Abstract
Purpose
Methotrexate (MTX), a potent anticancer drug, shows low oral bioavailability due to its low aqueous solubility and permeability. Moreover, its multidrug resistance (MDR) in cancer and toxic effects restricts its clinical applications. The aim of the study was to prepare novel MTX-loaded Poloxamer 407 and Gelucire®44/14 (GL44) mixed micelles (MTX-PGMM) to improve its oral bioavailability and cytotoxicity and to reduce its systemic toxicity.
Methods
MTX-PGMM was prepared by dialysis method, compared with blank or MTX-free blank mixed micelles (BMM) and aqueous dispersion of MTX (MTX-AD) with respect to morphology, size, zeta potential, entrapment efficiency, in vitro release, and in vitro cytotoxicity, bioavailability, and toxicity study in rats.
Results
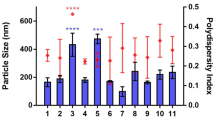
The developed MTX-PGMM exhibited small particle size, good encapsulation efficiency, and good stability in media simulating the physiological conditions of the gastrointestinal tract. The MTX-PGMM micelles demonstrated sustained release, enhanced (6.5-fold) oral bioavailability, slow plasma elimination, and long circulation of MTX. Biocompatibility was also established as there were no signs of tissue toxicity. Moreover, MTX-PGMM produced higher in vitro cytotoxicity (GI50 of 1.44 ± 0.33 μg/mL) than free MTX (GI50 13.71 ± 0.99 μg/mL) in human breast cancer MCF-7 cells.
Conclusion
Poloxamer 407 and Gelucire®44/14 (GL44) mixed micelles are promising carriers for oral delivery of MTX and can be utilized for other Biopharmaceutical Classification System class IV anticancer drugs having poor oral bioavailability and multidrug resistance in cancer.









Similar content being viewed by others
References
Bromberg L. Polymeric micelles in oral chemotherapy. J Control Release. 2008;128(2):99–112. https://doi.org/10.1016/j.jconrel.2008.01.018.
Abolmaali SS, Tamaddon AM, Dinarvand R. A review of therapeutic challenges and achievements of methotrexate delivery systems for treatment of cancer and rheumatoid arthritis. Cancer Chemother Pharmacol. 2013;71(5):1115–30. https://doi.org/10.1007/s00280-012-2062-0.
Braun J, Rau R. An update on methotrexate. Curr Opin Rheumatol. 2009;21(3):216–23. https://doi.org/10.1097/BOR.0b013e328329c79d.
Van Roon EN, Van de Laar MAFJ. Methotrexate bioavailability. Clin Exp Rheumatol. 2010;28(Suppl. 61):27–32.
Visser K, Van der Heijde DM. Risk and management of liver toxicity during methotrexate treatment in rheumatoid and psoriatic arthritis: a systematic review of the literature. Clin Exp Rheumatol. 2009;27(6):1017–25.
Paliwal R, Paliwal S, Mishra N, Mehta A, Vyas SP. Engineered chylomicron mimicking carrier emulsome for lymph targeted oral delivery of methotrexate. Int J Pharm. 2009a;380, 1–2:181–8.
MadhanKumar AB, Panduranga Rao K. Preparation and characterization of pH-sensitive proteinoid microspheres for the oral delivery of methotrexate. Biomaterials. 1998;19(19, 7–9):725–32. https://doi.org/10.1016/S0142-9612(97)00188-9.
Paliwal R, Rai S, Vyas SP. Lipid drug conjugate (LDC) nanoparticles as autolymphotrophs for oral delivery of methotrexate. J Biomed Nanotechnol. 2011;7(1):130–1. https://doi.org/10.1166/jbn.2011.1235.
Paliwal R, Rai S, Vaidya B, Khatri K, Goyal AK, Mishra N, et al. Effect of lipid core material on characteristics of solid lipid nanoparticles designed for oral lymphatic delivery. Nanomed. 2009b;5(2):184–91.
Bhaskaran S, Harish CG, Lakshmi PK. Methotrexate liposome as oral drug delivery carriers. J Pharm Res. 2011;4:3237.
Mi Y, Liu YT, Feng SS. Formulation of docetaxel loaded folic acid-conjugated D-tocopheryl polyethylene glycol succinate 2000 (vitamin ETPGS (2k)) micelles for targeted and synergistic chemotherapy. Biomaterials. 2011;32(16):4058–66. https://doi.org/10.1016/j.biomaterials.2011.02.022.
Zhang W, Shi Y, Chen Y, Ye J, Sha X, Fang X. Multifunctional Pluronic P123/F127 mixed polymeric micelles loaded with paclitaxel for the treatment of multidrug resistant tumors. Biomaterials. 2011;32(11):2894–906. https://doi.org/10.1016/j.biomaterials.2010.12.039.
Dou J, Haiqun Z, Xiuju L, Mengyu Z, Guangxi Z. Preparation and evaluation in vitro and in vivo of docetaxel loaded mixed micelles for oral administration. Colloids Surf B: Biointerfaces. 2014;114:20–7. https://doi.org/10.1016/j.colsurfb.2013.09.010.
Saxena V, Hussain MD. Polymeric mixed micelles for delivery of curcumin to multidrug resistant ovarian cancer. J Biomed Nanotechnol. 2013;9(7):1146–54. https://doi.org/10.1166/jbn.2013.1632.
Kulthe SS, Inamdar NN, Choudhari YM, Shirolikar SM, Borde LC, Mourya VK. Mixed micelle formation with hydrophobic and hydrophilic Pluronic block copolymers: implications for controlled and targeted drug delivery. Colloids and Surf B: Biointerfaces. 2011;88(2):691–6. https://doi.org/10.1016/j.colsurfb.2011.08.002.
Bothiraja C, Kapare HS, Pawar AP, Shaikh KS. Development of plumbagin-loaded phospholipid–Tween® 80 mixed micelles: formulation, optimization, effect on breast cancer cells and human blood/serum compatibility testing. Ther Deliv. 2013;4(10):1247–59. https://doi.org/10.4155/tde.13.92.
Li LB, Tan YB. Preparation and properties of mixed micelles made of Pluronic polymer and PEG-PE. J Colloid Interface Sci. 2008;317(1):326–31. https://doi.org/10.1016/j.jcis.2007.09.053.
Zhang Y, Jin T, Zhuo R. Methotrexate-loaded biodegradable polymeric micelles: preparation, physicochemical properties and in vitro drug release. Colloids Surf B: Biointerfaces. 2005;44(2–3):104–9. https://doi.org/10.1016/j.colsurfb.2005.06.004.
Batrakova EV, Li S, Alakhov VY, Miller DW, Kabanov AV. Optimal structure requirements for Pluronic block copolymers in modifying p-glycoprotein drug efflux transporter activity in bovine brain microvessel endothelial cells. J Pharmacol Exp Ther. 2003;304(2):845–54. https://doi.org/10.1124/jpet.102.043307.
Kawakami K, Oda N, Miyoshi K, Funki T, Eda Y. Solubilization behavior of poorly soluble drug under combined use of surfactants and co solvents. Eur J Pharm Sci. 2008;28:7–14.
Sachs-Barrable K, Thamboo A, Lee SD, Wasan KM. Lipid excipients Peceol and Gelucire 44/14 decrease P-glycoprotein mediated efflux of rhodamine 123 partially due to modifying P-glycoprotein protein expression within Caco-2 cells. J Pharm Sci. 2007;10(3):319–31.
Bansal T, Akhtar N, Jaggi M, Khar RK, Talegaonkar S. Novel formulation approaches for optimising delivery of anticancer drugs based on P-glycoprotein modulation. Drug Discov Today. 2009;14(21–22):1067–74. https://doi.org/10.1016/j.drudis.2009.07.010.
Bothiraja C, Pawar AP. Improved bioavailability of orally administered andrographolide from pH-sensitive nanoparticles. Eur J Drug Metab Pharmacokinet. 2011;35(3–4):123–9.
Wei Z, Hao J, Yuan S, Li Y, Juan W, Sha X, et al. Paclitaxel loaded Pluronic P123/F127 mixed polymeric micelles: formulation, optimization and in vitro characterization. Int J Pharm. 2009;376:176–85.
Dahmani F, Zohra H, Yang J, Zhou J, Yao T, Zhang Q. Enhanced oral bioavailability of paclitaxel in Pluronic/LHR mixed polymeric micelles: preparation, in vitro and in vivo evaluation. Eur J Pharm Sci. 2012;47(1):179–89. https://doi.org/10.1016/j.ejps.2012.05.015.
Pawar AP, Vinugala D, Bothiraja C. Nanocochleates derived from nanoliposomes for paclitaxel oral use: preparation, characterization, in vitro anticancer testing, bioavailability and biodistribution study in rats. Biomed Pharmacother. 2014;3502:1–9.
Devi KV, Raichur V. Development and validation of a highly sensitive high-performance liquid chromatography (HPLC) method for the estimation of methotrexate (MTX) pure drug and marketed formulation in spiked rat plasma. RJPPS. 2016;8(3):45–51.
Patil S, Choudhary B, Rathore A, Roya K, Mahadik K. Enhanced oral bioavailability and anticancer activity of novel curcumin loaded mixed micelles in human lung cancer cells. Phytomedicine. 2015;22(12):1103–11. https://doi.org/10.1016/j.phymed.2015.08.006.
Li X, Zhang Y, Fan Y, Zhou Y, Wang X, Fan C, et al. Preparation and evaluation of novel mixed micelles as nanocarriers for intravenous delivery of propofol. Nanoscale Res Lett. 2011;6:2–9.
Bothiraja C, Pawar A, Patel R, Arulmozhi S. D-a-Tocopheryl polyethylene glycol 1000 succinate conjugated folic acid nanomicelles: towards enhanced bioavailability, stability, safety, prolonged drug release and synergized anticancer effect of plumbagin. RSC Adv. 2016;6:78106.
Butt A, Masood M, Cairul I, Mohd A, Haliza K, Narong S, et al. In vitro characterization of Pluronic F127 and D-α-tocopheryl polyethylene glycol 1000 succinate mixed micelles as nanocarriers for targeted anticancer-drug delivery. J Nanomater. 2012;10:1155.
Gao Y, Li LB, Zhai G. Preparation and characterization of pluronic/TPGS mixed micelles for solubilization of camptothecin. Colloids Surf B. 2008;64(2):194–9. https://doi.org/10.1016/j.colsurfb.2008.01.021.
Vadia N, Rajput S. Study on formulation variables of methotrexate loaded mesoporous MCM-41 nanoparticles for dissolution enhancement. Eur J Pharm Sci. 2012;45(1-2):8–18. https://doi.org/10.1016/j.ejps.2011.10.016.
Mu L, Elbayoumi TA, Torchilin VP. Mixed micelles made of poly (ethylene glycol)-phosphatidylethanolamine conjugate and D-α-tocopheryl polyethylene glycol 1000 succinate as pharmaceutical nanocarriers for camptothecin. Int J Pharm. 2005;306(1–2):142–9. https://doi.org/10.1016/j.ijpharm.2005.08.026.
Song H, Geng H, Ruan J, Wang K, Bao C, Wang J, et al. Development of polysorbate 80 / phospholipid mixed micellar formation for docetaxel and assessment of its in vivo distribution in animal models. Nanoscale Res Lett. 2011;6(1):354. https://doi.org/10.1186/1556-276X-6-354.
Dumortier G, Grossiord JL, Florence A, Chaumeil JC. A review of Poloxamer 407 pharmaceutical and pharmacological characteristics. Pharm Res. 2006;23(12):2709–28. https://doi.org/10.1007/s11095-006-9104-4.
Acknowledgements
The authors are thankful to All India Council for Technical Education (AICTE), New Delhi, India, for providing financial support in the form of a Quality Improvement Programme (QIP) Fellowship to Mr. Prakash Jadhav.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All studies were approved by the Institutional Animal Ethics Committee of Poona College of Pharmacy (Pune, India, Registration number: 1703/PO/c/13/CPCSEA) with protocol approval number CPCSEA/PCT/15/2015 and were conducted under the provisions of the CPCSEA guidelines.
Rights and permissions
About this article
Cite this article
Jadhav, P., Bothiraja, C. & Pawar, A. Methotrexate-Loaded Nanomixed Micelles: Formulation, Characterization, Bioavailability, Safety, and In Vitro Anticancer Study. J Pharm Innov 13, 213–225 (2018). https://doi.org/10.1007/s12247-018-9314-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-018-9314-4