Abstract
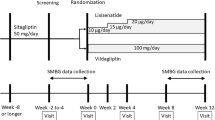
The safety and efficacy of sitagliptin as add-on therapy to glinides, rapid-acting insulin secretagogues, were evaluated for Japanese patients with type 2 diabetes mellitus. This 52-week study consisted of a 12-week double-blind period, followed by a 40-week open-label period. During the double-blind period, patients were randomized to sitagliptin 50 mg q.d. (S/S group) or placebo (P/S group) as add-on therapy to glinide monotherapy. During the open-label period, all patients in both groups were administered sitagliptin 50 mg q.d. (or 100 mg q.d. after up-titration). During the double-blind period, the overall occurrence of adverse experiences (AE) was similar in both treatment groups. The frequency of reported AE of hypoglycemia in both groups was low and not notably different. The nature of clinical AE during the open-label period for both groups was not notably different from that of clinical AE in the sitagliptin group during the double-blind period. The between-group difference in HbA1c least squares (LS) mean of change from baseline (95 % CI) at Week 12 was −1.1 % (−1.3, −0.8) in favor of sitagliptin (P < 0.001). LS mean of reductions from baseline of fasting plasma glucose and 2-h postmeal glucose were significantly greater in the sitagliptin group than in the placebo group: −23.1 mg/dL (−32.2, −13.9) and −51.2 mg/dL (−67.4, −35.0), respectively (both P < 0.001). The changes from baseline in glycemic data in the S/S group remained generally stable throughout the 52-week treatment period.





Similar content being viewed by others
References
Idris I, Donnelly R. Dipeptidyl peptidase-IV inhibitors: a major new class of oral antidiabetic drug. Diabetes Obes Metab. 2007;9:153–65.
Prato SD, Pulizzi N. The place of sulfonylureas in the therapy for type 2 diabetes mellitus. Metab Clin Exp. 2006;55(Suppl 1):S20–7.
Fukushima M, Suzuki H, Seino Y. Insulin secretion capacity in the development from normal glucose tolerance to type 2 diabetes. Diabetes Res Clin Pract. 2004;66S:S37–43.
Kashiwagi A, Kasuga M, Araki E, Oka Y, Hanafusa T, Ito H, Tominaga M, Oikawa S, Noda M, Kawamura T, Sanke T, Namba M, Hashiramoto M, Sasahara T, Nishio Y, Kuwa K, Ueki K, Takei I, Umemoto M, Murakami M, Yamakado M, Yatomi Y, Ohashi H, Committee on the standardization of diabetes mellitus related laboratory testing of Japan Diabetes Society. International clinical harmonization of glycated hemoglobin in Japan: from Japan diabetes society to national glycohemoglobin standardization program values. J Diabetes Investig. 2012;3:39–40.
Liang KY, Zeger SL. Longitudinal data analysis of continuous and discrete responses for pre-post designs. Sankhya Indian J Stat. 2000;62:134–48.
Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696–705.
Pi-Sunyer FX. The effects of pharmacologic agents for type 2 diabetes mellitus on body weight. Postgrad Med. 2008;120:5–17.
Nonaka K, Kakikawa T, Sato A, Okuyama K, Fujimoto G, Kato N, Suzuki H, Hirayama Y, Ahmed T, Davies MJ, Peter PS. Efficacy and safety of sitagliptin monotherapy in Japanese patients with type 2 diabetes. Diabetes Res Clin Pract. 2008;79:291–8.
Kadowaki T, Tajima N, Odawara M, Nishii M, Taniguchi T, Arjona Ferreira JC. Addition of sitagliptin to ongoing metformin monotherapy improves glycemic control in Japanese patients with type 2 diabetes over 52 weeks. J Diabetes Investig. 2013;4:174–81.
Kashiwagi A, Kadowaki T, Tajima N, Nonaka K, Taniguchi T, Nishii M, Arjona Ferreira JC, Amatruda JM. Sitagliptin added to treatment with ongoing pioglitazone for up to 52 weeks improves glycemic control in Japanese patients with type 2 diabetes. J Diabetes Investig. 2011;2:381–90.
Tajima N, Kadowaki T, Odawara M, Nishii M, Taniguchi T, Arjona Ferreira JC. Addition of sitagliptin to ongoing glimepiride therapy in Japanese patients with type 2 diabetes over 52 weeks leads to improved glycemic control. Diabetol Int. 2011;2:32–44.
Tajima N, Kadowaki T, Okamoto T, Sato A, Okuyama K, Minamide T, Arjona Ferreira JC. Sitagliptin added to voglibose monotherapy improves glycemic control in patients with type 2 diabetes. J Diabetes Investig. 2013;4:595–604.
Hermansen K, Kipnes M, Luo E, Fanurik D, Khatami H, Stein P, For the Sitagliptin Study 035 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, in patients with type 2 diabetes mellitus inadequately controlled on glimepiride alone or on glimepiride and metformin. Diabetes Obes Metab. 2007;9:733–45.
Aschner P, Kipnes MS, Lunceford JK, Sanchez M, Mickel C, Williams-Herman DE, For the Sitagliptin Study 021 Group. Effect of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care. 2006;29:2632–7.
The DECODE Study Group, On behalf of the European Diabetes Epidemiology Group. Glucose tolerance and cardiovascular mortality. Arch Intern Med. 2001;161:397–405.
Nakagami T, Qiao Q, Tuomilehto J, Balkau B, Tajima N, Hu G, Borch-Johnsen K. Screen-detected diabetes, hypertension and hypercholesterolemia as predictors of cardiovascular mortality in five populations of Asian origin: the DECODA study. Eur J Cardiovasc Prev Rehabil. 2005;13:555–61.
Dungan KM, Buse JB, Largay J, Kelly MM, Button EA, Kato S, Wittlin S. 1,5-Anhydroglucitol and postprandial hyperglycemia as measured by continuous glucose monitoring system in moderatory controlled patients with diabetes. Diabetes Care. 2006;29:1214–9.
Acknowledgments
The authors would like to thank M. Kawashima, D. Yanagida, M. Ohkubo, H. Shuno, M. Kondo, H. Suna, H. Maekawa, T. Yoshida, T. Kuramoto, S. Shiono, Y. Hatori, K. Nagasawa, Y. Kamehara, N. Inaba, and M. Odani (ONO Pharmaceutical Co., Ltd., Japan) for their assistance in writing and preparing this paper for submission and publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors Tajima and Kadowaki were coordinating investigators for this study. Author Odawara was the medical advisor for this study. Author Tajima has acted as advisory panel for Eli Lilly Japan K.K., Novo Nordisk Pharma Ltd. and Sanofi K.K.; as consultant for MSD K.K. and Ono Pharmaceutical Co., Ltd.; as a speaker for Astellas Pharma Inc., Daiichi-Sankyo, Co., Ltd., Eli Lilly Japan K.K., Kissei Pharmaceutical Co., Ltd., MSD K.K., Boehringer Ingelheim Japan Inc., Novo Nordisk Pharma Ltd., Ono Pharmaceutical Co., Ltd., Sanofi K.K., Taisho Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd. and Dainippon Sumitomo Pharma Co., Ltd. Author Kadowaki has acted as advisory panel for AstraZeneca K.K., Chugai Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Corp., Sanofi K.K., and Takeda Pharmaceutical Co., Ltd.; as consultant for MSD K.K., and Ono Pharmaceutical Co., Ltd.; as a speaker for Astellas Pharma, Inc., AstraZeneca K.K., Daiichi Sankyo Co., Ltd., Dainippon Sumitomo Pharma Co., Ltd., Eli Lilly Japan K.K., Kowa Pharmaceutical Co., Ltd., Kyowa Hakko Kirin Co., Ltd., Mitsubishi Tanabe Pharma Corp., MSD K.K., Nippon Boehringer Ingelheim Co., Ltd., Novartis Pharma K.K., Ono Pharmaceutical Co., Ltd., Sanofi K. K., Sanwa Kagaku Kenkyusho Co., Ltd., Taisho Pharmaceutical Co., Ltd., and Takeda Pharmaceutical Co., Ltd.; obtained research support from Daiichi Sankyo Co., Ltd., Dainippon Sumitomo Pharma Co., Ltd., MSD K.K., Novartis Pharma K.K., Novo Nordisk Pharma Ltd., Ono Pharmaceutical Co., Ltd., Sanofi K.K., and Takeda Pharmaceutical Co., Ltd.; and has been involved in research units endowed by MSD K.K., Nippon Boehringer Ingelheim Co., Ltd., Novo Nordisk Pharma Ltd., Takeda Pharmaceutical Co., Ltd., and Terumo Corp. Author Odawara has acted as consultant for MSD K.K. and Ono Pharmaceutical Co., Ltd.; as a speaker for MSD K.K. and Ono Pharmaceutical Co., Ltd.. Authors Minamide, Seki, and Oki are full-time employees of ONO Pharmaceutical Co., Ltd., Japan and authors Nagayasu is a full-time employee of MSD K.K.and Arjona Ferreira was a full-time employee of Merck Sharp & Dohme Corp. at the time of the study, and might potentially own stock and/or hold stock options in the company. No other potential conflicts of interest relevant to this article were reported. This study was sponsored by MSD K.K., Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, the manufacturer of sitagliptin, and by Ono Pharmaceutical Co. Ltd., Japan.
Human rights statement and informed consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later revision. Informed consent or substitute for it was obtained from all patients included in the study.
Additional information
This study is registered with ClinicalTrials.gov: NCT01517321, “MK-0431/ONO-5435 Phase III Clinical Trial-Rapid-acting Insulin Secretagogue Add-on Study in Patients With Type 2 Diabetes”, http://www.clinicaltrials.gov/ct2/show/NCT01517321?term=NCT01517321&rank=1.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix 1
Appendix 1
MK-0431 P282/ONO-5435-17 Primary Investigator list: Fuminobu Okuguchi (Okuguchi Clinic of Internal Medicine), Naoki Itabashi (Itabashi Clinic), Tomoyuki Arisaka (Arisaka Clinic), Takashi Ishii (Ishii Hospital), Yuichiro Makita (Koshigaya Municipal Hospital), Yoshihiko Suzuki (HDC Atlas Clinic), Yukiko Onishi (The Institute for Adult Diseases, Asahi Life Foundation), Masaharu Morohoshi (Sanraku Hospital, Lifestyle Diseases Clinic), Arihiro Kiyosue (Tokyo-Eki Center-Building Clinic), Masayo Yamada (Yokohama Sakae Kyosai Hospital), Taro Asakura (Tsuruma Kaneshiro Diabetes Clinic), Shin-ichiro Matsumura (Yokohama Cardiopulmonary Clinic), Ichitaro Takada (Takada Naika Clinic), Toshio Kiguchi (Kiguchi Clinic), Masaaki Ohashi (Saku Central Hospital), Akira Yamauchi (Suruga Clinic), Hideki Okamoto (Meitetsu Hospital), Genichi Watanabe (Watanabe Clinic), Mikihiro Nakayama (Nakayama Clinic), Masako Deguchi (Japanese Red Cross Kyoto Daini Hospital), Yasuro Kumeda (Minamiosaka Hospital), Soichi Kurioka (Komatsu Hospital), Takashi Ikawa (Osaka Rehabilitation Hospital), Kazushige Ejiri (Joyo Ejiri Hospital), Hidenori Ohno (Oofuji Clinic), Atsuyoshi Yuhara (Yuhara Clinic), Akira Matsutani (Shunan City Shin-nanyo Hospital), Makoto Kunisaki (Kunisaki Makoto Clinic), Shoichi Akazawa (Shinkoga Clinic), and Noriko Nakamura (Primula Clinic).
About this article
Cite this article
Tajima, N., Kadowaki, T., Odawara, M. et al. Safety and efficacy of addition of sitagliptin to rapid-acting insulin secretagogues for glycemic control, including post-prandial hyperglycemia, among Japanese with type 2 diabetes mellitus. Diabetol Int 7, 155–166 (2016). https://doi.org/10.1007/s13340-015-0230-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13340-015-0230-2