Abstract
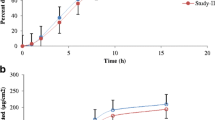
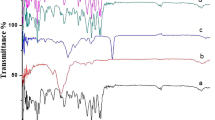
The first objective of the proposed research work includes comparative bioavailability and bioequivalence evaluation of oxybutynin transdermal patch with respect to different permeation enhancers. The second objective was to evaluate different in vitro methods along with synthetic membranes toward development of an in vitro–in vivo correlation. Oleic acid (fatty acid), Soluphor P (2-pyrrolidone, cosolvent), menthol (volatile oil), and dipropylene glycol (plasticizer) were selected as representatives from different classes of permeation enhancers. A random, crossover, single-dose pharmacokinetic study was carried out on male New Zealand white rabbits to determine bioavailability and bioequivalence. The obtained pharmacokinetic data were correlated with in vitro drug release using convolution–deconvolution approach. All developed formulations were found to be bioequivalent with respect to the marketed patch (Oxytrol®) on the basis of level of C max, AUC0–96, and AUCtotal (0.8–1.25). A biphasic linear correlation was obtained pertaining to differential diffusion behavior of the drug in vivo during the experimental timeframe. Because of close resemblance to skin, Cuprophan® membrane was found to be more suitable for developing an IVIVC than Millipore® membrane.






Similar content being viewed by others
References
Walters KA. Dermatological and transdermal formulations. New York: Marcel Dekker Inc; 2002.
Shaefer U, Hansen S, Schneider M, Contreras JL, Lehr CM. Models for skin absorption and skin toxicity testing. In: Errhardt C, Kim KJ, editors. Drug absorption studies: in situ, in vitro and in silico models. London: Springer; 2008. p. 4–20.
Surber C, Davis A. Bioavailability and bioequivalence of dermatological formulations. In: Walters KA, editor. Dermatological and transdermal formulations. New York: Marcel Dekker; 2002.
Skelly JP, Shah VP, Maibach HI, Guy RH, Wester RC, Flynn GL, et al. FDA and AAPS report of the workshop on principles and practices of in vitro percutaneous penetration studies: relevance to bioavailability and bioequivalence. Pharm Res. 1987;4:265–7.
Shah VP, Elkins J, Skelly JP. Relationship between in vivo skin blanching and in vitro release rate for betamethasone valerate creams. J Pharm Sci. 1992;81:104–6.
Tanaka S, Takashima Y, Murayama H, Tsuchiya S. Studies on release from ointments: V. Release of hydrocortisone butyrate propionate from topical dosage forms to silicone rubber. Int J Pharm. 1985;27:29–38.
Kaiho F, Nomura H, Makabe E, Kato Y. Percutaneous absorption of indomethacin from mixtures of fatty alcohol and propylene glycol (FAPG base) through rat skin: effects of fatty acid added to FAPG base. Chem Pharm Bull. 1987;35:2928–34.
Tojo K. Concentration profile in plasma after transdermal drug delivery. Int J Pharm. 1988;43:201–5.
Van Buskirk GA, Arsulowicz D, Basu P, Block L, Cai B, Cleary GW, et al. Passive transdermal systems whitepaper incorporating current chemistry, manufacturing and controls (CMC) development principles. AAPS PharmScitech. 2012;13:218–30.
Van Buskirk GA, González MA, Shah VP, Barnhardt S, Barrett C, Berge S, et al. Scale up of adhesive transdermal drug delivery systems. Pharm Res. 1997;14:848–52.
Mahayni H, Rekhi GS, Uppoor RS, et al. Evaluation of “external” predictability of an in vitro-in vivo correlation for an extended-release formulation containing metoprolol tartrate. J Pharm Sci. 2000;89:1354–61.
Gaynor C, Dunne A, Costello C, Davis J. A population approach to in vitro-in vivo correlation modeling for compounds with nonlinear kinetics. J Pharmacokinet Pharmacodyn. 2011;38:317–32.
Kortejärvi H, Malkki J, Marvola M, Urtti A, Yliperttula M, Pajunen P. Level A in vitro-in vivo correlation (IVIVC) model with Bayesian approach to formulation series. J Pharm Sci. 2006;95:1595–605.
Costello C, Rossenu S, Vermeulen A, Cleton A, Dunne A. A time scaling approach to develop an in vitro/in vivo correlation (IVIVC) model using a convolution-based technique. J Pharmacokinet Pharmacodyn. 2011;38:519–39.
Sutton SC, Hu M. An automated process for building reliable and optimal in vitro/in vivo correlation models based on Monte Carlo simulations. AAPS J. 2006;8:E307–13.
Polli JE. Dependence of in vitro-in vivo correlation analysis acceptability on model selections. Pharm Dev Technol. 1999;4:89–96.
Buchwald P. Direct, differential-equation-based in-vitro-in-vivo correlation (IVIVC) method. J Pharm Pharmacol. 2003;55:495–504.
Vitková Z, Vitko A, Zabka M, Cizmárik J. Alternative and generalized approach to in vitro-in vivo correlation. Acta Pol Pharm. 2011;68:417–21.
Lu Y, Kim S, Park K. In vitro-in vivo correlation: perspectives on model development. Int J Pharm. 2011;418:142–8.
Jacobs T, Rossenu S, Dunne A, Molenberghs G, Straetemans R, Bijnens L. Combined models for data from in vitro-in vivo correlation experiments. J Biopharm Stat. 2008;18:1197–211.
Parojcic J, Ibric S, Djuric Z, Jovanovic M, Corrigan OI. An investigation into the usefulness of generalized regression neural network analysis in the development of level A in vitro-in vivo correlation. Eur J Pharm Sci. 2007;30:264–72.
Dowell JA, Hussain A, Devane J, Young D. Artificial neural networks applied to the in vitro-in vivo correlation of an extended-release formulation: initial trials and experience. J Pharm Sci. 1999;88:154–60.
Gould AL, Agrawal NGB, Goel TV, Fitzpatrick S. A 1-step Bayesian predictive approach for evaluating in vitro in vivo correlation (IVIVC). Biopharm Drug Dispos. 2009;30:366–88.
Qi X, Liu R, Sun D, Ackermann C, Hou H. Convolution method to predict drug concentration profiles of 2,3,5,6-tetramethylpyrazine following transdermal application. Int J Pharm. 2003;259:39–45.
Sun L, Chun D, Yuan B, Cui H, Xi H, Mu L, et al. Formulation and in vitro/in vivo correlation of a drug-in-adhesive transdermal patch containing azasetron. J Pharm Sci. 2012;101:4540–8.
Zhao L, Li Y, Fang L, He Z, Liu X, Wang L, et al. Transdermal delivery of tolterodine by O-acylmenthol: in vitro/in vivo correlation. Int J Pharm. 2009;374:73–81.
Langenbucher F. Handling of computational in vitro/in vivo correlation problems by Microsoft Excel: III. Convolution and deconvolution. Eur J Pharm Biopharm. 2003;56:429–37.
Harushi M, Kazuya K, Koji A, Hitoshi I, Yasuhiko Y. Pharmacokinetics/pharmacodynamics analysis of the relationship between the in vivo micturition pressure and receptor occupancy of (R) -oxybutynin and its metabolite in rats. Biol Pharm Bull. 2007;30:955–62.
Acknowledgments
The authors would like to acknowledge the All India Council for Technical Education (AICTE) and TEQIP Phase II for providing lab facilities. The authors would like to thank CSIR, Govt. of India, for providing funding (letter no. 9/991 (0019) 2K12-EMR-I dated 28/02/2012). The authors would also like to thank Dr. Rahul Choudhari and Mahindra Ganu for teaching rabbit handling, physiology, and blood sampling from the ear vein of rabbits. The authors are also thankful to Watson Pharma, India, for providing the gift sample of oxybutynin.
Conflict of interest
This research work is part of doctoral studies of Mr. Achyut Sharad Khire. The authors declare no conflict of interest. The authors declare that the experiments performed on laboratory animals comply with the guidelines of the institutional animal ethics committee.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khire, A., Vavia, P. Bioavailability, bioequivalence, and in vitro–in vivo correlation of oxybutynin transdermal patch in rabbits. Drug Deliv. and Transl. Res. 4, 105–115 (2014). https://doi.org/10.1007/s13346-013-0170-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-013-0170-y