Abstract
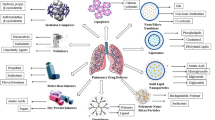
Delivering drugs through inhalation for systemic and local applications has been in practice since several decades to treat various diseases. In recent times, inhalation drug delivery is becoming one of the highly focused areas of research in the pharmaceutical industry. It is being considered as one of the major portals for delivering drugs because of its wide range of advantages like requirement of low concentrations of drug to reach therapeutic efficacy, surpassing first pass metabolism and a very low incidence of side effects as compared to conventional delivery of drugs. Owing to these favorable characteristics of pulmonary drug delivery, diverse pharmaceutical formulations like liposomes, nanoparticles, and microparticles are developed through consistent efforts for delivery drugs to lungs in suitable form. However, drug-loaded microparticles have displayed various advantages over the other pharmaceutical dosage forms which give a cutting edge over other inhalational drug delivery systems. Assuring results with respect to sustained release through inhalational delivery of drug-loaded microparticles from pre-clinical studies are anticipative of similar benefits in the clinical settings. This review centralizes partly on the advantages of inhalational microparticles over other inhalational dosage forms and largely on the therapeutic applications and future perspectives of inhalable microparticle drug delivery systems.




Similar content being viewed by others
References
Stein SW, Thiel CG. The history of therapeutic aerosols: a chronological review. J Aerosol Med Pulm Drug Deliv. 2017;30(1):20–41.
Sanders M. Inhalation therapy: an historical review. Prim Care Respir J. 2007;16(2):71–81.
Lee W-H, et al. Inhalation of nanoparticle-based drug for lung cancer treatment: advantages and challenges. Asian J Pharm Sci. 2015;10(6):481–9.
Gonda I. Systemic delivery of drugs to humans via inhalation. J Aerosol Med. 2006;19(1):47–53.
Patton JS, Byron PR. Inhaling medicines: delivering drugs to the body through the lungs. Nat Rev Drug Discov. 2007;6(1):67–74.
Agu RU, et al. The lung as a route for systemic delivery of therapeutic proteins and peptides. Respir Res. 2001;2(4):198.
Kim J, et al. Targeted delivery of liquid microvolumes into the lung. Proc Natl Acad Sci. 2015;112(37):11530–5.
Mohanty RR, Das S. Inhaled insulin-current direction of insulin research. J Clin Diagn Res. 2017;11(4):OE01–2.
Cukic V, Lovre V, Dragisic D, Ustamujic A. Asthma and chronic obstructive pulmonary disease (COPD)–differences and similarities. Materia socio-medica. 2012;24(2):100–5.
Bautista SC, et al. Administration of anti-infective agents through the inhaled route. Farm Hosp. 2007;31(2):112.
Zhou QT, et al. Inhaled formulations and pulmonary drug delivery systems for respiratory infections. Adv Drug Deliv Rev. 2015;85:83–99.
El-Sherbiny IM, El-Baz NM, Yacoub MH. Inhaled nano-and microparticles for drug delivery. Glob Cardiol Sci Pract. 2015;2015(1):2.
Labiris N, Dolovich M. Pulmonary drug delivery. Part I: physiological factors affecting therapeutic effectiveness of aerosolized medications. Br J Clin Pharmacol. 2003;56(6):588–99.
Darquenne C. Aerosol deposition in health and disease. J Aerosol Med Pulm Drug Deliv. 2012;25(3):140–7.
Ibrahim M, Verma R, Garcia-Contreras L. Inhalation drug delivery devices: technology update. Med Devices (Auckland, NZ). 2015;8:131.
Pandey R, Khuller G. Antitubercular inhaled therapy: opportunities, progress and challenges. J Antimicrob Chemother. 2005;55(4):430–5.
Ngan CL, Asmawi AA. Lipid-based pulmonary delivery system: a review and future considerations of formulation strategies and limitations. Drug Deliv Transl Res. 2018;8(5):1527–44.
Thomas RJ. Particle size and pathogenicity in the respiratory tract. Virulence. 2013;4(8):847–58.
Newman SP. Drug delivery to the lungs: challenges and opportunities. Ther Deliv. 2017;8(8):647–61.
Akbarzadeh A, et al. Liposome: classification, preparation, and applications. Nanoscale Res Lett. 2013;8(1):102.
Shade CW. Liposomes as advanced delivery systems for nutraceuticals. Integrative Medicine: A Clinician's Journal. 2016;15(1):33.
Cosco D, et al. Liposomes as multicompartmental carriers for multidrug delivery in anticancer chemotherapy. Drug Deliv Transl Res. 2011;1(1):66–75.
Sercombe L, et al. Advances and challenges of liposome assisted drug delivery. Front Pharmacol. 2015;6:286.
Thulasiramaraju T, et al. Liposome: a novel drug delivery system. Int J Biochem. 2012;2229:7499.
Grit M, Crommelin DJ. Chemical stability of liposomes: implications for their physical stability. Chem Phys Lipids. 1993;64(1–3):3–18.
Pattni BS, Chupin VV, Torchilin VP. New developments in liposomal drug delivery. Chem Rev. 2015;115(19):10938–66.
Gonzalez-Rothi RJ, et al. Liposomes and pulmonary alveolar macrophages: functional and morphologic interactions. Exp Lung Res. 1991;17(4):687–705.
Poelma D, Van Iwaarden J, Lachmann B. Surfactant metabolism: factors affecting lipid uptake in vivo and in vitro, in Anaesthesia, Pain, Intensive Care and Emergency Medicine—APICE. Milano: Springer; 2005. p. 259–77.
Schreier H, Gonzalez-Rothi RJ, Stecenko AA. Pulmonary delivery of liposomes. J Control Release. 1993;24(1–3):209–23.
Pagano RE, Weinstein JN. Interactions of liposomes with mammalian cells. Annu Rev Biophys Bioeng. 1978;7(1):435–68.
Szebeni J, Barenholz Y. Adverse immune effects of liposomes: complement activation. Immunogenicity and Immune Suppression. 2009. https://www.ncbi.nlm.nih.gov/pubmed/21787819. Accessed 26 Jan 2018.
Gregoriadis G, Florence AT. Liposomes in drug delivery. Drugs. 1993;45(1):15–28.
Elhissi A. Liposomes for pulmonary drug delivery: the role of formulation and inhalation device design. Curr Pharm Des. 2017;23(3):362–72.
De Leo V, et al. Preparation of drug-loaded small unilamellar liposomes and evaluation of their potential for the treatment of chronic respiratory diseases. Int J Pharm. 2018;545(1-2):378–88.
Rashid J, et al. Fasudil and DETA NONOate, loaded in a peptide-modified liposomal carrier, slow PAH progression upon pulmonary delivery. Mol Pharm. 2018;15(5):1755–65.
Riaz MK, et al. Pulmonary delivery of transferrin receptors targeting peptide surface-functionalized liposomes augments the chemotherapeutic effect of quercetin in lung cancer therapy. Int J Nanomedicine. 2019;14:2879.
Mudshinge SR, et al. Nanoparticles: emerging carriers for drug delivery. Saudi Pharm J. 2011;19(3):129–41.
Singh R, Lillard JW Jr. Nanoparticle-based targeted drug delivery. Exp Mol Pathol. 2009;86(3):215–23.
Desai N. Challenges in development of nanoparticle-based therapeutics. AAPS J. 2012;14(2):282–95.
Warheit DB, Reed KL, Sayes CM. A role for nanoparticle surface reactivity in facilitating pulmonary toxicity and development of a base set of hazard assays as a component of nanoparticle risk management. Inhal Toxicol. 2009;21(sup1):61–7.
Saptarshi SR, Duschl A, Lopata AL. Interaction of nanoparticles with proteins: relation to bio-reactivity of the nanoparticle. J Nanobiotechnol. 2013;11(1):26.
Bakand S, Hayes A. Toxicological considerations, toxicity assessment, and risk management of inhaled nanoparticles. Int J Mol Sci. 2016;17(6):929.
Papageorgiou I, et al. The effect of nano-and micron-sized particles of cobalt–chromium alloy on human fibroblasts in vitro. Biomaterials. 2007;28(19):2946–58.
Song Y, Li X, Du X. Exposure to nanoparticles is related to pleural effusion, pulmonary fibrosis and granuloma. Eur Respir J. 2009;34(3):559–67.
Madhav NS, Kala S. Review on microparticulate drug delivery system. Int J PharmTech Res. 2011;3(3):1242–4.
Kemala T, Budianto E, Soegiyono B. Preparation and characterization of microspheres based on blend of poly (lactic acid) and poly (ɛ-caprolactone) with poly (vinyl alcohol) as emulsifier. Arab J Chem. 2012;5(1):103–8.
Kohane DS. Microparticles and nanoparticles for drug delivery. Biotechnol Bioeng. 2007;96(2):203–9.
Doodipala, N.R., Polymeric matrices at micro and nanoscale for ocular drug delivery. 2017.
Siepmann J, Siepmann F. Microparticles used as drug delivery systems. In: Smart colloidal materials. Berlin: Springer; 2006. p. 15–21.
Heijerman H, et al. Inhaled medication and inhalation devices for lung disease in patients with cystic fibrosis: a European consensus. J Cyst Fibros. 2009;8(5):295–315.
Champion JA, Walker A, Mitragotri S. Role of particle size in phagocytosis of polymeric microspheres. Pharm Res. 2008;25(8):1815–21.
Hassan MS, Lau RWM. Effect of particle shape on dry particle inhalation: study of flowability, aerosolization, and deposition properties. AAPS PharmSciTech. 2009;10(4):1252–62.
Cai Y, et al. Porous microsphere and its applications. Int J Nanomedicine. 2013;8:1111.
Zhou M, et al. Design and pharmaceutical applications of porous particles. RSC Adv. 2017;7(63):39490–501.
Zdravkov BD, et al. Pore classification in the characterization of porous materials: a perspective. Cent Eur J Chem. 2007;5(2):385–95.
Edwards DA, Ben-Jebria A, Langer R. Recent advances in pulmonary drug delivery using large, porous inhaled particles. J Appl Physiol. 1998;85(2):379–85.
Edwards DA, et al. Large porous particles for pulmonary drug delivery. Science. 1997;276(5320):1868–72.
Tsapis N, et al. Trojan particles: large porous carriers of nanoparticles for drug delivery. Proc Natl Acad Sci. 2002;99(19):12001–5.
Jain A, Jain SK. In vitro release kinetics model fitting of liposomes: an insight. Chem Phys Lipids. 2016;201:28–40.
D’Souza S. A review of in vitro drug release test methods for nano-sized dosage forms. Adv Pharm. 2014;2014:1–12.
Shazly G, Nawroth T, Langguth P. Comparison of dialysis and dispersion methods for in vitro release determination of drugs from multilamellar liposomes. Dissolut Technol. 2008;15(2):7.
Han FY, et al. Bioerodable PLGA-based microparticles for producing sustained-release drug formulations and strategies for improving drug loading. Front Pharmacol. 2016;7:185.
Faisant N, Siepmann J, Benoit J. PLGA-based microparticles: elucidation of mechanisms and a new, simple mathematical model quantifying drug release. Eur J Pharm Sci. 2002;15(4):355–66.
Jain R, et al. Drug Nano-particle: a release kinetics. Journal of Nanomedicine & Nanotechnology. 2015;6(5):1.
Pai RV, et al. Development and evaluation of chitosan microparticles based dry powder inhalation formulations of rifampicin and rifabutin. J Aerosol Med Pulm Drug Deliv. 2016;29(2):179–95.
Fourie PB, Oluwarotimi S. Inhaled therapies for tuberculosis: a viable approach for spray-dried drugs delivered by handheld dry powder inhaler. Inhalation. 2015;9:1–5.
Kjellsson MC, et al. Pharmacokinetic evaluation of the penetration of antituberculosis agents in rabbit pulmonary lesions. Antimicrob Agents Chemother. 2012;56(1):446–57.
Sacks LV, et al. Adjunctive salvage therapy with inhaled aminoglycosides for patients with persistent smear-positive pulmonary tuberculosis. Clin Infect Dis. 2001;32(1):44–9.
Suarez S, et al. Airways delivery of rifampicin microparticles for the treatment of tuberculosis. J Antimicrob Chemother. 2001;48(3):431–4.
Parikh R, Patel L, Dalwadi S. Microparticles of rifampicin: comparison of pulmonary route with oral route for drug uptake by alveolar macrophages, phagocytosis activity and toxicity study in albino rats. Drug Deliv. 2014;21(6):406–11.
Garcia Contreras L, et al. Pharmacokinetics of inhaled rifampicin porous particles for tuberculosis treatment: insight into rifampicin absorption from the lungs of guinea pigs. Mol Pharm. 2015;12(8):2642–50.
Garcia-Contreras L, et al. Pharmacokinetics of ethionamide delivered in spray-dried microparticles to the lungs of guinea pigs. J Pharm Sci. 2017;106(1):331–7.
Verma RK, et al. Inhaled microparticles containing clofazimine are efficacious in treatment of experimental tuberculosis in mice. Antimicrob Agents Chemother. 2013;57(2):1050–2.
Organization, WH. http://www.who.int/mediacentre/factsheets/fs340/en. 2014, Accessed.
Torre LA, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108.
Ferlay J, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–86.
Zugazagoitia J, et al. Current challenges in cancer treatment. Clin Ther. 2016;38(7):1551–66.
Mokhtari RB, et al. Combination therapy in combating cancer. Oncotarget. 2017;8(23):38022.
Kyle AH, et al. Limited tissue penetration of taxanes: a mechanism for resistance in solid tumors. Clin Cancer Res. 2007;13(9):2804–10.
Kim I, et al. Doxorubicin-loaded highly porous large PLGA microparticles as a sustained-release inhalation system for the treatment of metastatic lung cancer. Biomaterials. 2012;33(22):5574–83.
Feng T, et al. Synergistic co-delivery of doxorubicin and paclitaxel by porous PLGA microspheres for pulmonary inhalation treatment. Eur J Pharm Biopharm. 2014;88(3):1086–93.
Sato T, et al. Intrapulmonary delivery of CpG microparticles eliminates lung tumors. Mol Cancer Ther. 2015;14(10):2198–205.
Zhu L, et al. Inhalable oridonin-loaded poly (lactic-co-glycolic) acid large porous microparticles for in situ treatment of primary non-small cell lung cancer. Acta Pharm Sin B. 2017;7(1):80–90.
Education NA, et al. Section 2, Definition, pathophysiology and pathogenesis of asthma, and natural history of asthma, in Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. 2007, National Heart, Lung, and Blood Institute (US).
Pawankar R. Allergic diseases and asthma: a global public health concern and a call to action. BioMed Central. 2014. 7(1):12, 1–3
Barnes PJ. Development of new drugs for COPD. Curr Med Chem. 2013;20(12):1531–40.
Moral VP, Donaire JG. Inhaled therapy in asthma. Medicina Clínica (English Edition). 2016;146(7):316–23.
Durham AL, et al. Targeted anti-inflammatory therapeutics in asthma and chronic obstructive lung disease. Transl Res. 2016;167(1):192–203.
Dekhuijzen PR, et al. Incidence of oral thrush in patients with COPD prescribed inhaled corticosteroids: effect of drug, dose, and device. Respir Med. 2016;120:54–63.
Cooper V, et al. Patient-reported side effects, concerns and adherence to corticosteroid treatment for asthma, and comparison with physician estimates of side-effect prevalence: a UK-wide, cross-sectional study. NPJ Prim Care Respir Med. 2015;25:15026.
Patel B, et al. Low-molecular-weight heparin-coated and montelukast-filled inhalable particles: a dual-drug delivery system for combination therapy in asthma. J Pharm Sci. 2017;106(4):1124–35.
Oh YJ, et al. Preparation of budesonide-loaded porous PLGA microparticles and their therapeutic efficacy in a murine asthma model. J Control Release. 2011;150(1):56–62.
Yang W-K, et al. Effects of inhalable microparticles of Seonpyejeongcheon-tang in an asthma mouse model:-effects of microparticles of SJT. J Pharm. 2016;19(4):303–11.
Dufour G, Bigazzi W, Wong N, Boschini F, de Tullio P, Piel G, et al. Interest of cyclodextrins in spray-dried microparticles formulation for sustained pulmonary delivery of budesonide. Int J Pharm. 2015;495(2):869–78.
de Oliveira JF, et al. Therapeutic potential of biodegradable microparticles containing Punica granatum L.(pomegranate) in murine model of asthma. Inflamm Res. 2013;62(11):971–80.
Dhoble S, Patravale V. Development of anti-angiogenic erlotinib liposomal formulation for pulmonary hypertension: a QbD approach. Drug Deliv Transl Res. 2019: 9(5):1–17.
Lai Y-C, et al. Pulmonary arterial hypertension: the clinical syndrome. Circ Res. 2014;115(1):115–30.
Raja SG, Raja SM. Treating pulmonary arterial hypertension: current treatments and future prospects. Ther Adv Chronic Dis. 2011;2(6):359–70.
Gupta V, et al. PLGA microparticles encapsulating prostaglandin E 1-hydroxypropyl-β-cyclodextrin (PGE 1-HPβCD) complex for the treatment of pulmonary arterial hypertension (PAH). Pharm Res. 2011;28(7):1733–49.
Gupta V, et al. Inhaled PLGA particles of prostaglandin E1 ameliorate symptoms and progression of pulmonary hypertension at a reduced dosing frequency. Mol Pharm. 2013;10(5):1655–67.
Anselmo AC, Mitragotri S. An overview of clinical and commercial impact of drug delivery systems. J Control Release. 2014;190:15–28.
Sánchez-Fueyo A, Strom TB. Immunologic basis of graft rejection and tolerance following transplantation of liver or other solid organs. Gastroenterology. 2011;140(1):51–64.e2.
Hartono C, Muthukumar T, Suthanthiran M. Immunosuppressive drug therapy. Cold Spring Harb Perspect Med. 2013;3(9):a015487.
Iacono AT, et al. A randomized trial of inhaled cyclosporine in lung-transplant recipients. N Engl J Med. 2006;354(2):141–50.
Karimi-Shah BA, Chowdhury BA. Forced vital capacity in idiopathic pulmonary fibrosis—FDA review of pirfenidone and nintedanib. N Engl J Med. 2015;372(13):1189–91.
da Silva Bitencourt C, et al. Hyaluronidase-loaded PLGA microparticles as a new strategy for the treatment of pulmonary fibrosis. Tissue Eng A. 2014;21(1–2):246–56.
Acknowledgments
The authors sincerely thank all of the research teams who contributed in the field of therapeutic applications of microparticle drug delivery system. We acknowledge DST-Science and Engineering Board-Early Career Research Award (SERB-ECR) Grant: ECR/2016/000007 and Department of Pharmaceuticals, Ministry of Chemicals and Fertilizers, Government of India.
Author information
Authors and Affiliations
Contributions
All the authors have significantly contributed to the concept and writing of manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pulivendala, G., Bale, S. & Godugu, C. Inhalation of sustained release microparticles for the targeted treatment of respiratory diseases. Drug Deliv. and Transl. Res. 10, 339–353 (2020). https://doi.org/10.1007/s13346-019-00690-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-019-00690-7