Abstract
Periodontal diseases are worldwide chronic inflammatory conditions that are associated with heavy production of reactive oxygen species followed by damage of the tooth-supporting tissues. Although the mechanical approach of scaling and root planing (SRP) for removing of plaque is considered as the key element for controlling periodontitis, the anatomical complexity of the teeth hinders accessibility to deeper points. The aim of this study was to design a micellar nanocarrier of coenzyme Q10 (Q10) to support the management of moderate periodontitis. Q10 was formulated in nanomicelles (NMQ10) and evaluated regarding encapsulation efficiency, loading efficiency, percent yield, hydrodynamic size (Dh), polydispersity index (PDI), and zeta potential (ζ potential). NMQ10 was incorporated to in situ gelling systems and the in vitro release of Q10 was studied. A clinical study including evaluation of periodontal parameters and biochemical assay of total antioxidant capacity (T-AOC) and lipid peroxide was achieved. Results revealed that Q10 was efficiently entrapped in spherical-shaped stable NMQ10 with Dh, PDI, and ζ potential of 154.0 nm, 0.108, and − 31.67 mV, respectively. The clinical study revealed that SRP only exhibited improvement of the periodontal parameters. Also, assay of T-AOC and lipid peroxide revealed that their values diminished by 21.5 and 23.8%, respectively. On the other hand, SRP combined with local application of NMQ10 resulted in a significant management of the periodontal parameters, and likewise, the assayed biomarkers proved enhanced antioxidant activity over SRP alone. In conclusion, NMQ10 can be suggested as a promising nanosystem as an approach to support the management of chronic periodontitis. Such results could be used to conduct larger clinical studies.

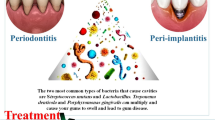
Graphical abstrac




Similar content being viewed by others
Abbreviations
- % LQ10 :
-
Percent coenzyme Q10 leaked out of the NMQ10
- % RQ10 :
-
% Retention of coenzyme Q10
- API:
-
Active pharmaceutical ingredient
- BOP:
-
Bleeding on probing
- CAL:
-
Clinical attachment level
- CGMP:
-
Current good manufacturing practices
- CMC:
-
Critical micelle concentration
- CP:
-
Carbopol 934
- CTM:
-
Clinical trial material
- DDS:
-
Drug delivery system
- D h :
-
Hydrodynamic size
- DIW:
-
Deionized water
- EE%:
-
Encapsulation efficiency
- EtQ10 :
-
Ethanolic solution of coenzyme Q10
- FNM :
-
NMQ10 dispersion
- FQ10 :
-
Coenzyme Q10 dispersion
- FRs:
-
Free radicals
- FT-IR:
-
Fourier-transform infrared spectroscopy
- GCF:
-
Gingival crevicular fluid
- GI:
-
Gingival index
- GLP:
-
Good laboratory practices
- HLB:
-
Hydrophilic–lipophilic balance
- HPMC:
-
Hydroxypropyl methyl cellulose K4M
- K407 :
-
Kolliphor® P 407
- K–P:
-
Korsmeyer–Peppas
- LE%:
-
Loading efficiency
- LPO:
-
Lipid peroxidation
- MC:
-
Methyl cellulose
- MDA:
-
Malondialdehyde
- NMP :
-
Coenzyme Q10-free nanomicelles
- NMQ10 :
-
Coenzyme Q10-loaded nanomicelles
- NMs:
-
Polymeric nanomicelles
- NLCs:
-
Nanostructured lipid carriers
- PB6.8 :
-
Phosphate buffer pH 6.8
- PDI:
-
Polydispersity index
- PDPs:
-
Periodontal pockets
- PEO:
-
Polyethylene oxide
- PI:
-
Plaque index
- PPD:
-
Probing pocket depth
- PPO:
-
Polypropylene oxide
- Q10 :
-
Coenzyme Q10
- ROS:
-
Reactive oxygen species
- SRP:
-
Scaling and root planing
- T-AOC:
-
Total antioxidant capacity
- TBA:
-
Thiobarbituric acid
- TEA:
-
Triethanolamine
- TEM:
-
Transmission electron microscopy
- Y%:
-
Percent yield
- ζ potential:
-
Zeta potential
References
Tóthová L, Celec P. Oxidative stress and antioxidants in the diagnosis and therapy of periodontitis. Front Physiol. 2017;8:1–14.
Jain N, Jain GK, Javed S, Iqbal Z, Talegaonkar S, Ahmad FJ, et al. Recent approaches for the treatment of periodontitis. Drug Discov Today. 2008;13:932–43.
Aminu N, Chan S-Y, Toh S-M. Roles of nanotechnological approaches in periodontal disease therapy. J Appl Pharm Sci. 2017;7:234–42.
Aminu N, Chan S-Y, Toh S-M. Formulation design and optimization of triclosan loaded nanoparticles for enhanced drug delivery across gingival sulcus by Resolution IV modeling of Design Expert®. JBCS. 2018;3:83–90.
Younis LT, Hassan MIA, Anuar SA, Yunus FA, Yusof N. The role of reactive oxygen species in initiation and progression of periodontal diseases. BJAST. 2015;8:541–9.
Chapple IL, Matthews JB. The role of reactive oxygen and antioxidant species in periodontal tissue destruction. Periodontol. 2007;43:160–232.
Nguyen TT, Ngo LQ, Promsudthi A, Surarit R. Salivary lipid peroxidation in patients with generalized chronic periodontitis and acute coronary syndrome. J Periodontol. 2016;87:134–41.
Salih TM, Mahmood MS. Evaluation of the effectiveness of coenzyme Q10 gel in management of patients with chronic periodontitis: I intra group comparison. J Bagh Coll Dent. 2015;27:130–5.
Ambati M, Rani KR, Reddy PV, Suryaprasanna J, Dasari R, Gireddy H. Evaluation of oxidative stress in chronic periodontitis patients following systemic antioxidant supplementation: a clinical and biochemical study. J Nat Sci Biol Med. 2017;8:99–103.
Todorovic K, Jovanovic G, Todorovic A, Mitic A, Stojiljkovic N, Ilic S, et al. Effects of coenzyme Q10 encapsulated in nanoliposomes on wound healing processes after tooth extraction. J Dent Sci. 2018;13:103–8.
Soni S, Agarwal PK, Sharma N, Chander S. Coenzyme Q10 and periodontal health—a review. Int J Oral Maxillofac Pathol. 2012;3:21–6.
Bhagavan HN, Chopra RK. Coenzyme Q10: Absorption, tissue uptake, metabolism and pharmacokinetics. Free Radic Res. 2006;40:445–53.
Hans M, Prakash S, Gupta S. Clinical evaluation of topical application of perio-Q gel (coenzyme Q10 ) in chronic periodontitis patients. J Indian Soc Periodontol. 2012;16:193.
Pitale UM, Khetarpal S, Peter KP, Pal V, Verma E, Gupta PK. Evaluation of efficacy of coenzyme Q 10 in management of gingivitis & slight periodontitis—a clinical study. Int J Curr Pharm Res. 2012;4:33–8.
Sale ST, Parvez H, Yeltiwar RKR, Vivekanandan G, Pundir AJ, Jain P. A comparative evaluation of topical and intrasulcular application of coenzyme Q10 (Perio Q™) gel in chronic periodontitis patients: a clinical study. J Indian Soc Periodontol. 2014;18:461–5.
Torchilin VP. Structure and design of polymeric surfactant-based drug delivery systems. J Control Release. 2001;73:137–72.
Kahraman E, Ÿzhan G, Ÿzsoy Y, Güngör S. Polymeric micellar nanocarriers of benzoyl peroxide as potential follicular targeting approach for acne treatment. Colloids Surf B: Biointerfaces. 2016;146:692–9.
Kong LX, Peng Z, Li S-D, Bartold PM. Nanotechnology and its role in the management of periodontal diseases. Periodontol. 2006;40:184–96.
Aminu N, Pramod K, Ali J. Targeted drug delivery systems for the treatment of periodontal infections. Biotechnology. Houston: Studium Press LLC; 2014. p. 97–128.
Dehghan Kelishady P, Saadat E, Ravar F, Akbari H, Dorkoosh F. Pluronic F127 polymeric micelles for co-delivery of paclitaxel and lapatinib against metastatic breast cancer: preparation, optimization and in vitro evaluation. Pharm Dev Technol. 2015;20:1009–17.
El-Far YM, Zakaria MM, Gabr MM, El Gayar AM, El-Sherbiny IM, Eissa LA. A newly developed silymarin nanoformulation as a potential antidiabetic agent in experimental diabetes. Nanomedicine. 2016;11:2581–602.
Chen S, Liu W, Wan J, Cheng X, Gu C, Zhou H, et al. Preparation of coenzyme Q10 nanostructured lipid carriers for epidermal targeting with high-pressure microfluidics technique. Drug Dev Ind Pharm. 2013;39:20–8.
Xia Q, Wang H. Preparation and characterization of coenzyme Q10-loaded nanostructured lipid carriers as delivery systems for cosmetic component. Nanotech. 2010;3:498–501.
Hsu C-H, Cui Z, Mumper RJ, Jay M. Preparation and characterization of novel coenzyme Q10 nanoparticles engineered from microemulsion precursors. AAPS PharmSciTech. 2003;4:24–35.
Zhao GD, Sun R, Ni SL, Xia Q. Development and characterisation of a novel chitosan-coated antioxidant liposome containing both coenzyme Q10 and alpha-lipoic acid. J Microencapsul. 2015;32:157–65.
Sahle FF, Gerecke C, Kleuser B, Bodmeier R. Formulation and comparative in vitro evaluation of various dexamethasone-loaded pH-sensitive polymeric nanoparticles intended for dermal applications. Int J Pharm. 2017;516:21–31.
Franzè S, Musazzi UM, Minghetti P, Cilurzo F. Drug-in-micelles-in-liposomes (DiMiL) systems as a novel approach to prevent drug leakage from deformable liposomes. Eur J Pharm Sci. 2019;130:27–35.
Wang J, Wang H, Zhou X, Tang Z, Liu G, Liu G, et al. Physicochemical characterization, photo-stability and cytotoxicity of coenzyme Q10-loading nanostructured lipid carrier. J Nanosci Nanotechnol. 2012;12:2136–48.
Yadav NK, Nanda S, Sharma G, Katare OP. Systematically optimized coenzyme Q10-loaded novel proniosomal formulation for treatment of photo-induced aging in mice: characterization, biocompatibility studies, biochemical estimations and anti-aging evaluation. J Drug Target. 2016;24:257–71.
Khattab A, Hassanin L, Zaki N. Self-nanoemulsifying drug delivery system of coenzyme (Q10) with improved dissolution, bioavailability, and protective efficiency on liver fibrosis. AAPS PharmSciTech. 2017;18:1657–72.
Schwarz JC, Baisaeng N, Hoppel M, Löw M, Keck CM, Valenta C. Ultra-small NLC for improved dermal delivery of coenyzme Q10. Int J Pharm. 2013;447:213–7.
Lohan SB, Bauersachs S, Ahlberg S, Baisaeng N, Keck CM, Müller RH, et al. Ultra-small lipid nanoparticles promote the penetration of coenzyme Q10 in skin cells and counteract oxidative stress. Eur J Pharm Biopharm. 2015;89:201–7.
Balakrishnan P, Lee B-J, Oh DH, Kim JO, Lee Y-I, Kim D-D, et al. Enhanced oral bioavailability of coenzyme Q10 by self-emulsifying drug delivery systems. Int J Pharm. 2009;374:66–72.
Nepal PR, Han H-K, Choi H-K. Preparation and in vitro–in vivo evaluation of Witepsol® H35 based self-nanoemulsifying drug delivery systems (SNEDDS) of coenzyme Q10. Eur J Pharm Sci. 2010;39:224–32.
Pitorre M, Gondé H, Haury C, Messous M, Poilane J, Boudaud D, et al. Recent advances in nanocarrier-loaded gels: which drug delivery technologies against which diseases? J Control Release. 2017;266:140–55.
Yang Y, Wang J, Zhang X, Lu W, Zhang Q. A novel mixed micelle gel with thermo-sensitive property for the local delivery of docetaxel. J Control Release. 2009;135:175–82.
de Graaf AJ, dos Santos AP II, Pieters EHE, Rijkers DTS, van Nostrum CF, Vermonden T, et al. A micelle-shedding thermosensitive hydrogel as sustained release formulation. J Control Release. 2012;162:582–90.
Gong C, Wu Q, Wang Y, Zhang D, Luo F, Zhao X, et al. A biodegradable hydrogel system containing curcumin encapsulated in micelles for cutaneous wound healing. Biomaterials. 2013;34:6377–87.
Kim Y-M, Song S-C. Targetable micelleplex hydrogel for long-term, effective, and systemic siRNA delivery. Biomaterials. 2014;35:7970–7.
Sheu M-T, Jhan H-J, Su C-Y, Chen L-C, Chang C-E, Liu D-Z, et al. Codelivery of doxorubicin-containing thermosensitive hydrogels incorporated with docetaxel-loaded mixed micelles enhances local cancer therapy. Colloid Surf B. 2016;143:260–70.
Bhowmik M, Bain MK, Ghosh LK, Chattopadhyay D. Effect of salts on gelation and drug release profiles of methylcellulose-based ophthalmic thermo-reversible in situ gels. Pharm Dev Technol. 2011;16:385–91.
Sherje AP, Londhe V. Development and evaluation of pH-responsive cyclodextrin-based in situ gel of paliperidone for intranasal delivery. AAPS PharmSciTech. 2018;19:384–94.
Silva MM, Calado R, Marto J, Bettencourt A, Almeida AJ, Gonçalves L. Chitosan nanoparticles as a mucoadhesive drug delivery system for ocular administration. Mar Drugs. 2017;15:370.
Higuchi T. Mechanism of sustained-action medication. Theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J Pharm Sci. 1963;52:1145–9.
Korsmeyer RW, Gurny R, Doelker E, Buri P, Peppas NA. Mechanisms of solute release from porous hydrophilic polymers. Int J Pharm. 1983;15:25–35.
Al-Shammari NM, Shafshak SM, Ali MS. Effect of 0.8% hyaluronic acid in conventional treatment of moderate to severe chronic periodontitis. J Contemp Dent Pract. 2018;19:527–34.
Löe H, Silness J. Periodontal disease in pregnancy I. Prevalence and severity. Acta Odontol Scand. 1963;21:533–51.
Silness J, Löe H. Periodontal disease in pregnancy II. Correlation between oral hygiene and periodontal condition. Acta Odontol Scand. 1964;22:121–35.
Christgau M, Palitzsch K-D, Schmalz G, Kreiner U, Frenzel S. Healing response to non-surgical periodontal therapy in patients with diabetes mellitus: clinical, microbiological, and immunologic results. J Clin Periodontol. 1998;25:112–24.
Chapple ILC, Landini G, Griffiths GS, Patel NC, Ward RSN. Calibration of the Periotron 8000® and 6000® by polynomial regression. J Periodontal Res. 1999;34:79–86.
Botsoglou NA, Fletouris DJ, Papageorgiou GE, Vassilopoulos VN, Mantis AJ, Trakatellis AG. Rapid, sensitive, and specific thiobarbituric acid method for measuring lipid peroxidation in animal tissue, food, and feedstuff samples. J Agric Food Chem. 1994;42:1931–7.
Zhang J, Wang S. Topical use of coenzyme Q10-loaded liposomes coated with trimethyl chitosan: tolerance, precorneal retention and anti-cataract effect. Int J Pharm. 2009;372:66–75.
Nehme H, Saulnier P, Ramadan AA, Cassisa V, Guillet C, Eveillard M, et al. Antibacterial activity of antipsychotic agents, their association with lipid nanocapsules and its impact on the properties of the nanocarriers and on antibacterial activity. PLoS One. 2018;13:e0189950.
Li P, Luo S, Xiao L, Tian B, Wang L, Zhang Z, et al. A novel gemcitabine derivative-loaded liposome with great pancreas-targeting ability. Acta Pharmacol Sin. 2019:1–9.
Bohley M, Haunberger A, Goepferich AM. Intracellular availability of poorly soluble drugs from lipid nanocapsules. Eur J Pharm Biopharm. 2019;139:23–32.
Fir MM, Smidovnik A, Milivojevic L, Zmitek J, Prosek M. Studies of CoQ10 and cyclodextrin complexes: solubility, thermo- and photo-stability. J Incl Phenom Macro. 2009;64:225–32.
Erickson HP. Size and shape of protein molecules at the nanometer level determined by sedimentation, gel filtration, and electron microscopy. Biol Proced Online. 2009;11:32–51.
Chen D-B, Yang T, Lu W-L, Zhang Q. In vitro and in vivo study of two types of long-circulating solid lipid nanoparticles containing paclitaxel. Chem Pharm Bull. 2001;49:1444–7.
Arranz-Romera A, Davis BM, Bravo-Osuna I, Esteban-Pérez S, Molina-Martínez IT, Shamsher E, et al. Simultaneous co-delivery of neuroprotective drugs from multi-loaded PLGA microspheres for the treatment of glaucoma. J Control Release. 2019;297:26–38.
Nayak K, Katiyar SS, Kushwah V, Jain S. Coenzyme Q10 and retinaldehyde co-loaded nanostructured lipid carriers for efficacy evaluation in wrinkles. J Drug Target. 2018;26:333–44.
Lee J-S, Suh JW, Kim ES, Lee HG. Preparation and characterization of mucoadhesive nanoparticles for enhancing cellular uptake of coenzyme Q10. J Agric Food Chem. 2017;65:8930–7.
Liu L, Mao K, Wang W, Pan H, Wang F, Yang M, et al. Kolliphor® HS 15 Micelles for the delivery of coenzyme Q10: preparation, characterization, and stability. AAPS PharmSciTech. 2016;17:757–66.
Shao Y, Yang L, Han H-K. TPGS-chitosome as an effective oral delivery system for improving the bioavailability of coenzyme Q10. Eur J Pharm Biopharm. 2015;89:339–46.
Mishra PR, Shaal LA, Müller RH, Keck CM. Production and characterization of hesperetin nanosuspensions for dermal delivery. Int J Pharm. 2009;371:182–9.
Müller RH, editor. Colloidal carriers for controlled drug delivery and targeting: modification, characterization and in vivo distribution. Milton Park: Taylor & Francis; 1991.
Bujňáková Z, Dutková E, Baláž M, Turianicová E, Baláž P. Stability studies of As 4 S 4 nanosuspension prepared by wet milling in poloxamer 407. Int J Pharm. 2015;478:187–92.
Saxena V, Hussain MD. Poloxamer 407/TPGS mixed micelles for delivery of gambogic acid to breast and multidrug-resistant cancer. Int J Nanomedicine. 2012;7:713–21.
de Graaf AJ, Boere KW, Kemmink J, Fokkink RG, van Nostrum CF, Rijkers DT, et al. Looped structure of flowerlike micelles revealed by 1H NMR relaxometry and light scattering. Langmuir. 2011;27:9843–8.
Naz G, Othaman Z, Shamsuddin M, Ghoshal SK. Aliquat 336 stabilized multi-faceted gold nanoparticles with minimal ligand density. Appl Surf Sci. 2016;363:74–82.
Bodnar M, Minko T, Hartmann JF, Borbely J. Fluorescent nanoparticles based on chitosan. NSTI-Nanotech. 2007;2:279–82.
Remya NS, Syama S, Sabareeswaran A, Mohanan PV. Toxicity, toxicokinetics and biodistribution of dextran stabilized iron oxide nanoparticles for biomedical applications. Int J Pharm. 2016;511:586–98.
Jadhav SV, Shewale PS, Shin BC, Patil MP, Kim GD, Rokade AA, et al. Study of structural and magnetic properties and heat induction of gadolinium-substituted manganese zinc ferrite nanoparticles for in vitro magnetic fluid hyperthermia. J Colloid Interface Sci. 2019;541:192–203.
Leach WT, Simpson DT, Val TN, Yu Z, Lim KT, Park EJ, et al. Encapsulation of protein nanoparticles into uniform-sized microspheres formed in a spinning oil film. AAPS PharmSciTech. 2005;6:E605–17.
Bule MV, Singhal RS, Kennedy JF. Microencapsulation of ubiquinone-10 in carbohydrate matrices for improved stability. Carbohydr Polym. 2010;82:1290–6.
Hwang SR, Lim S-J, Park J-S, Kim C-K. Phospholipid-based microemulsion formulation of all-trans-retinoic acid for parenteral administration. Int J Pharm. 2004;276:175–83.
Rowe RC, Sheskey PJ, Owen SC, American Pharmacists Association. Handbook of pharmaceutical excipients. London: Pharmaceutical Press; American Pharmacists Association; 2006.
Carlotti ME, Rossatto V, Gallarate M, Trotta M, Debernardi F. Vitamin A palmitate photostability and stability over time. Int J Cosmet Sci. 2004;26:270.
Kwon SS, Nam YS, Lee JS, Ku BS, Han SH, Lee JY, et al. Preparation and characterization of coenzyme Q10-loaded PMMA nanoparticles by a new emulsification process based on microfluidization. Colloids Surf A Physicochem Eng Asp. 2002;210:95–104.
El-Leithy ES, Abdel-Rashid RS. Lipid nanocarriers for tamoxifen citrate/coenzyme Q10 dual delivery. J Drug Deliv Sci Technol. 2017;41:239–50.
Phaechamud T, Jantadee T, Mahadlek J, Charoensuksai P, Pichayakorn W. Characterization of antimicrobial agent loaded Eudragit RS solvent exchange-induced in situ forming gels for periodontitis treatment. AAPS PharmSciTech. 2017;18:494–508.
Popa M, Ghica MV, Dinu-Pîrvu E. Periodontal chitosan-gels designed for improved local intra-pocket drug delivery. Farmacia. 2013;61:240–50.
Ankola DD, Viswanad B, Bhardwaj V, Ramarao P, Kumar MNVR. Development of potent oral nanoparticulate formulation of coenzyme Q10 for treatment of hypertension: can the simple nutritional supplements be used as first line therapeutic agents for prophylaxis/therapy? Eur J Pharm Biopharm. 2007;67:361–9.
Nazzal S, Guven N, Reddy IK, Khan MA. Preparation and characterization of coenzyme Q10–Eudragit® solid dispersion. Drug Dev Ind Pharm. 2002;28:49–57.
Sun J, Wang F, Sui Y, She Z, Zhai W, Wang C, et al. Effect of particle size on solubility, dissolution rate, and oral bioavailability: evaluation using coenzyme Q10 as naked nanocrystals. Int J Nanomedicine. 2012;7:5733–44.
Wang Y, Li Y, Wang Q, Wu J, Fang X. Pharmacokinetics and biodistribution of paclitaxel-loaded pluronic P105/L101 mixed polymeric micelles. Yakugaku Zasshi. 2008;128:941–50.
Yu H, Chen X, Cai J, Ye D, Wu Y, Liu P. Dual controlled release nanomicelle-in-nanofiber system for long-term antibacterial medical dressings. J Biomater Sci Polym Ed. 2018:1–13.
Jadhav P, Bothiraja C, Pawar A. Methotrexate-loaded nanomixed micelles: formulation, characterization, bioavailability, safety, and in vitro anticancer study. J Pharm Innov. 2018;13:213–25.
Xue B, Wang Y, Tang X, Xie P, Wang Y, Luo F, et al. Biodegradable self-assembled MPEG-PCL micelles for hydrophobic oridonin delivery in vitro. J Biomed Nanotechnol. 2012;8:80–9.
Wang Y, Lapitsky Y, Kang CE, Shoichet MS. Accelerated release of a sparingly soluble drug from an injectable hyaluronan–methylcellulose hydrogel. J Control Release. 2009;140:218–23.
Heng D, Cutler DJ, Chan H-K, Yun J, Raper JA. What is a suitable dissolution method for drug nanoparticles? Pharm Res. 2008;25:1696–701.
Hoskins C, Cheng WP. Hydrophobic drug solubilisation. In: Uchegbu IF, Schätzlein AG, Cheng WP, Aikaterini L, editors. Fundamentals of pharmaceutical nanoscience. New York: Springer; 2013. p. 375–98.
Boogaerts JG, Lafont ND, Declercq AG, Luo HC, Gravet ET, Bianchi JA, et al. Epidural administration of liposome associated bupivacaine for the management of postsurgical pain: a first study. J Clin Anesth. 1994;6:315–20.
Barat R, Srinatha A, Pandit JK, Ridhurkar D, Balasubramaniam J, Mittal N, et al. Niridazole biodegradable inserts for local long-term treatment of periodontitis: possible new life for an orphan drug. Drug Deliv. 2006;13:365–73.
Pignatello R, Stancampiano AHS, Ventura CA, Puglisi G. Dexamethasone sodium phosphate-loaded chitosan based delivery systems for buccal application. J Drug Target. 2007;15:603–10.
Franz-Montan M, Cereda CMS, Gaspari A, da Silva CMG, de Araújo DR, Padula C, et al. Liposomal-benzocaine gel formulation: correlation between in vitro assays and in vivo topical anesthesia in volunteers. J Liposome Res. 2013;23:54–60.
Gadagi JS, Chava VK, Reddy VR. Green tea extract as a local drug therapy on periodontitis patients with diabetes mellitus: a randomized case–control study. J Indian Soc Periodontol. 2013;17:198–203.
Stephon D. Clinical trial supplies. In: Bunn GP, editor. Good manufacturing practices for pharmaceuticals. 7th ed. Boca Raton: CRC, Taylor & Francis Group; 2019. p. 127–36.
Goodson JM. Pharmacokinetic principles controlling efficacy of oral therapy. J Dent Res. 1989;68:1625–32.
Pradeep AR, Sagar SV, Daisy H. Clinical and microbiologic effects of subgingivally delivered 0.5% azithromycin in the treatment of chronic periodontitis. J Periodontol. 2008;79:2125–35.
Acknowledgments
We gratefully acknowledge Dr. Mohammad El Nabalawy, lecturer at the Department of Medical Biochemistry, Faculty of Medicine, Mansoura University, for his support in the biochemical assay.
Author information
Authors and Affiliations
Contributions
Conceptualization: SHE, MMA, and FBB; methodology: MAS and NMS; formal analysis and investigation: MAS and NMS; writing—original draft preparation: NMS; writing—review and editing: SHE, MMA, and NMS; supervision: SHE, MMA, and FBB.
Corresponding author
Ethics declarations
The experiments comply with the current laws of the country in which they were performed. All procedures were conducted in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 (5). Informed consents were obtained from all patients for being included in the study.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shaheen, M.A., Elmeadawy, S.H., Bazeed, F.B. et al. Innovative coenzyme Q10-loaded nanoformulation as an adjunct approach for the management of moderate periodontitis: preparation, evaluation, and clinical study. Drug Deliv. and Transl. Res. 10, 548–564 (2020). https://doi.org/10.1007/s13346-019-00698-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-019-00698-z