Abstract
Background
Combination therapies of drugs with distinct mechanisms of action are emerging as ways to achieve strict glycemic control, thus preventing the onset and progression of diabetic complications in type 2 diabetes patients. A rapid-acting insulin secretagog, nateglinide, and a potent dipeptidyl peptidase-4 inhibitor, sitagliptin, meet such criteria.
Methods
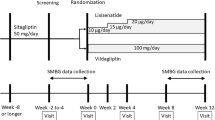
A total of 121 patients inadequately controlled with sitagliptin monotherapy received 52-week combination therapy (nateglinide + sitagliptin). The primary endpoint was the safety of the therapy, and its efficacy was also evaluated. A meal tolerance test was performed 4 weeks before the start of combination therapy (week −4) and at week 24 and week 52 after the start of combination therapy.
Results
HbA1c levels were lower at week 52 than at week 0 [−0.42% (95% confidence interval −0.53, −0.31)]. Fasting plasma glucose levels tended to decrease from baseline (week 0) to week 52 [−4.8 mg/dl (−9.4, −0.2)]. In the meal tolerance test, postprandial plasma glucose levels and area under the curve of glucose from before to 2 h after the meal load were lower at week 24 and week 52 than at week −4. In addition, the levels of insulin and active glucagon-like peptide-1 were higher at week 52 than at week −4. Furthermore, the incidence of adverse events in combination therapy with sitagliptin was similar to those previously shown in nateglinide monotherapy.
Conclusion
Compared with sitagliptin monotherapy, the combination therapy of nateglinide plus sitagliptin was more effective in type 2 diabetes patients at improving glycemic control while showing similar safety.






Similar content being viewed by others
References
Ikenoue T, Akiyoshi M, Fujitani S, Okazaki K, Kondo N, Maki T. Hypoglycaemic and insulinotropic effects of a novel oral antidiabetic agent, (−)-N-(trans-4-isopropylcyclohexanecarbonyl)-d-phenylalanine (A-4166). Br J Pharmacol. 1997;120(1):137–45.
Kosaka K, Kikuchi M, Kuzuya T, Akanuma Y, Ohashi Y. Clinical effect and safety of AY4166 in patients with non-insulin-dependent diabetes mellitus. Yakuri Rinsho. 1997;7:635–51 (in Japanese).
Kosaka K, Kikuchi M, Kuzuya T, Akanuma Y, Takiguchi K, Ishihara H, Ohashi Y. Change in postprandial plasma glucose and pharmacodynamics in patients with non-insulin-dependent diabetes mellitus by administration with AY4166. Yakuri Rinsho. 1997;7:653–68 (in Japanese).
Kaku K, Inagaki N, Kobayashi N. Long-term effects of mitiglinide in Japanese diabetics inadequately controlled with DPP-4 inhibitor or biguanide monotherapy. Diabetes Ther. 2014;5(1):97–111.
Committee on Proper Use of Incretins (GLP-1 Receptor Agonists and DPP-4 Inhibitors). The Japan Diabetes Society. 2011. http://www.fa.kyorin.co.jp/jds/uploads/photos/797.pdf. Accessed 11 Oct 2017 (in Japanese).
Glucose tolerance and mortality: comparison of WHO and American Diabetes Association diagnostic criteria. The DECODE study group. European Diabetes Epidemiology Group. Diabetes epidemiology: collaborative analysis of diagnostic criteria in Europe. Lancet. 1999;354(9179):617–21.
Tominaga M, Eguchi H, Manaka H, Igarashi K, Kato T, Sekikawa A. Impaired glucose tolerance is a risk factor for cardiovascular disease, but not impaired fasting glucose. The Funagata Diabetes Study. Diabetes Care. 1999;22(6):920–4.
Nakagami T, Qiao Q, Tuomilehto J, Balkau B, Tajima N, Hu G, Borch-Johnsen K. Screen-detected diabetes, hypertension and hypercholesterolemia as predictors of cardiovascular mortality in five populations of Asian origin: the DECODA study. Eur J Cardiovasc Prev Rehabil. 2006;13(4):555–61.
Kodama K, Tojjar D, Yamada S, Toda K, Patel CJ, Butte AJ. Ethnic differences in the relationship between insulin sensitivity and insulin response: a systematic review and meta-analysis. Diabetes Care. 2013;36(6):1789–96.
Pharmaceutical and Food Safety Bureau. Guideline for clinical evaluation of oral hypoglycemic agents. 2010. http://www.pmda.go.jp/files/000208194.pdf. Accessed 22 Feb 2017.
Duffy NA, Green BD, Irwin N, Gault VA, McKillop AM, O’Harte FP, Flatt PR. Effects of antidiabetic drugs on dipeptidyl peptidase IV activity: nateglinide is an inhibitor of DPP IV and augments the antidiabetic activity of glucagon-like peptide-1. Eur J Pharmacol. 2007;568(1–3):278–86.
Kitahara Y, Miura K, Yasuda R, Kawanabe H, Ogawa S, Eto Y. Nateglinide stimulates glucagon-like peptide-1 release by human intestinal L cells via a K(ATP) channel-independent mechanism. Biol Pharm Bull. 2011;34(5):671–6.
Bell PM, Cuthbertson J, Patterson S, O’Harte FP. Additive hypoglycaemic effect of nateglinide and exogenous glucagon-like peptide-1 in type 2 diabetes. Diabetes Res Clin Pract. 2011;91(3):e68–70.
Kudo-Fujimaki K, Hirose T, Yoshihara T, Sato F, Someya Y, Ohmura C, Kanazawa A, Fujitani Y, Watada H. Efficacy and safety of nateglinide plus vildagliptin combination therapy compared with switching to vildagliptin in type 2 diabetes patients inadequately controlled with nateglinide. J Diabetes Investig. 2014;5(4):400–9.
Kosaka K, Kikuchi M, Tarui S, Shigeta Y, Kuzuya T, Akanuma Y, Toyota T, Ohashi Y. Long-term administration of fast and short-acting insulin secretagogue AY4166 to non-insulin-dependent diabetes mellitus. Yakuri Rinsho. 1997;7:797–818 (in Japanese).
Kuzuya T, Oka Y, Yaga K, Inoue Y, Matsutani A, Kosaka K. Utility of long-term administration of fast and short-acting insulin secretagogue AY4166 to non-insulin-dependent diabetes mellitus. Yakuri Rinsho. 1997;7:819–32 (in Japanese).
Kikuchi M. Safety and efficacy of long-term combined therapy of nateglinide and metformin hydrochloride in type 2 diabetes patients inadequately controlled with monotherapy of metformin hydrochloride: multicenter, open-label trial. Rinsho Iyaku. 2008;24:741–60 (in Japanese).
Kikuchi M. Utility of long-term combined therapy of nateglinide and pioglitazone hydrochloride in type 2 diabetes patients inadequately controlled with monotherapy of pioglitazone hydrochloride (extended administration from phase II/phase III double blind parallel group comparison trials): multicenter, open-label trial. Rinsho Iyaku. 2009;25:57–75 (in Japanese).
Tajima N, Kadowaki T, Odawara M, Minamide T, Seki A, Oki K, Nagayasu R, Arjona Ferreira JC. Safety and efficacy of addition of sitagliptin to rapid-acting insulin secretagogues for glycemic control, including post-prandial hyperglycemia, among Japanese with type 2 diabetes mellitus. Diabetol Int. 2016;7(2):155–66.
Acknowledgements
The authors thank all of the patients and investigators who took part in this study. The principal investigators in this study were: Dr. Keiichi Oikawa, Oikawa Medical Clinic; Dr. Shigeru Hirano, Hirano Medical Clinic; Dr. Masayuki Noritake, Noritake Clinic; Dr. Naoki Itabashi, Itabashi Diabetes and Dermatology Clinic; Dr. Takahiko Tokuyama, Tokuyama Clinic; Dr. Munehiro Honda, KKR Mishuku Hospital; Dr. Marohito Murakami, Hino Municipal Hospital; Drs. Yasushi Iwaita and Yoshihiro Takamiya, Koukan Clinic; Dr. Toshiki Fukui, NTT West Takamatsu Hospital; Dr. Satoshi Murao, KKR Takamatsu Hospital. Nateglinide was jointly developed by EA Pharma Co., Ltd., and Astellas Pharma Inc. This study was primarily sponsored and funded by EA Pharma Co., Ltd.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
T. Hirose received consultancy fees from EA Pharma Co., Ltd.; lecture fees from MSD K.K., Eli Lilly Japan K.K., Takeda Pharmaceutical Co., Ltd., Novartis Pharma K.K., Dainippon Sumitomo Pharma Co., Ltd., Novo Nordisk Pharma Ltd, Sanofi K.K., AstraZeneca K.K., and Daiichi Sankyo Co., Ltd.; and research funds from MSD K.K., Eli Lilly Japan K.K., Takeda Pharmaceutical Co., Ltd., Novo Nordisk Pharma Ltd, Kissei Pharmaceutical Co., Ltd., Boehringer Ingelheim, Mitsubishi Tanabe Pharma Corporation, Dainippon Sumitomo Pharma Co., Ltd., Sanofi K.K., Ono pharmaceutical Co., Ltd., AstraZeneca K.K., and Daiichi Sankyo Co., Ltd. C. Saitoh and I. Oikawa are employees of EA Pharma Co., Ltd. N. Kondo was an employee of EA Pharma Co., Ltd.
Human rights statement
This research involves human participants. The study protocol was approved by the Sakayori Clinic Institutional Review Board on July 23, 2012 (approval number is not applicable).
Ethical standards
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions.
Informed consent
Informed consent was obtained from all patients for being included in the study.
About this article
Cite this article
Hirose, T., Saitoh, C., Oikawa, I. et al. Efficacy and safety of nateglinide plus sitagliptin combination therapy in type 2 diabetes patients inadequately controlled by sitagliptin monotherapy: a phase 3, multicenter, open-label, long-term study. Diabetol Int 9, 168–178 (2018). https://doi.org/10.1007/s13340-017-0341-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13340-017-0341-z