Abstract
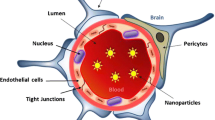
Search for efficient therapeutic agents for central nervous system (CNS) disorders has been extensive. Nevertheless, blood–brain barrier (BBB) is an obstacle that prevents the majority of compounds to act in these diseases. It is, thus, of extreme relevance the BBB overcome, in order to deliver a drugs therapeutically active concentration to the action site, with the least losses and interaction with other organs, tissues, or cells. The present study aimed to investigate the potential protective effect of quercetin-biapigenin encapsulated into poly(Ɛ-polycaprolactone) (PCL) nanoparticles against t-BOOH-induced oxidative stress in several brain cell lines, as well as evaluate the permeability of those active molecules through an in vitro BBB model. The three cell lines under study (BV-2, hcmec/D3, and U87) presented different reactions to t-BOOH. In general, quercetin-biapigenin PCL-loaded nanoparticles were able to minimize compound toxicity they convey, regardless the cell line. Quercetin-biapigenin PCL-loaded nanoparticles (Papp of approximately 80 × 10–6 cm/s) revealed to be more permeable than free compounds (Papp of approximately 50 × 10–6 cm/s). As of our knowledge, this is the first report of quercetin-biapigenin PCL-loaded nanoparticle activity in brain cells. It is also the first determining its permeability through BBB, as an effective nanocarrier for brain delivery
Graphical Abstract







Similar content being viewed by others
Data Availability
Data can be obtained from the authors upon request.
References
Floyd RA, Hensley K. Oxidative stress in brain aging. Implications for therapeutics of neurodegenerative diseases. Neurobiol Aging. 2002;23(5):795–807.
Pang Z, Lu W, Gao H, Hu K, Chen J, Zhang C, Gao X, Jiang X, Zhu C. Preparation and brain delivery property of biodegradable polymersomes conjugated with OX26. J Control Release. 2008. https://doi.org/10.1016/j.jconrel.2008.03.007.
Emerit J, Edeas M, Bricaire F. Neurodegenerative diseases and oxidative stress. Biomed Pharmacother. 2004;58(1):39–46.
Gilgun-Sherki Y, Melamed E, Offen D. Oxidative stress induced-neurodegenerative diseases: the need for antioxidants that penetrate the blood brain barrier. Neuropharmacology. 2001;40(8):959–75.
Pietta PG. Flavonoids as antioxidants. J Nat Prod. 2000. https://doi.org/10.1021/np9904509.
Silva BA, Ferreres F, Malva JO, Dias ACP. Phytochemical and antioxidant characterization of Hypericum perforatum alcoholic extracts. Food Chem. 2005;90:157–67.
Silva BA, Malva JO, Dias ACP. St John’s Wort (Hypericum perforatum) extracts and isolated phenolic compounds are effective antioxidants in several in vitro models of oxidative stress. Food Chem. 2008;110:611–9.
Barbu E, Molnar E, Tsibouklis J, Gorecki DC. The potential for nanoparticle-based drug delivery to the brain: overcoming the blood-brain barrier. Expert Opin Drug Deliv. 2009. https://doi.org/10.1517/17425240902939143.
Alam MI, Beg S, Samad A, Baboota S, Kohli K, Ali J, Akbar M. Strategy for effective brain drug delivery. Eur J Pharm Sci. 2010. https://doi.org/10.1016/j.ejps.2010.05.003.
Wohlfart S, Gelperina S, Kreuter J. Transport of drugs across the blood-brain barrier by nanoparticles. J Control Release. 2012. https://doi.org/10.1016/j.jconrel.2011.08.017.
Yang H. Nanoparticle-mediated brain-specific drug delivery, imaging, and diagnosis. Pharm Res. 2010. https://doi.org/10.1007/s11095-010-0141-7.
Blasi E, Barluzzi R, Bocchini V, Mazzolla R, Bistoni F. Immortalization of murine microglial cells by a v-raf/v-myc carrying retrovirus. J Neuroimmunol. 1990;27(2–3):229–37.
Dok-Go H, Lee KH, Kim HJ, Lee EH, Lee J, Song YS, Lee Y, Jin C, Lee YS, Cho J. Neuroprotective effects of antioxidative flavonoids, quercetin, (+)-dihydroquercetin and quercetin 3-methyl ether, isolated from Opuntia ficus-indica var. Saboten Brain Res. 2003;965(1–2):130–6.
Arredondo F, Echeverry C, Abin-Carriquiry JA, Blasina F, Antunez K, Jones DP, Go YM, Liang YL, Dajas F. After cellular internalization, quercetin causes Nrf2 nuclear translocation, increases glutathione levels, and prevents neuronal death against an oxidative insult. Free Radic Biol Med. 2010. https://doi.org/10.1016/j.freeradbiomed.2010.05.020.
Suematsu N, Hosoda M, Fujimori K. Protective effects of quercetin against hydrogen peroxide-induced apoptosis in human neuronal SH-SY5Y cells. Neurosci Lett. 2011. https://doi.org/10.1016/j.neulet.2011.09.028.
Sasaki N, Toda T, Kaneko T, Baba N, Matsuo M. Protective effects of flavonoids on the cytotoxicity of linoleic acid hydroperoxide toward rat pheochromocytoma PC12 cells. Chem Biol Interact. 2003;145(1):101–16.
Silva JP, Gomes AC, Coutinho OP. Oxidative DNA damage protection and repair by polyphenolic compounds in PC12 cells. Eur J Pharmacol. 2008. https://doi.org/10.1016/j.ejphar.2008.10.046.
Ansari MA, Abdul HM, Joshi G, Opii WO, Butterfield DA. Protective effect of quercetin in primary neurons against Abeta(1–42): relevance to Alzheimer’s disease. J Nutr Biochem. 2009. https://doi.org/10.1016/j.jnutbio.2008.03.002.
Silva B, Oliveira PJ, Dias A, Malva JO. Quercetin, kaempferol and biapigenin from Hypericum perforatum are neuroprotective against excitotoxic insults. Neurotox Res. 2008;13(3–4):265–79.
Silva BA, Oliveira PJ, Cristovao A, Dias AC, Malva JO. Biapigenin modulates the activity of the adenine nucleotide translocator in isolated rat brain mitochondria. Neurotox Res. 2010. https://doi.org/10.1007/s12640-009-9082-5.
Oliveira AI, Pinho C, Fonte P, Sarmento B, Dias ACP. Development, characterization, antioxidant and hepatoprotective properties of poly (Ɛ-caprolactone) nanoparticles loaded with a neuroprotective fraction of Hypericum perforatum. Int J Biol Macromol. 2018. https://doi.org/10.1016/j.ijbiomac.2017.10.103.
Dias ACP, Seabra RM, Andrade PB, Fernandes-Ferreira M. The development and evaluation of an HPLC-DAD method for the analysis of the phenolic fractions from in vivo and in vitro biomass of Hypericum species. Journal of Liquid Chromatoghrafy & Related Technology. 1999;22(2):215–27.
Lima CF, Valentao PC, Andrade PB, Seabra RM, Fernandes-Ferreira M, Pereira-Wilson C. Water and methanolic extracts of Salvia officinalis protect hepg2 cells from t-BHP induced oxidative damage. Chem Biol Interact. 2007. https://doi.org/10.1016/j.cbi.2007.01.020.
Carvalho AC, Gregory F, Dias ACP, Lima CF. Methanolic extract of Hypericum perforatum cells elicited with Agrobacterium tumefaciens provides protection against oxidative stress induced in human hepg2 cells. Ind Crops Prod. 2014;59:177–83.
Mendes B, Marques C, Carvalho I, Costa P, Martins S, Ferreira D, Sarmento B. Influence of glioma cells on a new co-culture in vitro blood-brain barrier model for characterization and validation of permeability. Int J Pharm. 2015. https://doi.org/10.1016/j.ijpharm.2015.05.027.
Mohanraj VJ, Chen YN. Nanoparticles - a review. Trop J Pharm Res. 2006. https://doi.org/10.4314/tjpr.v5i1.14634.
Fessi H, Puisieux J, Devissaguet JPH, Ammoury N, Benita S. Nanocapsule formation by interfacial polymer deposition following solvent displacement. Int J Pharm. 1989. https://doi.org/10.1016/0378-5173(89)90281-0.
Vauthier C, Bouchemal K. Methods for the preparation and manufacture of polymeric nanoparticles. Pharm Res. 2009. https://doi.org/10.1007/s11095-008-9800-3.
Kumari A, Yadav SK, Yadav SC. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf B Biointerfaces. 2010. https://doi.org/10.1016/j.colsurfb.2009.09.001.
Hu Y, Jiang X, Ding Y, Zhang L, Yang C, Zhang J, Chen J, Yang Y. Preparation and drug release behaviors of nimodipine-loaded poly(caprolactone)-poly(ethylene oxide)-polylactide amphiphilic copolymer nanoparticles. Biomaterials. 2003. https://doi.org/10.1016/S0142-9612(03)00021-8.
Wilkening S, Stahl F, Bader A. Comparison of primary human hepatocytes and hepatoma cell line Hepg2 with regard to their biotransformation properties. Drug Metab Dispos. 2003. https://doi.org/10.1124/dmd.31.8.1035.
Blasi P, Giovagnoli S, Schoubben A, Ricci M, Rossi C. Solid lipid nanoparticles for targeted brain drug delivery. Adv Drug Deliv Rev. 2007. https://doi.org/10.1016/j.addr.2007.04.01.
Wolburg H, Lippoldt A. Tight junctions of the blood-brain barrier: development, composition and regulation. Vascul Pharmacol. 2002;38(6):323–37.
Weksler BB, Subileau EA, Perriere N, Charneau P, Holloway K, Leveque M, Tricoire-Leignel H, Nicotra A, Bourdoulous S, Turowski P, Male DK, Roux F, Greenwood J, Romero IA, Couraud PO. Blood-brain barrier-specific properties of a human adult brain endothelial cell line. FASEB J. 2005. https://doi.org/10.1096/fj.04-3458fje.
Weksler B, Romero IA, Couraud PO. The hcmec/D3 cell line as a model of the human blood brain barrier. Fluids Barriers CNS. 2013. https://doi.org/10.1186/2045-8118-10-16.
Yu SC, Ping YF, Yi L, Zhou ZH, Chen JH, Yao XH, Gao L, Wang JM, Bian XW. Isolation and characterization of cancer stem cells from a human glioblastoma cell line U87. Cancer Lett. 2008. https://doi.org/10.1016/j.canlet.2008.02.010.
Clark MJ, Homer N, O’Connor BD, Chen Z, Eskin A, Lee H, Merriman B, Nelson SF. U87MG decoded: the genomic sequence of a cytogenetically aberrant human cancer cell line. Plos Genet. 2010. https://doi.org/10.1371/journal.pgen.1000832.
Kim SS, Lim J, Bang Y, Gal J, Lee SU, Cho YC, Yoon G, Kang BY, Cheon SH, Choi HJ. Licochalcone E activates Nrf2/antioxidant response element signaling pathway in both neuronal and microglial cells: therapeutic relevance to neurodegenerative disease. J Nutr Biochem. 2012. https://doi.org/10.1016/j.jnutbio.2011.07.012.
Prochazkova D, Bousova I, Wilhelmova N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia. 2011. https://doi.org/10.1016/j.fitote.2011.01.018.
Banati RB, Gehrmann J, Schubert P, Kreutzberg GW. Cytotoxicity of microglia. Glia. 1993. https://doi.org/10.1002/glia.440070117.
Nelson PT, Soma LA, Lavi E. Microglia in diseases of the central nervous system. Ann Med. 2002;34(7–8):491–500.
Weber CC, Kressmann S, Fricker G, Muller WE. Modulation of P-glycoprotein function by St John’s wort extract and its major constituents. Pharmacopsychiatry. 2004. https://doi.org/10.1055/s-2004-832686.
Mitsunaga Y, Takanaga H, Matsuo H, Naito M, Tsuruo T, Ohtani H, Sawada Y. Effect of bioflavonoids on vincristine transport across blood-brain barrier. Eur J Pharmacol. 2000;395(3):193–201.
Siegelin MD, Reuss DE, Habel A, Rami A, von Deimling A. Quercetin promotes degradation of survivin and thereby enhances death-receptor-mediated apoptosis in glioma cells. Neuro Oncol. 2009. https://doi.org/10.1215/15228517-2008-085.
Long TC, Saleh N, Tilton RD, Lowry GV, Veronesi B. Titanium dioxide (P25) produces reactive oxygen species in immortalized brain microglia (BV2): implications for nanoparticle neurotoxicity. Environ Sci Technol. 2006;40(14):4346–52.
Sohn JH, Han KL, Lee SH, Hwang JK. Protective effects of panduratin A against oxidative damage of tert-butylhydroperoxide in human hepg2 cells. Biol Pharm Bull. 2005;28(6):1083–6.
Kang CH, Choi YH, Moon SK, Kim WJ, Kim GY. Quercetin inhibits lipopolysaccharide-induced nitric oxide production in BV2 microglial cells by suppressing the NF-kappab pathway and activating the Nrf2-dependent HO-1 pathway. Int Immunopharmacol. 2013. https://doi.org/10.1016/j.intimp.2013.09.009.
Zamin LL, Filippi-Chiela EC, Dillenburg-Pilla P, Horn F, Salbego C, Lenz G. Resveratrol and quercetin cooperate to induce senescence-like growth arrest in C6 rat glioma cells. Cancer Sci. 2009. https://doi.org/10.1111/j.1349-7006.2009.01215.x.
Sang DP, Li RJ, Lan Q. Quercetin sensitizes human glioblastoma cells to temozolomide in vitro via inhibition of Hsp27. Acta Pharmacol Sin. 2014. https://doi.org/10.1038/aps.2014.22.
Xia S, Rosen EM, Laterra J. Sensitization of glioma cells to Fas-dependent apoptosis by chemotherapy-induced oxidative stress. Cancer Res. 2005;65(12):5248–55.
Kraus B, Wolff H, Heilmann J, Elstner EF. Influence of Hypericum perforatum extract and its single compounds on amyloid-beta mediated toxicity in microglial cells. Life Sci. 2007. https://doi.org/10.1016/j.lfs.2007.07.020.
Wolff A, Antfolk M, Brodin B, Tenje M. In Vitro Blood-Brain Barrier Models-An Overview of Established Models and New Microfluidic Approaches. J Pharm Sci. 2015. https://doi.org/10.1002/jps.24329.
Hatherell K, Couraud PO, Romero IA, Weksler B, Pilkington GJ. Development of a three-dimensional, all-human in vitro model of the blood-brain barrier using mono-, co-, and tri-cultivation Transwell models. J Neurosci Methods. 2011;199(2):223–9.
Madara JL. Regulation of the movement of solutes across tight junctions. Annu Rev Physiol. 1998. https://doi.org/10.1146/annurev.physiol.60.1.143.
Gomes MJ, Kennedy PJ, Martins S, Sarmento B. Delivery of sirna silencing P-gp in peptide-functionalized nanoparticles causes efflux modulation at the blood–brain barrier. Nanomedicine. 2017. https://doi.org/10.2217/nnm-2017-0023.
Gaillard PJ, de Boer AG. Relationship between permeability status of the blood-brain barrier and in vitro permeability coefficient of a drug. Eur J Pharm Sci. 2000;12(2):95–102.
Mahar Doan KM, Humphreys JE, Webster LO, Wring SA, Shampine LJ, Serabjit-Singh CJ, Adkison KK, Polli JW. Passive permeability and P-glycoprotein-mediated efflux differentiate central nervous system (CNS) and non-CNS marketed drugs. J Pharmacol Exp Ther. 2002. https://doi.org/10.1124/jpet.102.039255.
Faria A, Meireles M, Fernandes I, Santos-Buelga C, Gonzalez-Manzano S, Duenas M, Freiras V, Mateus N, Calhau C. Flavonoid metabolites transport across a human BBB model. Food Chem. 2014. https://doi.org/10.1016/j.foodchem.2013.10.095.
Funding
This paper was financed by Portuguese funds through FCT—Fundação para a Ciência e a Tecnologia/Ministério da Ciência, Tecnologia e Ensino Superior in the framework of the project “Institute for Research and Innovation in Health Sciences” UID/BIM/04293/2019. It was also supported by the “Contrato-Programa” UIDB/04050/2020 funded by national funds through the FCT I.P. Ana Isabel Oliveira was supported by Escola Superior de Saúde do Porto and Instituto Politécnico do Porto (Programa de Formação Avançada de Docentes).
Author information
Authors and Affiliations
Contributions
As for different contributions for this manuscript, Ana Isabel Oliveira had intervention in all the practical parts of the study and on the elaboration of the paper. Cláudia Pinho intervened on specific parts of the study (cell line assays) aiding on the elaboration of the paper on those specific parts. Bruno Sarmento and Alberto Dias intervened on the structure and elaboration of the article and review it. All authors have knowledge and approve the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Oliveira, A.I., Pinho, C., Sarmento, B. et al. Quercetin-biapigenin nanoparticles are effective to penetrate the blood–brain barrier. Drug Deliv. and Transl. Res. 12, 267–281 (2022). https://doi.org/10.1007/s13346-021-00917-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-021-00917-6