrane targeting platform that also enables EVs to carry targeting peptides has been established, called cloaking (Fig. 3), in which biotinylated molecules can be linked to glycerol-phospholipid-PEG conjugates (DMPE-PEG) inserted into EVs phospholipid bilayers, thereby enhancing EVs uptake by damaged tissues. Antes et al. compared the cloaking with the surface display and found that there was no significant difference in targeting efficacy, and both had their own advantages [27], the specific limitations of which are discussed further below (see Section "Post-isolation methods").
The clocking platform enhances the uptake of extracellular vesicles by the injured heart. A Schematic diagram of the construction of the clocking platform. B In the rat (I/R) model, IschCDC-EV exhibited significant cardiac targeting. C IschHEK-EV s exhibit enhanced targeting ability to damaged myocardium [27].
Researchers have discovered an advanced method for improving the targeting function of EVs: chemically attaching some functional groups to the surface of EV membranes. Copper-catalyzed azide-alkyne cycloaddition (click chemistry) is an efficient and promising technique for introducing various functional azide groups into EVs for subsequent bioorthogonal reactions. Smith et al. applied this click chemistry approach and demonstrated that the method not only allows EVs to be more easily targeted to recipient cells by chemical bonding, but also does not alter the size of the EVs or affect the endocytosis of the target cells [26]. Jia et al. used click chemistry to bind EV membranes to RGE peptides and then produced engineered EVs called RGE-Exo-SPION/Cur with strong glioma targeting ability [110]. However, click chemistry is not applicable to somatic-derived EVs and is non-specific, which limits the recognition of EVs with target cells [117].
Recently, a more effective, simpler and newer technology for actively targeted EV delivery has emerged: nanoengineering of aptamer-targeted EV delivery. Compared with the previously used antibodies and peptides, aptamers have some significant advantages, including higher stability, stronger specificity, less toxicity, and superb tissue penetration [118]. Considering the property that aptamers can bind to the corresponding ligands with high affinity, they have promising applications in the field of targeted therapeutics. E3 aptamers have been shown to specifically target prostate cancer cells in previous studies, so coupling E3 aptamers to the surface of EVs carrying siRNA reduced prostate cancer proliferation and metastasis both in vitro and in vivo [119] (Fig. 4). In addition, Zou and colleagues developed an Apt-Exos nanoplatform for targeted delivery of anticancer drugs in which the cancer-specific nucleic acid aptamer Sgc8 is modified on the exosome surface by hydrophobic interaction [120]. The ability of Apt-Exos to target delivery of DOX was subsequently verified by fluorescent labeling (Fig. 4). Xiang et al. compared the effects of aptamers and peptides on tumor penetration and found that epithelial cell adhesion molecule (EpCAM) aptamers can effectively penetrate tumors and improve circulatory stability [121]. Moreover, Macdonald and colleagues developed a dual-functional aptamer combining EpA and TfR, which successfully delivered the drug to the brain through the blood–brain barrier [122]. RNA aptamer and aptamer-binding protein (ABP) interactions can be used to actively enrich Cas9 ribonucleoproteins from different species into vesicles, and fusion of Com to the N-terminus and C-terminus of CD63 to further increase the activity of gene editing [123]. This contributes to reducing the off-target effects of gene editing. In terms of disease treatment, EVs and aptamers also play a critical role. For example, Tran et al. introduced aptamer-guided EVs diagnostics and therapeutics in detail based on the targeted EV drug treatment system [124]. The study by Luo et al. provided an Apt-bone marrow stromal cell (ST) -derived EVs complex, which is an aptamer-based bone-specific targeting method for EVs delivery [125]. RVG-EVs loaded with aptamers targeted α-synuclein aggregates and eventually save synaptic protein loss and neuronal death in the parkinson disease (PD) mouse model [126]. The most critical advantage of these aptamers, which are called "chemical antibodies," is that they don’t have to consider the differences between batches.

Copyright 2021, Theranostics. C Use of Aptamer-Modified Dox-Encapsulated EVs (Apt-Exos-D) for effective drug delivery to target cancer [120]. Copyright 2019, Analytical Chemistry
Aptamers as "chemical antibodies" for penetration and accumulation in tumors for targeted drug delivery. A Schematic diagram of the use of aptamer-PEG-cholesterol coupling to modify siRNA-loaded EVs (EXOApt-siRNA). B Confocal images showing that EXOApt prefers to accumulate in prostate cancer cells [119].
For precisely targeted drugs to treat disease, CP05 peptide was turned out. CP05 peptide can bind to CD63, a marker on the surface of EVs, with high affinity to the second extracellular loop (Fig. 5). Based on this, CP05 can capture EVs in the circulation and deliver a variety of cargos into specific tissues without any toxicity [127]. This method avoids the modification of therapeutic drugs into EVs, but directly fusion with CP05 peptide. CP05 was used as an anchor peptide to be fused with KV11 and incubated with EVs for 6 h to produce EXOKV11, which could efficiently deliver an anti-angiogenic peptide and suppresses neovascularization of the retina [128]. Similarly, using this method to anchor myostatin propeptide to the surface of EVs can improve muscle function and provide new possibilities for treating duchenne muscular dystrophy (DMD) [115].
Through the specific binding of the CP05 peptide to CD63 on the surface of EVs, the anti-angiogenic peptide was bound to the surface of EVs to generate an EXOKV11 delivery system that targets retinal blood vessels. A Schematic illustration of the preparation of EXOKV11. B Flow cytometry results show that 83.1% of KV11 peptides were bound to EVs based on the effect of CP05. C Mice were injected with KV11 and EXOKV11, respectively, and treated with FITC labeling observed fluorescence. D Under the action of CP05, EXOKV11 entered the eye more efficiently compared with KV11 alone. E In the OIR mouse model, retinal cryosections shows that EXOKV11 targets retinal vessels more efficiently [128].
Some features of the EV surface, such as negative charge, can also be used to produce targeted EVs. To make the EVs more aggregate in the liver tissue, Ryo Tamura et al. efficiently bound positively charged pullulan complexes to EVs with negatively charged surfaces via electrostatic interactions. The modification with cationized pullulan enhances the internalization of the modified EVs via asialoglycoprotein receptor (ASGPR) in hepatocytes or HepG2 cells. The disadvantage of targeting EVs based on electrostatic interactions is the cytotoxicity of pullulan [129]. After treating with positively charged polyethyleneimine (PEI), the intraocular lens surface can be targeted to fix with the negatively charged EVs by electrostatic interactions, and this method is effective in preventing posterior capsular opacification [130]. Nakase and colleagues reported a technique in which EVs were modified with GALA peptides and cationic lipids to effectively deliver macromolecular cargoes, such as dextran and saponin. Specifically, cationic lipids counteract some of the negative charges on the EV membrane surface and enhance their uptake by target cells, while GALA peptides also exhibit high affinity for target cell membranes under the low pH conditions of the tumor microenvironment [131].
The surface of EVs can be modified with the receptors of some targeting ligands to enhance their targeting capability. It is well known that folate (FA) receptor is overexpressed on the surface of the majority of cancer cells. Based on this, Zheng et al. constructed FA/exosome to deliver siRNA for effective cancer inhibition [132]. Yang et al. developed a pH-sensitive superparamagnetic nanoparticle cluster for the precise separation of blood EVs based on the specific recognition of Tf and TfR, and these pH changes do not cause damage to EVs [34]. The main drawback of this approach is the challenge and cost of synthesizing functional ligands rather than simple chemicals such as azide as in click chemistry [133]. With several strategies for nanoengineering EVs as described above, EVs are now achieving breakthroughs targeting multiple organs (Table 2).
Limitations of various modification methods
Although nanoengineered EVs have been extensively tested for targeted therapies, there are limitations to their various approaches. The typical approach to nanoengineered EVs is to transfect genes into the parental cells of the EVs so that the secreted EVs membrane expresses the target protein. The EVs subsequently bind specifically to receptors that are highly expressed at the site of the lesion. However, this gene modification method has some problems, such as safety issues, complex and expensive gene transfection, and unstable transfection, targeting peptides may affect the structure of the EVs surface [118]. One limitation of expressing antibodies on the EVs surface for targeting is the need to use known, validated single-stranded variable fragment sequences to fuse to a display domain such as C1C2 and express in the generating cells. L17E peptide was shown to increase the release of antibodies for targeting the cytosol [82]. The advantage of using EVs of specific parental cell origin is that the secreted EVs carry some of the properties of the parental cells, however, the modification of the secreted EVs can alter or even lose these endogenous properties [145]. The use of click chemistry allows a selective, rapid and effective method that is independent of the origin of the EV cells and is suitable for clinical applications but may inactivate EV surface proteins [146]. The overall negative charge of EVs is critical for uptake by target cells, and targeting approaches developed based on multiple charge interactions can reduce non-specific uptake by receptor cells. Current studies show that cationized pullulan can accelerate endocytosis, but the concern is the cytotoxicity of cationic nanomaterials and the risk of lysosomal degradation after loading is endocytosed into the receptor cell [147] (Fig. 6).
Design methods and characteristics of targeted EVs. EVs can be nanoengineered by modifying parental cells as well as by directly modifying isolated EVs. By fusing the peptides of interest into plasmids and transfecting them into donor cells, the secreted EVs express specific proteins and bind to recipient cells through receptor-ligand interactions (left). Once the EVs are isolated, they can be modified by click chemistry, CP05 peptides, aptamers, electrostatic interactions, and other strategies (right). Therapeutic cargoes can also be encapsulated into nanoengineered EVs. Finally, the advantages and disadvantages of various design approaches are presented so that researchers can choose the appropriate design
Assistance: what external factors facilitate targeting effectiveness?
Magnetic therapy
Surface modification of EVs has already yielded many remarkable results in improving targeting specificity, and surprisingly, researchers have found that targeting effects will be aided by synergistic external stimulation. Several researchers have considered the use of external magnetic fields to enhance the targeting of EVs. For example, magnetically functionalized blood EVs can be effectively targeted to treat cancer by preparing superparamagnetic iron oxide nanoparticles (SPIONs). The surface of EVs produced by reticulocytes within blood contains membrane proteins including TfR that can bind to superparamagnetic nanoparticles coupled to Tf. [28] Harvesting SMNC-EXO based on superparamagnetic magnetite colloidal nanocrystal clusters (SMCNCs) after loading Dox in the presence of magnetic fields (MFs) increases its internalization by tumors [148]. Kim et al. prepared MSC-generated magnetic nanovesicles, and encapsulated iron oxide nanoparticles (IONP) into the vesicles, which can target the site of cerebral ischemic injury with the assistance of a magnetic field [149]. Labeling astrocyte-derived EV with ultrasmall superparamagnetic iron oxide (USPIO) by intranasal perfusion can be distributed to the brain and produce fewer side effects. This provides a new idea for EV-targeted treatment of central system diseases [150].
Photothermal therapy
To achieve precise treatment of cancer, Wang et al. provided a revolutionary strategy, which can achieve nanoengineered EVs active targeting and controlled drug release: using RGD and FA as double ligand modification, combined gold nanorods (AuNRs) to selectively eliminate tumor cells under the irradiation of near-infrared light without affecting the surrounding normal tissues [151]. An emerging technique for EVs includes loading protein into the cytoplasm of target cells through EXPLORE technology (Fig. 7). Yim et al. developed an emerging technique for loading protein into the cytoplasm of target cells via light-inducible reversible protein–protein interactions (PPI). HEK293T cells were exposed to continuous blue light illumination, and the isolated EXPLORs contained significantly higher content of mCherry-cryptochrome 2(mCherry-CRY2) protein. In this system, a truncated version of CRY-interacting basic-helix-loop-helix 1 (CIBN) binds to CD9, cargo protein binds to CRY2, and the cargo protein was guided into the cell membrane surface under blue light irradiation. It is easy to separate the EVs carrying cargo protein from the cell. The subsequent series of experiments proved that using EXPLOR-based therapeutics can more effectively deliver proteins and transcription factors [152].
Schematic diagram of EXPLOR technology. In EXPLOR-producing donor cells, CRY2 protein was fused to a cargo protein, and CIBN was conjugated with a representative marker of EVs, CD9 protein. Blue light illumination induces the reversible PPI between CIBN and CRY2 fusion proteins. With continuous blue light irradiation, the cargo proteins are guided to the inner surface of the cell membrane or the surface of early endosomes. Mature multi-vesicular bodies (MVBs) then readily secrete cargo protein-carrying EVs (EXPLORs) from the cells by membrane fusion with the plasma membrane. After exocytosis, EXPLORs can be easily isolated and purified in vitro. Purified EXPLORs can be used for delivery of the cargo proteins into target cells via membrane fusion or endocytosis processes. Bottom grey boxes highlight the essential steps from EXPLORs biogenesis to target cell delivery [152].
Sonodynamic therapy
Ultrasound can enhance cell membrane permeability and promote the uptake of drugs by cancer cells and is commonly employed as an auxiliary tool for tumor treatment [153]. Liu et al. designed an advanced EVs-based drug delivery system: sinoporphyrin sodium as a sonosensitizer was encapsulated in EV and accumulated in large quantities at the tumor site. Under ultrasound stimulation, sinoporphyrin sodium is continuously released at the desired locations and generates reactive oxygen species (ROS) for therapeutic and imaging effects. As mentioned above, EVs had the highest uptake rate in homologous tumors, which was 2.35 times higher than that of other tumor cell lines. The in vivo fluorescence signal demonstrated the targeting of EVs to the tissues under the action of ultrasound, which ultimately exhibited excellent anti-tumor effects [154]. Similarly, Wang et al. demonstrated the significant role of ultrasound in EVs-targeted tumor therapy. They designed nanoengineered EVs carrying a payload to target tumor tissues with the assistance of ultrasound, improving the tumor immune microenvironment and working together with the EPR effect to inhibit tumor growth [100].
Challenges and outlook
Quantifying targeting efficiency
Current studies of EVs-targeted therapies typically use EVs as nanocarriers loading proteins or therapeutic RNAs that bind specifically to target cells, but little attention has been paid to the evaluation of various nanoengineered EVs-targeted therapeutic approaches. Commonly used methods for observing the distribution patterns of EVs include lipophilic fluorescently labeled EVs, X-ray, and positron emission tomography (PET) [155, 156]. Fluorescence imaging-based EVs monitoring is the most commonly used method for EVs tracking, which shows the accumulation of EVs modification groups over controls, but the technique is not very sensitive and does not provide absolute quantitative results. Therefore, a gold standard for quantifying the efficiency of targeted therapies is lacking.
Adequate dosage of targeted EVs
An ongoing clinical trial for Alzheimer's disease has shown that MSCs-Exos are nasally dropped in amounts of 5–20 μg and is required twice a week for 12 weeks. These exosomes take several weeks to extract (ClinicalTrials.gov Identifier: NCT04388982). The selection of a suitable source of EVs for the experimental purpose was mentioned above as an effective way to address the low EV production. There is no clear method for meeting the volume of EVs required for large-scale clinical trials, and every process for producing therapeutically targeted EVs requires a safe protocol that reduces batch-to-batch variability. Alternatively, EVs mimics that are structurally and functionally similar to EVs but with higher yields are gaining interest in the field of cancer therapy [157]. Heterologous EV hybrids obtained by hybridizing macrophage-derived small cell EVs with synthetic liposomes using extruded membrane fusion techniques to facilitate the specific delivery of oncology drugs [158]. The integration of proteins associated with homing action in EVs mimics successfully confers corresponding tumor homing properties and can be used as an alternative to EVs [159]. Additionally, this approach is simpler, more convenient and high yielding, making it a promising method for targeted delivery of biomolecules.
Environmental effects on EVs targeting
The environment to which EVs are exposed (pH and temperature) can significantly affect their uptake by the cells. Although low pH decreases the stability of EVs to some extent, it increases the uptake of EVs [160]. It has also been shown that an acidic environment alters the lipid composition of EVs and results in EVs possessing a more abundant negative charge on their surface, which allows EVs to better integrate with target cells [161]. It has been shown that the particle size of EVs increases as the temperature decreases. Compared to the freshly isolated EVs, the particle size of EVs increased by 10% and 25% at 4 °C and − 80 °C, respectively, however, the size of EVs decreased at 37 °C. Further characterization of the contents of the EVs at different temperatures showed that the decrease in temperature resulted in the leakage of some proteins [162]. Ge et al. demonstrated that the stability of miRNA at low temperatures was only slightly affected [163]. EVs are taken up more at 37 °C and 60 °C compared to 4 °C [160].
Purity of EVs affects targeting
Although many studies have demonstrated that EVs are promising therapeutic vehicles for targeted drug delivery, the EVs that are currently isolated often contain "impurities" such as apoptotic vesicles or microvesicles [8]. Most people believe that the different biological components of vesicles affect the accuracy and reliability of EV-targeted therapies and that the quality and purity of EVs should be strictly required [164]. Therefore, the method of EV isolation may also be a factor affecting the distribution, as different isolation methods result in different protein/vesicle ratios [165]. The precise isolation of EVs from numerous vesicles has encountered critical challenges today. Current methods of EV isolation include the commonly used ultracentrifugation, gradient ultracentrifugation, and size-exclusion chromatography. Because to the lack of yield and purity of EVs, new enrichment methods have been developed recently that may better preserve the targeting function, such as microfluidic filtering, contact-free sorting, and immunoaffinity enrichment [166]. However, it has also been shown that these "impurities" in the EV extraction process can be involved in targeted therapies along with the EVs [164].
Others
Some studies have also shown that the environment in which cells are cultured, such as hypoxia and low PH, can also enhance the EV production [167] and even enhance the targeting ability [10, 161, 168]. However, the composition of EV produced following cellular stress may be altered and the safety of EVs for therapeutic use at this time should be carefully assessed [160]. Additionally, in the EV-assisted targeting approach, the introduction of exogenous superparamagnetic nanoparticles to the surface of EVs does enhance the targeting properties [169], but it is unknown whether their biocompatibility is affected compared to that of the EVs themselves. Moreover, proteins, lipids, and glycans on the surface of EV membranes all play important roles in the targeting process, but most of the current research has focused on the surface modification of proteins [170]. Therefore, the modification methods for lipids and glycans should be further explored subsequently. Finally, it is uncertain whether the various modification methods we mentioned above for EVs will cause damage to the membrane proteins on their surface and thus affect the targeted delivery function of EVs. Therefore, subsequent experiments are needed to further study these issues and to develop safe and non-toxic strategies for the design of targeted EVs.
Conclusions
EVs, as cell-released vesicles, are emerging as promising nanoplatforms for drug delivery. Natural EVs themselves abundantly express unique membrane surface molecules including proteins, lipids, and glycans that facilitate EV targeting mission. Moreover, EVs injection modalities and parent cells determine the fate of EVs after release into the extracellular matrix. In order to upgrade natural EV-targeted drug delivery capabilities, researchers explored a range of nanoengineering approaches, including surface display and post-isolation methods. We further compared different nanoengineered EVs strategies. Several non-invasive external stimuli can assist natural and nanoengineering EVs in targeted drug delivery. Aggregation of therapeutic EVs to the injured tissue by magnetism, ultrasound or photothermal can reduce off-target effects of EVs and avoid damage to normal tissues. Despite these encouraging achievements, there are still some obstacles to the application of EVs in precision delivery platforms, such as the lack of a gold standard for measuring EVs targeting efficiency and the need to overcome the problem of insufficient EVs yield. Thus, more advanced approaches are still needed to expand the potential of EVs in targeted therapies. In the future, we expect a multidisciplinary effort to achieve a scale-up production of quality-controlled targeted EVs and break the therapeutic bottleneck of EVs in targeted therapy.
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Abbreviations
- AA-PEG:
-
Aminoethylanisamide-polyethylene glycol
- ABP:
-
Aptamer-binding protein
- AnV:
-
Annexin V
- ASGPR:
-
Asialoglycoprotein receptor
- AuNRs:
-
Gold nanorods
- BBB:
-
Blood–brain barrier
- BMSC:
-
Bone marrow mesenchymal stem cells
- BSM:
-
Bone-marrow derived macrophages
- CAP:
-
Chondrocyte-affinity peptide
- CCL18:
-
Chemokine (C–C motif) ligand 18
- CCR8:
-
Chemokine (C–C motif) receptor 8
- CD47:
-
Cluster of differentiation 47
- CDCs:
-
Cardiosphere-derived cells
- CIBN:
-
CRY-interacting basic-helix-loop-helix 1
- CML:
-
Chronic myeloid leukemia
- CMP:
-
Cardiomyocyte specific peptide
- CPCs:
-
Cardiac progenitor cells
- Cur:
-
Curcuminoid
- CXCR4:
-
C-X-C motif chemokine receptor 4
- DC:
-
Dendritic cell
- DC-SIGN:
-
Dendritic cell-specific ICAM-grabbing non-integrin
- DMD:
-
Duchenne muscular dystrophy
- DMPE-PEG:
-
Glycerol-phospholipid-PEG conjugates
- Dox:
-
Doxorubicin
- EC:
-
Endothelial cells
- EGF:
-
Epidermal growth factor
- EGFR:
-
Epidermal growth factor receptor
- EpCAM:
-
Epithelial cell adhesion molecule
- EPR:
-
Enhanced permeability and retention
- EVs:
-
Extracellular vesicles
- EXO:
-
Exosome
- EXOKV11 :
-
EXO-linked KV11 peptide
- FA:
-
Folate
- GAP43:
-
Growth-associated protein-43
- HCT-1165FR:
-
5-FU-resistant derivative of the HCT-116 human colon cancer cell line
- HDEA:
-
Hyaluronic acid grafted with 3-(diethylamino) propylamine
- HEK293:
-
Human embryonic kidney 293
- HuR:
-
Human antigen R
- ICAM-1:
-
Intercellular-adhesion molecule 1
- ICDCs:
-
Cardiosphere-derived cells
- IL3:
-
Interleukin 3
- IL-4:
-
Interleukin-4
- imDC:
-
Immature dendritic cells
- IMTP:
-
Ischemic myocardium-targeting peptide
- IONP:
-
Iron oxide nanoparticles
- KV11:
-
A known anti-angiogenic factor
- LA:
-
Lactadherin
- Lamp-2b:
-
Lysosome-associated membrane protein-2b
- LFA-1:
-
Lymphocyte function associated antigen 1
- mCherry-CRY2:
-
MCherry-cryptochrome 2
- MFs:
-
Magnetic fields
- MHC:
-
Major histocompatibility complex
- MOR:
-
Opioid receptor Mu
- MSC:
-
Mesenchymal stem cells
- MUC1:
-
Mucin 1(MUC1)
- MVBs:
-
Multi-vesicular bodies
- NE:
-
Neutrophil
- NRP1:
-
Neuropilin1
- NRP2:
-
Neuropilin2
- OA:
-
Osteoarthritis
- OIR:
-
Oxygen-induced retinopathy
- PD:
-
Parkinson disease
- PDGFR:
-
Platelet-derived growth factor receptor
- PEG:
-
Polyethylene glycol
- PEI:
-
Polyethyleneimine
- PET:
-
Positron emission tomography
- PPI:
-
Protein–protein interactions
- PS:
-
Phosphatidylserine
- PSR:
-
Phosphatidylserine receptor
- PTX:
-
Paclitaxel
- Que:
-
Quercetin
- Raw264.7:
-
Leukemia cells in mouse macrophage
- RES:
-
Reticuloendothelial system
- RGD:
-
Arg-Gly-Asp
- RGE:
-
Neuropilin-1-targeted peptide
- ROS:
-
Reactive oxygen species
- RVG:
-
Rabies viral glycoprotein
- SD rat:
-
Sprague–Dawley rat
- SF-MSC:
-
Synovial fluid-derived mesenchymal stem cells
- SIRPα:
-
Signal regulatory protein-α
- SMCNCs:
-
Superparamagnetic magnetite colloidal nanocrystal clusters
- SPIONs:
-
Superparamagnetic iron oxide nanoparticles
- ST:
-
Stromal cell
- Tf:
-
Transferrin
- TfR:
-
Transferrin receptors
- TIM4:
-
T-cell immunoglobulin mucin 4
- TRAIL:
-
TNF-related apoptosis-inducing ligand
- Tstan8:
-
Tetraspanin-8
- USPIO:
-
Ultrasmall superparamagnetic iron oxide
References
Manzari MT, Shamay Y, Kiguchi H, Rosen N, Scaltriti M, Heller DA. Targeted drug delivery strategies for precision medicines. Nat Rev Mater. 2021;6:351–70.
Arias-Alpizar G, Kong L, Vlieg RC, Rabe A, Papadopoulou P, Meijer MS, et al. Light-triggered switching of liposome surface charge directs delivery of membrane impermeable payloads in vivo. Nat Commun. 2020;11:3638.
Sun T, Zhang Y, Power C, Alexander PM, Sutton JT, Aryal M, et al. Closed-loop control of targeted ultrasound drug delivery across the blood–brain/tumor barriers in a rat glioma model. Proc Natl Acad Sci USA. 2017;114:E10281–90.
Erel-Akbaba G, Carvalho LA, Tian T, Zinter M, Akbaba H, Obeid PJ, et al. Radiation-induced targeted nanoparticle-based gene delivery for brain tumor therapy. ACS Nano. 2019;13:4028–40.
Kooijmans SAA, Aleza CG, Roffler SR, van Solinge WW, Vader P, Schiffelers RM. Display of GPI-anchored anti-EGFR nanobodies on extracellular vesicles promotes tumour cell targeting. J Extracell Vesicles. 2016;5:31053.
Martins-Marques T, Pinho MJ, Zuzarte M, Oliveira C, Pereira P, Sluijter JPG, et al. Presence of Cx43 in extracellular vesicles reduces the cardiotoxicity of the anti-tumour therapeutic approach with doxorubicin. J Extracell Vesicles. 2016;5:32538.
Nojima H, Freeman CM, Schuster RM, Japtok L, Kleuser B, Edwards MJ, et al. Hepatocyte exosomes mediate liver repair and regeneration via sphingosine-1-phosphate. J Hepatol. 2016;64:60–8.
Lötvall J, Hill AF, Hochberg F, Buzás EI, Di Vizio D, Gardiner C, et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles. 2014;3:26913.
Cheng Q, Shi X, Han M, Smbatyan G, Lenz H-J, Zhang Y. Reprogramming exosomes as nanoscale controllers of cellular immunity. J Am Chem Soc. 2018;140:16413–7.
Jung KO, Jo H, Yu JH, Gambhir SS, Pratx G. Development and MPI tracking of novel hypoxia-targeted theranostic exosomes. Biomaterials. 2018;177:139–48.
Guo X, Lv X, Ru Y, Zhou F, Wang N, Xi H, et al. Circulating exosomal gastric cancer-associated long noncoding RNA1 as a biomarker for early detection and monitoring progression of gastric cancer: a multiphase study. JAMA Surg. 2020;155:572–9.
Thakur A, Qiu G, Xu C, Han X, Yang T, Ng SP, et al. Label-free sensing of exosomal MCT1 and CD147 for tracking metabolic reprogramming and malignant progression in glioma. Sci Adv. 2020;6:eaaz6119.
Azizi M, Dianat-Moghadam H, Salehi R, Farshbaf M, Iyengar D, Sau S, et al. Interactions between tumor biology and targeted nanoplatforms for imaging applications. Adv Func Mater. 2020;30:1910402.
Rosenblum D, Joshi N, Tao W, Karp JM, Peer D. Progress and challenges towards targeted delivery of cancer therapeutics. Nat Commun. 2018;9:1410.
Yang Z, Shi J, Xie J, Wang Y, Sun J, Liu T, et al. Large-scale generation of functional mRNA-encapsulating exosomes via cellular nanoporation. Nat Biomed Eng. 2020;4:69–83.
Rana S, Yue S, Stadel D, Zöller M. Toward tailored exosomes: the exosomal tetraspanin web contributes to target cell selection. Int J Biochem Cell Biol. 2012;44:1574–84.
Näslund TI, Paquin-Proulx D, Paredes PT, Vallhov H, Sandberg JK, Gabrielsson S. Exosomes from breast milk inhibit HIV-1 infection of dendritic cells and subsequent viral transfer to CD4+ T cells. AIDS. 2014;28:171–80.
Kowal J, Tkach M. Dendritic cell extracellular vesicles. Int Rev Cell Mol Biol. 2019;349:213–49.
Wang Y, Cao Z, Wei Q, Ma K, Hu W, Huang Q, et al. VH298-loaded extracellular vesicles released from gelatin methacryloyl hydrogel facilitate diabetic wound healing by HIF-1α-mediated enhancement of angiogenesis. Acta Biomater. 2022;147:342–55.
Gong M, Wang M, Xu J, Yu B, Wang Y-G, Liu M, et al. Nano-sized extracellular vesicles secreted from GATA-4 modified mesenchymal stem cells promote angiogenesis by delivering Let-7 miRNAs. Cells. 2022;11:1573.
Garofalo M, Villa A, Crescenti D, Marzagalli M, Kuryk L, Limonta P, et al. Heterologous and cross-species tropism of cancer-derived extracellular vesicles. Theranostics. 2019;9:5681–93.
Kojima R, Bojar D, Rizzi G, Hamri GC-E, El-Baba MD, Saxena P, et al. Designer exosomes produced by implanted cells intracerebrally deliver therapeutic cargo for Parkinson’s disease treatment. Nat Commun. 2018;9:1305.
Yerneni SS, Lathwal S, Shrestha P, Shirwan H, Matyjaszewski K, Weiss L, et al. Rapid on-demand extracellular vesicle augmentation with versatile oligonucleotide tethers. ACS Nano. 2019;13:10555–65.
Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJA. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29:341–5.
Liang Y, Xu X, Li X, Xiong J, Li B, Duan L, et al. Chondrocyte-targeted microRNA delivery by engineered exosomes toward a cell-free osteoarthritis therapy. ACS Appl Mater Interfaces. 2020;12:36938–47.
Smyth T, Petrova K, Payton NM, Persaud I, Redzic JS, Graner MW, et al. Surface functionalization of exosomes using click chemistry. Bioconjug Chem. 2014;25:1777–84.
Antes TJ, Middleton RC, Luther KM, Ijichi T, Peck KA, Liu WJ, et al. Targeting extracellular vesicles to injured tissue using membrane cloaking and surface display. J Nanobiotechnol. 2018;16:61.
Zhuang M, Du D, Pu L, Song H, Deng M, Long Q, et al. SPION-decorated exosome delivered BAY55-9837 targeting the pancreas through magnetism to improve the blood GLC response. Small. 2019;15: e1903135.
Ma Y, Zhang Y, Han R, Li Y, Zhai Y, Qian Z, et al. A cascade synergetic strategy induced by photothermal effect based on platelet exosome nanoparticles for tumor therapy. Biomaterials. 2022;282: 121384.
Pluchino S, Smith JA. Explicating exosomes: reclassifying the rising stars of intercellular communication. Cell. 2019;177:225–7.
Jeppesen DK, Fenix AM, Franklin JL, Higginbotham JN, Zhang Q, Zimmerman LJ, et al. Reassessment of exosome composition. Cell. 2019;177:428-45.e18.
Nazarenko I, Rana S, Baumann A, McAlear J, Hellwig A, Trendelenburg M, et al. Cell surface tetraspanin Tspan8 contributes to molecular pathways of exosome-induced endothelial cell activation. Can Res. 2010;70:1668–78.
Hoshino A, Costa-Silva B, Shen T-L, Rodrigues G, Hashimoto A, Tesic Mark M, et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527:329–35.
Yang L, Han D, Zhan Q, Li X, Shan P, Hu Y, et al. Blood TfR+ exosomes separated by a pH-responsive method deliver chemotherapeutics for tumor therapy. Theranostics. 2019;9:7680–96.
Koning N, Kessen SFM, Van Der Voorn JP, Appelmelk BJ, Jeurink PV, Knippels LMJ, et al. Human milk blocks DC-SIGN-pathogen interaction via MUC1. Front Immunol. 2015;6:112.
Sukreet S, Silva BVRE, Adamec J, Cui J, Zempleni J. Galactose and sialo-galactose modifications in glycoproteins on the surface of bovine milk exosome are essential for exosome uptake in non-bovine species (OR34-07-19). Curr Develop Nutr. 2019;3:Supplement_1.
Park M, Kang KW. Phosphatidylserine receptor-targeting therapies for the treatment of cancer. Arch Pharmacal Res. 2019;42:617–28.
Bhatta M, Shenoy GN, Loyall JL, Gray BD, Bapardekar M, Conway A, et al. Novel phosphatidylserine-binding molecule enhances antitumor T-cell responses by targeting immunosuppressive exosomes in human tumor microenvironments. J Immunother Cancer. 2021;9:e003148.
Flannagan RS, Canton J, Furuya W, Glogauer M, Grinstein S. The phosphatidylserine receptor TIM4 utilizes integrins as coreceptors to effect phagocytosis. Mol Biol Cell. 2014;25:1511–22.
Matsumoto A, Takahashi Y, Nishikawa M, Sano K, Morishita M, Charoenviriyakul C, et al. Role of phosphatidylserine-derived negative surface charges in the recognition and uptake of intravenously injected B16BL6-derived exosomes by macrophages. J Pharm Sci. 2017;106:168–75.
Berenguer J, Lagerweij T, Zhao XW, Dusoswa S, van der Stoop P, Westerman B, et al. Glycosylated extracellular vesicles released by glioblastoma cells are decorated by CCL18 allowing for cellular uptake via chemokine receptor CCR8. J Extracell Vesicles. 2018;7:1446660.
Wan Z, Zhao L, Lu F, Gao X, Dong Y, Zhao Y, et al. Mononuclear phagocyte system blockade improves therapeutic exosome delivery to the myocardium. Theranostics. 2020;10:218–30.
Koh E, Lee EJ, Nam G-H, Hong Y, Cho E, Yang Y, et al. Exosome-SIRPα, a CD47 blockade increases cancer cell phagocytosis. Biomaterials. 2017;121:121–9.
Belhadj Z, He B, Deng H, Song S, Zhang H, Wang X, et al. A combined “eat me/don’t eat me” strategy based on extracellular vesicles for anticancer nanomedicine. J Extracell Vesicles. 2020;9:1806444.
Ma Q, Liang M, Wu Y, Dou C, Xu J, Dong S, et al. Small extracellular vesicles deliver osteolytic effectors and mediate cancer-induced osteolysis in bone metastatic niche. J Extracell Vesicles. 2021;10: e12068.
Liang G, Zhu Y, Ali DJ, Tian T, Xu H, Si K, et al. Engineered exosomes for targeted co-delivery of miR-21 inhibitor and chemotherapeutics to reverse drug resistance in colon cancer. J Nanobiotechnol. 2020;18:10.
Wolfram J, Ferrari M. Clinical cancer nanomedicine. Nano Today. 2019;25:85–98.
Shi Y, van der Meel R, Chen X, Lammers T. The EPR effect and beyond: strategies to improve tumor targeting and cancer nanomedicine treatment efficacy. Theranostics. 2020;10:7921–4.
Tu Y, Dong Y, Wang K, Shen S, Yuan Y, Wang J. Intercellular delivery of bioorthogonal chemical receptors for enhanced tumor targeting and penetration. Biomaterials. 2020;259: 120298.
Walker S, Busatto S, Pham A, Tian M, Suh A, Carson K, et al. Extracellular vesicle-based drug delivery systems for cancer treatment. Theranostics. 2019;9:8001–17.
Woo H-K, Cho YK, Lee CY, Lee H, Castro CM, Lee H. Characterization and modulation of surface charges to enhance extracellular vesicle isolation in plasma. Theranostics. 2022;12:1988–98.
Qiao L, Hu S, Huang K, Su T, Li Z, Vandergriff A, et al. Tumor cell-derived exosomes home to their cells of origin and can be used as Trojan horses to deliver cancer drugs. Theranostics. 2020;10:3474–87.
Hercher D, Nguyen MQ, Dworak H. Extracellular vesicles and their role in peripheral nerve regeneration. Exp Neurol. 2022;350: 113968.
Zhang L, Wei W, Ai X, Kilic E, Hermann DM, Venkataramani V, et al. Extracellular vesicles from hypoxia-preconditioned microglia promote angiogenesis and repress apoptosis in stroke mice via the TGF-β/Smad2/3 pathway. Cell Death Dis. 2021;12:1068.
Gong C, Zhang X, Shi M, Li F, Wang S, Wang Y, et al. Tumor exosomes reprogrammed by low pH are efficient targeting vehicles for smart drug delivery and personalized therapy against their homologous tumor. Adv Sci. 2021;8:2002787.
Pan S, Zhang Y, Huang M, Deng Z, Zhang A, Pei L, et al. Urinary exosomes-based engineered nanovectors for homologously targeted chemo-chemodynamic prostate cancer therapy via abrogating EGFR/AKT/NF-kB/IkB signaling. Biomaterials. 2021;275: 120946.
Zhu J-Y, Zheng D-W, Zhang M-K, Yu W-Y, Qiu W-X, Hu J-J, et al. Preferential cancer cell self-recognition and tumor self-targeting by coating nanoparticles with homotypic cancer cell membranes. Nano Lett. 2016;16:5895–901.
Li Y, Gao Y, Gong C, Wang Z, Xia Q, Gu F, et al. A33 antibody-functionalized exosomes for targeted delivery of doxorubicin against colorectal cancer. Nanomed Nanotechnol Biol Med. 2018;14:1973–85.
Chen W, Wang J, Shao C, Liu S, Yu Y, Wang Q, et al. Efficient induction of antitumor T cell immunity by exosomes derived from heat-shocked lymphoma cells. Eur J Immunol. 2006;36:1598–607.
Ye Y, Shi Q, Yang T, Xie F, Zhang X, Xu B, et al. Visualized tracking of tumor-derived extracellular vesicles using CRISPR-Cas9 system. Technol Cancer Res Treat. 2022;21:15330338221085370.
Buschow SI, van Balkom BWM, Aalberts M, Heck AJR, Wauben M, Stoorvogel W. MHC class II-associated proteins in B-cell exosomes and potential functional implications for exosome biogenesis. Immunol Cell Biol. 2010;88:851–6.
Montecalvo A, Shufesky WJ, Stolz DB, Sullivan MG, Wang Z, Divito SJ, et al. Exosomes as a short-range mechanism to spread alloantigen between dendritic cells during T cell allorecognition. J Immunol. 2008;180:3081–90.
André F, Chaput N, Schartz NEC, Flament C, Aubert N, Bernard J, et al. Exosomes as potent cell-free peptide-based vaccine. I. Dendritic cell-derived exosomes transfer functional MHC class I/peptide complexes to dendritic cells. J Immunol. 2004;172:2126–36.
Segura E, Nicco C, Lombard B, Véron P, Raposo G, Batteux F, et al. ICAM-1 on exosomes from mature dendritic cells is critical for efficient naive T-cell priming. Blood. 2005;106:216–23.
Cherry LK, Li X, Schwab P, Lim B, Klickstein LB. RhoH is required to maintain the integrin LFA-1 in a nonadhesive state on lymphocytes. Nat Immunol. 2004;5:961–7.
Makgoba MW, Sanders ME, Ginther Luce GE, Dustin ML, Springer TA, Clark EA, et al. ICAM-1 a ligand for LFA-1-dependent adhesion of B, T and myeloid cells. Nature. 1988;331:86–8.
Yuan D, Zhao Y, Banks WA, Bullock KM, Haney M, Batrakova E, et al. Macrophage exosomes as natural nanocarriers for protein delivery to inflamed brain. Biomaterials. 2017;142:1–12.
Kaur S, Singh SP, Elkahloun AG, Wu W, Abu-Asab MS, Roberts DD. CD47-dependent immunomodulatory and angiogenic activities of extracellular vesicles produced by T cells. Matrix Biol. 2014;37:49–59.
Momen-Heravi F, Bala S, Bukong T, Szabo G. Exosome-mediated delivery of functionally active miRNA-155 inhibitor to macrophages. Nanomed Nanotechnol Biol Med. 2014;10:1517–27.
Fischer UM, Harting MT, Jimenez F, Monzon-Posadas WO, Xue H, Savitz SI, et al. Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first-pass effect. Stem Cells Develop. 2009;18:683–92.
Su T, Xiao Y, Xiao Y, Guo Q, Li C, Huang Y, et al. Bone marrow mesenchymal stem cells-derived exosomal MiR-29b-3p regulates aging-associated insulin resistance. ACS Nano. 2019;13:2450–62.
Willis GR, Fernandez-Gonzalez A, Anastas J, Vitali SH, Liu X, Ericsson M, et al. Mesenchymal stromal cell exosomes ameliorate experimental bronchopulmonary dysplasia and restore lung function through macrophage immunomodulation. Am J Respir Crit Care Med. 2018;197:104–16.
Riazifar M, Mohammadi MR, Pone EJ, Yeri A, Lässer C, Segaliny AI, et al. Stem cell-derived exosomes as nanotherapeutics for autoimmune and neurodegenerative disorders. ACS Nano. 2019;13:6670–88.
Shimoda A, Tahara Y, Sawada S-I, Sasaki Y, Akiyoshi K. Glycan profiling analysis using evanescent-field fluorescence-assisted lectin array: importance of sugar recognition for cellular uptake of exosomes from mesenchymal stem cells. Biochem Biophys Res Commun. 2017;491:701–7.
Wiklander OPB, Nordin JZ, O’Loughlin A, Gustafsson Y, Corso G, Mäger I, et al. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J Extracell Vesicles. 2015;4:26316.
Lee C, Mitsialis SA, Aslam M, Vitali SH, Vergadi E, Konstantinou G, et al. Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertension. Circulation. 2012;126:2601–11.
Naseri Z, Oskuee RK, Forouzandeh-Moghadam M, Jaafari MR. Delivery of LNA-antimiR-142-3p by mesenchymal stem cells-derived exosomes to breast cancer stem cells reduces tumorigenicity. Stem Cell Rev Rep. 2020;16:541–56.
Lou G, Chen L, Xia C, Wang W, Qi J, Li A, et al. MiR-199a-modified exosomes from adipose tissue-derived mesenchymal stem cells improve hepatocellular carcinoma chemosensitivity through mTOR pathway. J Exp Clin Cancer Res. 2020;39:4.
Hu Y, Li X, Zhang Q, Gu Z, Luo Y, Guo J, et al. Exosome-guided bone targeted delivery of antagomir-188 as an anabolic therapy for bone loss. Bioact Mater. 2021;6:2905–13.
Song H, Li X, Zhao Z, Qian J, Wang Y, Cui J, et al. Reversal of osteoporotic activity by endothelial cell-secreted bone targeting and biocompatible exosomes. Nano Lett. 2019;19:3040–8.
Qu M, Lin Q, Huang L, Fu Y, Wang L, He S, et al. Dopamine-loaded blood exosomes targeted to brain for better treatment of Parkinson’s disease. J Control Release. 2018;287:156–66.
Zhan Q, Yi K, Qi H, Li S, Li X, Wang Q, et al. Engineering blood exosomes for tumor-targeting efficient gene/chemo combination therapy. Theranostics. 2020;10:7889–905.
Zhan Q, Yi K, Cui X, Li X, Yang S, Wang Q, et al. Blood exosomes-based targeted delivery of cPLA2 siRNA and metformin to modulate glioblastoma energy metabolism for tailoring personalized therapy. Neuro Oncol. 2022;noac071.
Melnik BC, John SM, Schmitz G. Milk: an exosomal microRNA transmitter promoting thymic regulatory T cell maturation preventing the development of atopy? J Transl Med. 2014;12:43.
Chen W, Wang R, Li D, Zuo C, Wen P, Liu H, et al. Comprehensive analysis of the glycome and glycoproteome of bovine milk-derived exosomes. J Agric Food Chem. 2020;68:12692–701.
Agrawal AK, Aqil F, Jeyabalan J, Spencer WA, Beck J, Gachuki BW, et al. Milk-derived exosomes for oral delivery of paclitaxel. Nanomed Nanotechnol Biol Med. 2017;13:1627–36.
Brossa A, Fonsato V, Grange C, Tritta S, Tapparo M, Calvetti R, et al. Extracellular vesicles from human liver stem cells inhibit renal cancer stem cell-derived tumor growth in vitro and in vivo. Int J Cancer. 2020;147:1694–706.
Németh K, Varga Z, Lenzinger D, Visnovitz T, Koncz A, Hegedűs N, et al. Extracellular vesicle release and uptake by the liver under normo- and hyperlipidemia. Cell Mol Life Sci. 2021;78:7589–604.
Wei Z, Chen Z, Zhao Y, Fan F, Xiong W, Song S, et al. Mononuclear phagocyte system blockade using extracellular vesicles modified with CD47 on membrane surface for myocardial infarction reperfusion injury treatment. Biomaterials. 2021;275: 121000.
Zhang K, Chen S, Sun H, Wang L, Li H, Zhao J, et al. two-photon microscopy reveals the contribution of Sox9 cell to kidney regeneration in a mouse model with extracellular vesicle treatment. J Biol Chem. 2020;295:12203–13.
Hong CM, Gangadaran P, Oh JM, Rajendran RL, Gopal A, Zhu L, et al. Radioiodine labeling and in vivo trafficking of extracellular vesicles. Sci Rep. 2021;11:5041.
Heidari N, Abbasi-Kenarsari H, Namaki S, Baghaei K, Zali MR, Ghaffari Khaligh S, et al. Adipose-derived mesenchymal stem cell-secreted exosome alleviates dextran sulfate sodium-induced acute colitis by Treg cell induction and inflammatory cytokine reduction. J Cell Physiol. 2021;236:5906–20.
Nojehdehi S, Soudi S, Hesampour A, Rasouli S, Soleimani M, Hashemi SM. Immunomodulatory effects of mesenchymal stem cell-derived exosomes on experimental type-1 autoimmune diabetes. J Cell Biochem. 2018;119:9433–43.
Zhou X, Li Z, Sun W, Yang G, Xing C, Yuan L. Delivery efficacy differences of intravenous and intraperitoneal injection of exosomes: perspectives from tracking dye labeled and MiRNA encapsulated exosomes. Curr Drug Deliv. 2020;17:186–94.
Arntz OJ, Pieters BCH, Oliveira MC, Broeren MGA, Bennink MB, de Vries M, et al. Oral administration of bovine milk derived extracellular vesicles attenuates arthritis in two mouse models. Mol Nutr Food Res. 2015;59:1701–12.
Nazimek K, Bryniarski K, Ptak W, Groot Kormelink T, Askenase PW. Orally administered exosomes suppress mouse delayed-type hypersensitivity by delivering miRNA-150 to antigen-primed macrophage APC targeted by exosome-surface anti-peptide antibody light chains. Int J Mol Sci. 2020;21:5540.
Umezu T, Takanashi M, Murakami Y, Ohno S-I, Kanekura K, Sudo K, et al. Acerola exosome-like nanovesicles to systemically deliver nucleic acid medicine via oral administration. Mol Ther Methods Clin Dev. 2021;21:199–208.
Rivoltini L, Chiodoni C, Squarcina P, Tortoreto M, Villa A, Vergani B, et al. TNF-related apoptosis-inducing ligand (TRAIL)-armed exosomes deliver proapoptotic signals to tumor site. Clin Cancer Res. 2016;22:3499–512.
Smyth T, Kullberg M, Malik N, Smith-Jones P, Graner MW, Anchordoquy TJ. Biodistribution and delivery efficiency of unmodified tumor-derived exosomes. J Control Release. 2015;199:145–55.
Wang D, Wan Z, Yang Q, Chen J, Liu Y, Lu F, et al. Sonodynamical reversion of immunosuppressive microenvironment in prostate cancer via engineered exosomes. Drug Delivery. 2022;29:702–13.
Wilke S, Krausze J, Büssow K. Crystal structure of the conserved domain of the DC lysosomal associated membrane protein: implications for the lysosomal glycocalyx. BMC Biol. 2012;10:62.
Hung ME, Leonard JN. Stabilization of exosome-targeting peptides via engineered glycosylation. J Biol Chem. 2015;290:8166–72.
Li Z, Zhou X, Wei M, Gao X, Zhao L, Shi R, et al. In vitro and in vivo RNA inhibition by CD9-HuR functionalized exosomes encapsulated with miRNA or CRISPR/dCas9. Nano Lett. 2019;19:19–28.
Ohno S-I, Takanashi M, Sudo K, Ueda S, Ishikawa A, Matsuyama N, et al. Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol Ther. 2013;21:185–91.
Rountree RB, Mandl SJ, Nachtwey JM, Dalpozzo K, Do L, Lombardo JR, et al. Exosome targeting of tumor antigens expressed by cancer vaccines can improve antigen immunogenicity and therapeutic efficacy. Can Res. 2011;71:5235–44.
Tian T, Cao L, He C, Ye Q, Liang R, You W, et al. Targeted delivery of neural progenitor cell-derived extracellular vesicles for anti-inflammation after cerebral ischemia. Theranostics. 2021;11:6507–21.
Wang J, Li W, Lu Z, Zhang L, Hu Y, Li Q, et al. The use of RGD-engineered exosomes for enhanced targeting ability and synergistic therapy toward angiogenesis. Nanoscale. 2017;9:15598–605.
Wang X, Chen Y, Zhao Z, Meng Q, Yu Y, Sun J, et al. Engineered exosomes with ischemic myocardium-targeting peptide for targeted therapy in myocardial infarction. J Am Heart Assoc. 2018;7: e008737.
Mentkowski KI, Lang JK. Exosomes engineered to express a cardiomyocyte binding peptide demonstrate improved cardiac retention in vivo. Sci Rep. 2019;9:10041.
Jia G, Han Y, An Y, Ding Y, He C, Wang X, et al. NRP-1 targeted and cargo-loaded exosomes facilitate simultaneous imaging and therapy of glioma in vitro and in vivo. Biomaterials. 2018;178:302–16.
Kim G, Kim M, Lee Y, Byun JW, Hwang DW, Lee M. Systemic delivery of microRNA-21 antisense oligonucleotides to the brain using T7-peptide decorated exosomes. J Control Release. 2020;317:273–81.
Bai J, Duan J, Liu R, Du Y, Luo Q, Cui Y, et al. Engineered targeting tLyp-1 exosomes as gene therapy vectors for efficient delivery of siRNA into lung cancer cells. Asian J Pharm Sci. 2020;15:461–71.
Xu X, Liang Y, Li X, Ouyang K, Wang M, Cao T, et al. Exosome-mediated delivery of kartogenin for chondrogenesis of synovial fluid-derived mesenchymal stem cells and cartilage regeneration. Biomaterials. 2021;269: 120539.
Bellavia D, Raimondo S, Calabrese G, Forte S, Cristaldi M, Patinella A, et al. Interleukin 3- receptor targeted exosomes inhibit and chronic myelogenous leukemia cell growth. Theranostics. 2017;7:1333–45.
Ran N, Gao X, Dong X, Li J, Lin C, Geng M, et al. Effects of exosome-mediated delivery of myostatin propeptide on functional recovery of mdx mice. Biomaterials. 2020;236: 119826.
Kanuma T, Yamamoto T, Kobiyama K, Moriishi E, Masuta Y, Kusakabe T, et al. CD63-mediated antigen delivery into extracellular vesicles via DNA vaccination results in robust CD8 T cell responses. J Immunol. 2017;198:4707–15.
Xu L, Faruqu FN, Liam-Or R, Abu Abed O, Li D, Venner K, et al. Design of experiment (DoE)-driven and uptake studies of exosomes for pancreatic cancer delivery enabled by copper-free click chemistry-based labelling. J Extracell Vesicles. 2020;9:1779458.
Tran PHL, Xiang D, Tran TTD, Yin W, Zhang Y, Kong L, et al. Exosomes and nanoengineering: a match made for precision therapeutics. Adv Mater. 2020;32: e1904040.
Han Q, Xie QR, Li F, Cheng Y, Wu T, Zhang Y, et al. Targeted inhibition of SIRT6 via engineered exosomes impairs tumorigenesis and metastasis in prostate cancer. Theranostics. 2021;11:6526–41.
Zou J, Shi M, Liu X, Jin C, Xing X, Qiu L, et al. Aptamer-functionalized exosomes: elucidating the cellular uptake mechanism and the potential for cancer-targeted chemotherapy. Anal Chem. 2019;91:2425–30.
Xiang D, Zheng C, Zhou S-F, Qiao S, Tran PH-L, Pu C, et al. Superior performance of aptamer in tumor penetration over antibody implication of aptamer-based theranostics in solid tumors. Theranostics. 2015;5:1083–97.
Macdonald J, Henri J, Goodman L, Xiang D, Duan W, Shigdar S. Development of a bifunctional aptamer targeting the transferrin receptor and epithelial cell adhesion molecule (EpCAM) for the treatment of brain cancer metastases. ACS Chem Neurosci. 2017;8:777–84.
Yao X, Lyu P, Yoo K, Yadav MK, Singh R, Atala A, et al. Engineered extracellular vesicles as versatile ribonucleoprotein delivery vehicles for efficient and safe CRISPR genome editing. J Extracell Vesicles. 2021;10: e12076.
Tran PHL, Xiang D, Nguyen TNG, Tran TTD, Chen Q, Yin W, et al. Aptamer-guided extracellular vesicle theranostics in oncology. Theranostics. 2020;10:3849–66.
Luo Z-W, Li F-X-Z, Liu Y-W, Rao S-S, Yin H, Huang J, et al. Aptamer-functionalized exosomes from bone marrow stromal cells target bone to promote bone regeneration. Nanoscale. 2019;11:20884–92.
Ren X, Zhao Y, Xue F, Zheng Y, Huang H, Wang W, et al. Exosomal DNA aptamer targeting α-synuclein aggregates reduced neuropathological deficits in a mouse Parkinson’s disease model. Mol Ther Nucleic Acids. 2019;17:726–40.
Gao X, Ran N, Dong X, Zuo B, Yang R, Zhou Q, et al. Anchor peptide captures, targets, and loads exosomes of diverse origins for diagnostics and therapy. Sci Transl Med. 2018;10:aat0195.
Dong X, Lei Y, Yu Z, Wang T, Liu Y, Han G, et al. Exosome-mediated delivery of an anti-angiogenic peptide inhibits pathological retinal angiogenesis. Theranostics. 2021;11:5107–26.
Tamura R, Uemoto S, Tabata Y. Augmented liver targeting of exosomes by surface modification with cationized pullulan. Acta Biomater. 2017;57:274–84.
Zhu S, Huang H, Liu D, Wen S, Shen L, Lin Q. Augmented cellular uptake and homologous targeting of exosome-based drug loaded IOL for posterior capsular opacification prevention and biosafety improvement. Bioact Mater. 2022;15:469–81.
Nakase I, Futaki S. Combined treatment with a pH-sensitive fusogenic peptide and cationic lipids achieves enhanced cytosolic delivery of exosomes. Sci Rep. 2015;5:10112.
Zheng Z, Li Z, Xu C, Guo B, Guo P. Folate-displaying exosome mediated cytosolic delivery of siRNA avoiding endosome trapping. J Control Release. 2019;311–312:43–9.
Armstrong JPK, Holme MN, Stevens MM. Re-engineering extracellular vesicles as smart nanoscale therapeutics. ACS Nano. 2017;11:69–83.
Youn S-W, Li Y, Kim Y-M, Sudhahar V, Abdelsaid K, Kim HW, et al. Modification of cardiac progenitor cell-derived exosomes by miR-322 provides protection against myocardial infarction through Nox2-dependent angiogenesis. Antioxidants. 2019;8:18.
Zhu L-P, Tian T, Wang J-Y, He J-N, Chen T, Pan M, et al. Hypoxia-elicited mesenchymal stem cell-derived exosomes facilitates cardiac repair through miR-125b-mediated prevention of cell death in myocardial infarction. Theranostics. 2018;8:6163–77.
Hu S, Wang X, Li Z, Zhu D, Cores J, Wang Z, et al. Platelet membrane and stem cell exosome hybrid enhances cellular uptake and targeting to heart injury. Nano Today. 2021;39:101210.
Tian T, Zhang H-X, He C-P, Fan S, Zhu Y-L, Qi C, et al. Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy. Biomaterials. 2018;150:137–49.
Liu Y, Li D, Liu Z, Zhou Y, Chu D, Li X, et al. Targeted exosome-mediated delivery of opioid receptor Mu siRNA for the treatment of morphine relapse. Sci Rep. 2015;5:17543.
Guo L, Huang Z, Huang L, Liang J, Wang P, Zhao L, et al. Surface-modified engineered exosomes attenuated cerebral ischemia/reperfusion injury by targeting the delivery of quercetin towards impaired neurons. J Nanobiotechnol. 2021;19:141.
Wang J, Tang W, Yang M, Yin Y, Li H, Hu F, et al. Inflammatory tumor microenvironment responsive neutrophil exosomes-based drug delivery system for targeted glioma therapy. Biomaterials. 2021;273: 120784.
Kim MS, Haney MJ, Zhao Y, Yuan D, Deygen I, Klyachko NL, et al. Engineering macrophage-derived exosomes for targeted paclitaxel delivery to pulmonary metastases: in vitro and in vivo evaluations. Nanomed Nanotechnol Biol Med. 2018;14:195–204.
Cho BS, Kim JO, Ha DH, Yi YW. Exosomes derived from human adipose tissue-derived mesenchymal stem cells alleviate atopic dermatitis. Stem Cell Res Ther. 2018;9:187. https://doi.org/10.1186/s13287-018-0939-5.
Tian Y, Li S, Song J, Ji T, Zhu M, Anderson GJ, et al. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials. 2014;35:2383–90.
Lee H, Park H, Noh GJ, Lee ES. pH-responsive hyaluronate-anchored extracellular vesicles to promote tumor-targeted drug delivery. Carbohydrate Polym. 2018;202:323–33. https://doi.org/10.1016/j.carbpol.2018.08.141.
de Abreu RC, Fernandes H, da Costa Martins PA, Sahoo S, Emanueli C, Ferreira L. Native and bioengineered extracellular vesicles for cardiovascular therapeutics. Nat Rev Cardiol. 2020;17:685–97.
Zhang E, Liu Y, Han C, Fan C, Wang L, Chen W, et al. Visualization and Identification of bioorthogonally labeled exosome proteins following systemic administration in mice. Front Cell Dev Biol. 2021;9: 657456.
Xia T, Kovochich M, Liong M, Zink JI, Nel AE. Cationic polystyrene nanosphere toxicity depends on cell-specific endocytic and mitochondrial injury pathways. ACS Nano. 2008;2:85–96.
Qi H, Liu C, Long L, Ren Y, Zhang S, Chang X, et al. Blood exosomes endowed with magnetic and targeting properties for cancer therapy. ACS Nano. 2016;10:3323–33.
Kim HY, Kim TJ, Kang L, Kim Y-J, Kang MK, Kim J, et al. Mesenchymal stem cell-derived magnetic extracellular nanovesicles for targeting and treatment of ischemic stroke. Biomaterials. 2020;243: 119942.
Kutchy NA, Ma R, Liu Y, Buch S, Hu G. Extracellular vesicle-mediated delivery of ultrasmall superparamagnetic iron oxide nanoparticles to mice brain. Front Pharmacol. 2022;13: 819516.
Wang J, Wang H, Yan L, Hu Z, Wu X, Li F. Dual targeted and pH-responsive gold nanorods with improved chemotherapy and photothermal ablation for synergistic cancer treatment. RSC Adv. 2019;9:5270–81.
Yim N, Ryu S-W, Choi K, Lee KR, Lee S, Choi H, et al. Exosome engineering for efficient intracellular delivery of soluble proteins using optically reversible protein-protein interaction module. Nat Commun. 2016;7:12277.
Foglietta F, Canaparo R, Cossari S, Panzanelli P, Dosio F, Serpe L. Ultrasound triggers hypericin activation leading to multifaceted anticancer activity. Pharmaceutics. 2022;14:1102.
Liu Y, Bai L, Guo K, Jia Y, Zhang K, Liu Q, et al. Focused ultrasound-augmented targeting delivery of nanosonosensitizers from homogenous exosomes for enhanced sonodynamic cancer therapy. Theranostics. 2019;9:5261–81.
Ma N, Wu C, Meng Z. In vivo imaging and tracking of exosomes for theranostics. J Innov Opt Health Sci. 2021;14:2130005.
Verweij FJ, Balaj L, Boulanger CM, Carter DRF, Compeer EB, D’Angelo G, et al. The power of imaging to understand extracellular vesicle biology in vivo. Nat Methods. 2021;18:1013–26.
Lai J, Huang C, Guo Y, Rao L. Engineered extracellular vesicles and their mimics in cardiovascular diseases. J Control Release. 2022;347:27–43.
Rayamajhi S, Nguyen TDT, Marasini R, Aryal S. Macrophage-derived exosome-mimetic hybrid vesicles for tumor targeted drug delivery. Acta Biomater. 2019;94:482–94.
Li Y-J, Wu J-Y, Liu J, Xu W, Qiu X, Huang S, et al. Artificial exosomes for translational nanomedicine. J Nanobiotechnol. 2021;19:242.
Cheng Y, Zeng Q, Han Q, Xia W. Effect of pH, temperature and freezing-thawing on quantity changes and cellular uptake of exosomes. Protein Cell. 2019;10:295–9.
Parolini I, Federici C, Raggi C, Lugini L, Palleschi S, De Milito A, et al. Microenvironmental pH is a key factor for exosome traffic in tumor cells. J Biol Chem. 2009;284:34211–22.
Maroto R, Zhao Y, Jamaluddin M, Popov VL, Wang H, Kalubowilage M, et al. Effects of storage temperature on airway exosome integrity for diagnostic and functional analyses. J Extracell Vesicles. 2017;6:1359478.
Ge Q, Zhou Y, Lu J, Bai Y, Xie X, Lu Z. miRNA in plasma exosome is stable under different storage conditions. Molecules. 2014;19:1568–75.
Noren Hooten N, Yáñez-Mó M, DeRita R, Russell A, Quesenberry P, Ramratnam B, et al. Hitting the bullseye: are extracellular vesicles on target? J Extracell Vesicles. 2020;10: e12032.
Nordin JZ, Lee Y, Vader P, Mäger I, Johansson HJ, Heusermann W, et al. Ultrafiltration with size-exclusion liquid chromatography for high yield isolation of extracellular vesicles preserving intact biophysical and functional properties. Nanomed Nanotechnol Biol Med. 2015;11:879–83.
Shao H, Im H, Castro CM, Breakefield X, Weissleder R, Lee H. New technologies for analysis of extracellular vesicles. Chem Rev. 2018;118:1917–50.
Ban J-J, Lee M, Im W, Kim M. Low pH increases the yield of exosome isolation. Biochem Biophys Res Commun. 2015;461:76–9.
Taraboletti G, D’Ascenzo S, Giusti I, Marchetti D, Borsotti P, Millimaggi D, et al. Bioavailability of VEGF in tumor-shed vesicles depends on vesicle burst induced by acidic pH. Neoplasia. 2006;8:96–103.
Zhuang M, Chen X, Du D, Shi J, Deng M, Long Q, et al. SPION decorated exosome delivery of TNF-α to cancer cell membranes through magnetism. Nanoscale. 2020;12:173–88.
Shi X, Cheng Q, Hou T, Han M, Smbatyan G, Lang JE, et al. Genetically engineered cell-derived nanoparticles for targeted breast cancer immunotherapy. Mol Ther. 2020;28:536–47.
Acknowledgements
Not applicable.
Funding
This work was supported by National Natural Science Foundation of China (82071155, 82271023), the Shanxi Province Key Research and Development Program (201903D321148), the Shanxi Applied Basic Research Program Science-Youth Technology Research Fund [Grant numbers 20210302124398 and 202103021223218], The Project of Shanxi Province Key Laboratory of Oral Diseases Prevention and New Materials [Grant number RC2021-02 and KF2020-07].
Author information
Authors and Affiliations
Contributions
XW selected the topic and guided the review, HS and XC wrote the manuscript, YH, JW and QX searched the references and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
None.
Consent for publication
All authors have given consent for publication.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Song, H., Chen, X., Hao, Y. et al. Nanoengineering facilitating the target mission: targeted extracellular vesicles delivery systems design. J Nanobiotechnol 20, 431 (2022). https://doi.org/10.1186/s12951-022-01638-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12951-022-01638-9