Abstract
Background and Objectives
Acute inflammation caused by infections or sepsis can impact pharmacokinetics. We used a model-based analysis to evaluate the effect of acute inflammation as represented by interleukin-6 (IL-6) levels on drug clearance, focusing on renal glomerular filtration rate (GFR) and cytochrome P450 3A4 (CYP3A4)-mediated metabolism.
Methods
A physiologically based model incorporating renal and hepatic drug clearance was implemented. Functions correlating IL-6 levels with GFR and in vitro CYP3A4 activity were derived and incorporated into the modeling framework. We then simulated treatment scenarios for hypothetical drugs by varying the IL-6 levels, the contribution of renal and hepatic drug clearance, and protein binding. The relative change in observed area under the concentration-time curve (AUC) was computed for these scenarios.
Results
Inflammation showed opposite effects on drug exposure for drugs eliminated via the liver and kidney, with the effect of inflammation being inversely proportional to the extraction ratio (ER). For renally cleared drugs, the relative decrease in AUC was close to 30% during severe inflammation. For CYP3A4 substrates, the relative increase in AUC could exceed 50% for low-ER drugs. Finally, the impact of inflammation-induced changes in drug clearance is smaller for drugs with a larger unbound fraction.
Conclusion
This analysis demonstrates differences in the impact of inflammation on drug clearance for different drug types. The effects of inflammation status on pharmacokinetics may explain the inter-individual variability in pharmacokinetics in critically ill patients. The proposed model-based analysis may be used to further evaluate the effect of inflammation, i.e., by incorporating the effect of inflammation on other drug-metabolizing enzymes or physiological processes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Acute inflammation is associated with an increase in drug exposure for drugs metabolized mainly through cytochrome P450 3A4, while drug exposure is decreased for drugs cleared through renal glomerular filtration, whereas the extent of these effects is driven by both drug- and system-specific properties. |
Physiologically based modeling enables evaluation of the impact of inflammation on pharmacokinetics by considering multiple biological processes through the integration of experimental data from various sources, and may support the rational design of drug treatments during acute inflammation. |
1 Introduction
Acute severe inflammation can alter drug exposure [1, 2] in patients and thereby potentially impact drug efficacy and toxicity, especially for drugs with narrow therapeutic windows, e.g., antimicrobials [3] and immunosuppressants [4]. Studies in critically ill or septic patients, where acute inflammation is often present, show large inter-individual variability [5] in pharmacokinetics, e.g., clearance and distribution volume [6]. Several clinical pharmacokinetics studies investigating patient populations suffering from acute inflammation by empirically employing population pharmacokinetics modeling approaches have found that increased levels of inflammatory biomarkers such as C-reactive protein (CRP) or interleukin-6 (IL-6) may contribute to the observed variability in pharmacokinetics [7]. However, various other population studies in similar patients have failed to identify relationships between inflammatory biomarkers and variability in pharmacokinetic parameters [8]. A systematic investigation of the effect of inflammation on pharmacokinetics in patients is complicated by differences in health state between and within patients, as well as practical limitations in study designs.
The effects of inflammation on pharmacokinetics processes include alterations in the activities of various drug-metabolizing enzymes and transporters [9], plasma protein expression and drug–protein binding [10], and kidney function [11, 12]. Most of these effects have been studied in vitro or in animal and/or in healthy volunteers[13]. Scaling the contribution of such isolated effects to the observed pharmacokinetics of drugs during different levels of inflammation is not trivial. Moreover, depending on the drug’s properties, e.g., its plasma protein binding, lipophilicity, or blood-plasma ratio, the effect of inflammation-induced changes may lead to different effects on pharmacokinetics. Given the variable and inconclusive results of patient studies [8, 14, 15], there is a need to develop further insight into the likely effect of inflammation on drugs with different pharmacokinetic properties. This would allow for the identification of the types of drugs for which dosing adaptations and/or prospective clinical studies may be warranted. Physiologically based pharmacokinetic models could be used to integrate and scale experimentally derived data on inflammation-induced effects on pharmacokinetic parameters in a mechanistic manner [16], incorporating both drug- and biological-system-specific parameters, to facilitate the derivation of expected pharmacokinetics profiles in patients [17].
In the current analysis, we applied a physiologically based modeling approach to systematically explore inflammation-induced effects on drug clearance. As a proof-of-concept study, we focussed on elimination through glomerular filtration (GFR) and/or through hepatic cytochrome P450 3A4 (CYP3A4) metabolism, which are considere two major clinically relevant drug elimination pathways .
2 Methods
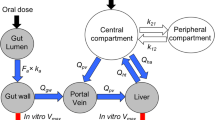
A physiologically based modeling framework was established by first deriving functions relating inflammation-associated reported IL-6 levels to CYP3A4 activity and GFR, as IL-6 has been suggested to be the most important inflammatory cytokine when considering the effect of cytokine modulators on the pharmacokinetics of small drug molecules [18]. These functions were incorporated in mechanism-based equations for hepatic and renal drug clearance. Simulations were then performed for different drug types, by varying IL-6 level, the extent of renal or CYP3A4-hepatic clearance, and protein binding, to evaluate the resulting changes in drug exposure. The workflow is summarized in Fig. 1.
2.1 Effect of Inflammation on CYP3A4 Activity and GFR
To investigate the effect of inflammation on drug exposure, IL-6 levels were used as an inflammation indicator. We digitized data from previously published studies characterizing CYP3A4 activity in relation to IL-6 levels in human hepatocytes [19, 20]. For GFR, we used a healthy volunteer endotoxemia study [12], which described the relationship between GFR and IL-6 levels. The data from these studies were digitized using PlotDigitizer (version 3.1.5) (Fig. S1 in the Supplementary Information). Nonlinear and linear relationships were fitted to describe the relationships between IL-6 and CYP3A4 activity (Eq. 1) and GFR (Eq. 2), respectively. The fitting was performed using the “optim” and “lm” functions in the statistical software R (version 4.2.1).
Here, Imax represents the maximum inhibitory effect of IL-6 on CYP3A4 activity as a percentage, IC50 is the IL-6 concentration ([IL6]) that achieves half of the maximum effect, and γ is the Hill coefficient. GFRbase is the average GFR in healthy volunteers, while the constant α represents the slope factor between IL-6 plasma levels and the increase in GFR.
2.2 Hepatic CYP3A4-Mediated Clearance
The intrinsic clearance (CLint) associated with inflammation was calculated by relating the relative change in CYP3A4 activity for different IL-6 levels to the basal in vitro CLint value. The latter value was derived based on the metabolic enzyme activity and scaled by microsomal protein per gram of liver and human liver weight [21]. The extrapolation from in vitro CLint to in vivo hepatic clearance was performed using a dispersion liver model [22] (Eqs. 3–6), which has been reported to more accurately predict hepatic clearance of drugs for which the hepatic route is dominant, while it provides equivalent clearance predictions to the well-stirred models for other drugs [23].
Here, CLhep is the hepatic plasma clearance, QH is the hepatic blood flow, which is nearly 25% of the cardiac output (Qco) [24], ER is the hepatic extraction ratio, and BP is the blood to plasma ratio. QH and BP were assumed to be constant. ER was derived using the dispersion model, where the axial dispersion number (DN) was estimated to be 0.27 for microsomal data [23]. The efficiency number (RN) was a measure of the removal rate of solutes by hepatocytes [25] and was derived from the scaled unbound fraction in plasma (fu), the in vitro CLint, and the hepatic blood flow.
2.3 Total Body Clearance
The total body clearance (CL) was defined as the sum of the hepatic plasma clearance and renal plasma clearance (Eq. 7). The hepatic clearance was exclusively based on the CYP3A4-mediated metabolism and the renal clearance was based on the GFR-mediated elimination of the unbound drug (Eq. 8):
where fcl_h is the index of composition of total body clearance related to the hepatic clearance, and CLre is the renal plasma clearance, which was derived from the production of the unbound fraction and the GFR.
2.4 Drug Properties and Simulation Scenarios
A panel of hypothetical drugs with drug-specific properties covering realistic ranges was evaluated (Table 1). The values of in vitro CLint included ranged between 0.5 and 189 ml/min/kg, which were based on a previous study examining hepatic metabolism [26] for a panel of drugs representing a broad variety of structures, including basic, acidic, and neutral compounds. IL-6 was evaluated for the clinically relevant range of 0–2500 pg/mL. Other important drug-specific properties like fcl_h and fu were set to range between 0 or 0.01 and 1, respectively.
A standard unit dose was given of each hypothetical drug. Drug exposure was represented by the area under the concentration-time curve (AUC) derived from the dose and total body clearance (Eq. 9):
where F is the bioavailability (assumed to be 1), and we assumed a standard unit dose of 1.
Concentration–time profiles generated using one-compartment models for intravenously (Eqs. 10 and 13) and orally (Eqs. 11–13) administered drugs were used to evaluate the inflammation-associated alterations in maximum drug concentration (Cmax) and time to maximum concentration (Tmax).
For i.v.-bolus-administered drugs,
For orally administered drugs,
where Cp is the plasma drug concentration, Ca is the drug concentration at the absorption site, ka is the absorption rate constant, k is the elimination rate constant, and Vd is the volume of distribution.
2.5 Evaluation of Effects
The simulated exposure metrics for all simulated hypothetical drugs were grouped into several categories for comparison purposes. ER was grouped into low (ER < 0.3), intermediate (0.3–0.7), or high ER (ER > 0.7). Clearance pathways were simplified into three classes according to the value of fcl_h, namely the kidney (fcl_h = 0), hybrid (fcl_h = 0.5), and liver (fcl_h = 1) elimination pathways. Protein binding (fu) was grouped into the following categories: 0.01, 0.25, 0.5, 0.75, and 1.
The inflammatory effects on drug exposure were assessed by comparing the relative changes in the AUC under different IL-6 levels with respect to AUC under no inflammation (Eq. 14).
All the above-mentioned parameters and the values they were set to are shown in Table 1.
3 Results
3.1 Quantifying Inflammation Effects on CYP3A4 Activity and GFR
The relationship between IL-6 level and CYP3A4 activity was quantitatively characterized using a sigmoid model with an estimated maximum inhibition of 73.26% and a half-maximal inhibitory concentration of 43.65 pg/mL, while the correlation between IL-6 level and increase in GFR was described using a linear function with a slope factor that was estimated to be 0.016. All the estimated parameters and standard errors are shown in Table 2. The developed models described observations accurately (Fig. S1).
3.2 Elimination route
We calculated the hepatic ER category based on Eq. (4) for each hypothetical drug with or without inflammation. Importantly, the ER was found to be impacted by the extent of inflammation due to the negative relationship between CLint and inflammation (Figs. 2, S2–S5). Consequently, the ER category was not treated as fixed, and a transition between categories was observed for drugs in all three investigated elimination pathway classes, i.e., renal, hybrid, and hepatic.
Relative change in area under the concentration–time curve (AUC) in relation to severity of inflammation, i.e., interleukin-6 (IL-6) levels, for different elimination pathways with the unbound fraction equal to 0.5. Dashed gray lines are reference lines representing no AUC change. Dotted lines connect predictions for different individual drugs, and points represent the predicted relative changes in AUC in relation to inflammation for different individual drugs. Colors indicate the actual ER categories for each drug in different inflammation states, while initial ER refers to the ER status in absence of inflammation. ER extraction ratio
Opposite effects of inflammation on the relative change in AUC were found in the hepatic and renal elimination pathways, whilst these effects were larger for drugs with a low ER than for drugs with a higher ER (Figs. 2, S2–S5). For drugs primarily eliminated renally, a relative decrease in AUC of close to 30% was observed in cases of severe inflammation (IL-6 levels of 2500 pg/mL). For drugs primarily eliminated hepatically, relative changes in AUC were positively correlated with inflammation, with an increase of over 50% for low-ER drugs observed during severe inflammation.
Effects of inflammation on the AUCs of drugs eliminated by hybrid pathways (e.g., both hepatic and renal elimination) were more complex. In the less severe stages of inflammation (IL-6 < 1000 pg/ml), the inflammation-induced decrease in hepatic metabolism is the main driver of changes in AUC. When inflammation became severe (IL-6 > 1000 pg/ml), the impact of changes in GFR increases to the point where it can even (partially) reverse the increase in AUC by metabolism. Therefore, the relative increase in AUC became smaller and is even associated with a decreased AUC for high-ER drugs (Figs. 2, S2–S5).
To further assess the effect of inflammation on drug exposure, additional drug concentration–time profiles were generated for the hypothetical drugs after oral administration. Similarly, opposing effects were found for drugs eliminated through hepatic or renal pathways. For renally eliminated drugs, both Cmax and Tmax decreased with increasing severity of inflammation, while for hepatically eliminated drugs, both Cmax and Tmax increased with inflammation (Figs. 3, S3). For hybrid pathways, the gradual increases in Cmax and Tmax observed in cases of less severe inflammation (e.g., at IL-6 levels of less than 1000 pg/mL) were partially reversed in more severe inflammation states. The changes in Cmax and Tmax caused by inflammation were greater for lower-ER drugs than for higher-ER drugs (Figs. 3, S6).
Concentration–time profiles of orally administered hypothetical drugs with only total body clearance affected in inflammation. For each extraction ratio (ER) category, one typical drug was selected with its ER category kept unchanged regardless of inflammation severity (e.g., in vitro intrinsic clearance CLint = 0.5, 54.4, and 189.0 mL/min/kg body weight for low-, intermediate-, and high-ER categories, respectively).Lines with colors represent the concentration–time profiles obtained with different interleukin-6 (IL-6) levels representing different severities of inflammation
3.3 Protein Binding
The unbound fraction was identified as important mediator of inflammation effects on drug exposure. The relative changes in AUC were smaller for drugs with larger unbound fractions (Figs. 4, S7–S8). This effect was less pronounced for high-ER drugs since the main factor limiting clearance for this type of drug is blood flow, which is assumed to be stable in inflammation.
Relative change in area under the concentration–time curve (AUC) in relation to inflammation, i.e., interleukin-6 (IL-6) concentration, for different unbound fractions (fu) of drugs eliminated hepatically.Dashed gray lines are reference lines representing no AUC change.Dotted lines are individual drugs, andpoints represent the relative changes in AUC in relation to inflammation.Colors indicate the actual extraction ratio (ER) categories for each drug in different inflammation states, while initial ER for in the absence inflammation
4 Discussion
In this study, we used a physiologically based modeling approach incorporating relationships between inflammation and CYP3A4-mediated hepatic metabolism and between inflammation and GFR-mediated renal excretion to investigate the effect of inflammation on drug exposure. We demonstrated how the influence of inflammation on drug exposure was different for different ER categories, protein binding properties, and elimination pathways.
In our analysis, we have quantified the correlation between IL-6 concentration and CYP3A4 expression based on experimental data from published in vitro studies. As large variability has been observed between donors and/or studies regarding the effects of inflammation on CYP3A4 activity [29], pooled data from different in vitro studies with various experimental designs were included to estimate parameters (e.g., Imax and IC50). Systematic studies of the effects of inflammation on other drug-metabolizing enzymes are, moreover, scarce and diverse [19, 30]. As such, our findings should not be extrapolated to other cytochrome P450 (CYP) enzymes.
For hepatically cleared drugs, we focused on CYP3A4, a clinically important drug-metabolizing enzyme. Our results suggest that for drugs cleared through CYP3A4-mediated hepatic metabolism, exposure increases with increased levels of inflammation. This prediction is qualitatively in line with clinical data [9, 31, 32], where the plasma levels of the CYP3A4-cleared drugs clindamycin [31] and quinine [32] have been observed to increase in patients with inflammation. Notably, CYP3A4 enzymes are primarily located in the liver, but are also present at the small intestine [33], which could influence the bioavailability of orally administered drugs. However, in this study, we disregarded the possible effects on bioavailability to focus on hepatic metabolism.
For hepatically cleared drugs with a high ER (>0.7), the change in hepatic clearance is mainly limited by hepatic blood flow. For drugs with a low ER (<0.3), hepatic clearance is limited by protein binding and changes in intrinsic clearance. We assumed that the hepatic blood flow was stable while incorporating CYP3A4-mediated effects of inflammation into intrinsic clearance. Therefore, inflammation generally has a lower impact on drug exposure for drugs with a high ER compared to drugs with a low ER. Previous clinical data showed an observation of a significant increase in total liver blood flow in patients during early sepsis [34], while another study reported no significant increase in total liver blood flow in critically ill patients compared to control groups in the first 5 days after admission [35]. Due to this inconsistency in reported results related to the effect of inflammation on hepatic blood flow, its clinical relevance is currently unclear and might be limited.
For predominantly renally cleared drugs, we found that drug exposure can be decreased during severe inflammation. This observation is in line with previously reported clinical data for beta-lactams [36] and aminoglycosides [37, 38], where the inflammation-induced reduction in drug exposure could impact the efficacy of the antibiotic treatment, and a prolonged infusion [36] and a higher dose [37] were therefore recommended in patients with sepsis. Notably, in our study, we only evaluated the effect of inflammation on glomerular filtration, while ignoring active processes like tubular secretion and reabsorption. Quantitative data on the effect of inflammation on such processes are currently lacking but could be included in this framework once they become available.
We have demonstrated the impact of the level of plasma protein binding for different drugs: the inflammation affected the relative change in drug exposure less with a larger unbound fraction, especially for high-ER drugs. In our analysis, we assumed plasma protein levels, and therefore the unbound fraction, to be unaffected by inflammation. This assumption can be disputed, as inflammatory states are known to be associated with altered protein levels, especially in moderate inflammation or the early stage of sepsis [39]. Unfortunately, the relationship between the two main plasma proteins, human serum albumin and α1-acid glycoprotein, and inflammation levels could not be characterized due to the current lack of sufficient quantitative data. Conducting further studies that systematically characterize the effects of inflammation on plasma protein levels would aid in deriving a mechanistic model of protein-specific protein binding which can be integrated into the current model framework.
Our work demonstrates how a physiologically based modeling workflow can help to quantitatively evaluate the effects of inflammation on body clearance and ultimate drug exposure, considering common differences in drug- and system-specific parameters. This model-based analysis demonstrates the importance of an integrative evaluation of multiple physiological processes [40] to understand the ultimate impact of inflammation on drug clearance, which is not possible using in vitro experimental assays alone. Still, large knowledge gaps currently exist regarding the effect of inflammation on such physiological processes, e.g., drug-metabolizing enzymes and transporters, organ blood flows, and dynamic plasma protein levels. Available data are, moreover, highly variable in terms of methodologies and data quality, indicating the need for a more systematic and consistent approach to in vitro studies of the effect of inflammation.
The focus of our analysis is on acute inflammation effects, with IL-6 used as a marker for inflammation. Our findings should not be directly extrapolated to various chronic inflammatory conditions [41]. Systematic experimental and clinical studies to evaluate to what extent our predictions can be extrapolated to a chronic inflammatory condition represent a potential next step. Furthermore, extending them to other more long-term inflammatory markers, such as CRP, could expand the applicability of the framework and provide deeper insight into the effects of inflammation on pharmacokinetics.
Although the current study evaluated hypothetical drugs only, ultimately, a physiologically based pharmacokinetic modeling framework could also be used to derive predictions of the impact of inflammation on drug exposure for specific drugs and to rationally derive dose adjustments, which would potentially be informed by inflammation-associated biomarkers measured in individual patients.
5 Conclusion
In conclusion, the developed physiologically based modeling workflow demonstrated, as a proof of concept, a pronounced impact of inflammation on drug exposure for drugs primarily cleared through hepatic CYP3A4 metabolism or through renal glomerular filtration. Further experimental data incorporating the effects on inflammation on other drug-metabolizing enzymes, transporters, and plasma proteins are needed to obtain more generalized insights into the effects of inflammation on drug pharmacokinetics. Ultimately, the developed framework may support the rational design of optimized and/or individualized drug treatments in patients.
References
Medzhitov R. Inflammation 2010: new adventures of an old flame. Cell. 2010;140:771–6.
Schmith VD, Foss JF. Effects of inflammation on pharmacokinetics/pharmacodynamics: increasing recognition of its contribution to variability in response. Clin Pharmacol Ther. 2008;83:809–11.
Guo T, van Hest RM, Zwep LB, Roggeveen LF, Fleuren LM, Bosman RJ, et al. Optimizing predictive performance of Bayesian forecasting for vancomycin concentration in intensive care patients. Pharm Res. 2020;37:171.
Andrews LM, Li Y, De Winter BCM, Shi Y-Y, Baan CC, Van Gelder T, et al. Pharmacokinetic considerations related to therapeutic drug monitoring of tacrolimus in kidney transplant patients. Expert Opin Drug Metab Toxicol. 2017;13:1225–36.
Guo T, Abdulla A, Koch BCP, van Hasselt JGC, Endeman H, Schouten JA, et al. Pooled population pharmacokinetic analysis for exploring ciprofloxacin pharmacokinetic variability in intensive care patients. Clin Pharmacokinet. 2022;61:869–79.
Felton TW, Hope WW, Roberts JA. How severe is antibiotic pharmacokinetic variability in critically ill patients and what can be done about it? Diagn Microbiol Infect Dis. 2014;79:441–7.
Aulin LBS, de Lange DW, Saleh MAA, van der Graaf PH, Völler S, van Hasselt JGC. Biomarker-guided individualization of antibiotic therapy. Clin Pharmacol Ther. 2021;110:346–60.
Venuto CS, Lim J, Messing S, Hunt PW, McComsey GA, Morse GD. Inflammation investigated as a source of pharmacokinetic variability of atazanavir in AIDS Clinical Trials Group protocol A5224s. Antivir Ther. 2018;23:345.
Stanke-Labesque F, Gautier-Veyret E, Chhun S, Guilhaumou R. Inflammation is a major regulator of drug metabolizing enzymes and transporters: consequences for the personalization of drug treatment. Pharmacol Ther. 2020;215: 107627.
Roberts JA, Pea F, Lipman J. The clinical relevance of plasma protein binding changes. Clin Pharmacokinet. 2013;52:1–8.
Costello-White R, Ryff CD, Coe CL. Aging and low-grade inflammation reduce renal function in middle-aged and older adults in Japan and the USA. Age (Dordr). 2015;37(4):9808. https://doi.org/10.1007/s11357-015-9808-7.
Beunders R, Schütz MJ, van Groenendael R, Leijte GP, Kox M, van Eijk LT, et al. Endotoxemia-induced release of pro-inflammatory mediators are associated with increased glomerular filtration rate in humans in vivo. Front Med. 2020;7:760.
Liu F, Aulin LBS, Guo T, Krekels EHJ, Moerland M, van der Graaf PH, et al. Modelling inflammatory biomarker dynamics in a human lipopolysaccharide (LPS) challenge study using delay differential equations. Br J Clin Pharmacol. 2022;88:5420–7.
Slaviero KA, Clarke SJ, Rivory LP. Inflammatory response: An unrecognised source of variability in the pharmacokinetics and pharmacodynamics of cancer chemotherapy. Lancet Oncol. 2003;4:224–32.
Aulin LBS, De Paepe P, Dhont E, de Jaeger A, Vande Walle J, Vandenberghe W, et al. Population pharmacokinetics of unbound and total teicoplanin in critically ill pediatric patients. Clin Pharmacokinet. 2021;60:353–63.
MacHavaram KK, Almond LM, Rostami-Hodjegan A, Gardner I, Jamei M, Tay S, et al. A physiologically based pharmacokinetic modeling approach to predict disease-drug interactions: suppression of CYP3A by IL-6. Clin Pharmacol Ther. 2013;94:260–8.
Rostami-Hodjegan A. Physiologically based pharmacokinetics joined with in vitro–in vivo extrapolation of ADME: a marriage under the arch of systems pharmacology. Clin Pharmacol Ther. 2012;92:50–61.
Chen KF, Jones HM, Gill KL. Physiologically based pharmacokinetic modeling to predict drug-biologic interactions with cytokine modulators: are these relevant and is interleukin-6 enough? Drug Metab Dispos. 2022;50:1322–31.
Dickmann LJ, Patel SK, Rock DA, Wienkers LC, Slatter JG. Effects of interleukin-6 (IL-6) and an anti-IL-6 monoclonal antibody on drug-metabolizing enzymes in human hepatocyte culture. Drug Metab Dispos. 2011;39:1415–22.
Long TJ, Cosgrove PA, Dunn RT, Stolz DB, Hamadeh H, Afshari C, et al. Modeling therapeutic antibody-small molecule drug–drug interactions using a three-dimensional perfusable human liver coculture platform. Drug Metab Dispos. 2016;44:1940–8.
Obach RS, Baxter JG, Liston TE, Silber BM, et al. The prediction of human pharmacokinetic parameters from preclinical and in vitro metabolism data. J Pharmacol Exp Ther. 1997;283:46–58.
Roberts MS, Rowland M. A dispersion model of hepatic elimination: 1. Formulation of the model and bolus considerations. J Pharmacokinet Biopharm. 1986;14:227–6260.
Ridgway D, Tuszynski JA, Tam YK. Reassessing models of hepatic extraction. J Biol Phys. 2003;29:1.
Eipel C, Abshagen K, Vollmar B. Regulation of hepatic blood flow: the hepatic arterial buffer response revisited. World J Gastroenterol. 2010;16:6046.
Roberts MS, Rowland M. Hepatic elimination–dispersion model. J Pharm Sci. 1985;74(5):585–7.
Obach RS. Prediction of human clearance of twenty-nine drugs from hepatic microsomal intrinsic clearance data: an examination of in vitro half-life approach and nonspecific binding to microsomes. Drug Metab Dispos. 1999;27:1350–9.
King J, Lowery DR. Physiology, cardiac output. StatPearls. Treasure Island: StatPearls Publishing; 2022. Available from https://www.ncbi.nlm.nih.gov/books/NBK470455/. Accessed 30 March 2023.
Sterner G, Frennby B, Mansson S, Nyman U, Van Westen D, Almén T. Determining ‘true’ glomerular filtration rate in healthy adults using infusion of inulin and comparing it with values obtained using other clearance techniques or prediction equations. Scand J Urol Nephrol. 2009;42:278–85.
de Jong LM, Jiskoot W, Swen JJ, Manson ML. Distinct effects of inflammation on cytochrome P450 regulation and drug metabolism: lessons from experimental models and a potential role for pharmacogenetics. Genes (Basel). 2020;11:1–30.
Lenoir C, Daali Y, Rollason V, Curtin F, Gloor Y, Bosilkovska M, et al. Impact of acute inflammation on cytochromes P450 activity assessed by the Geneva cocktail. Clin Pharmacol Ther. 2021;109:1668.
Gatti G, Flaherty J, Bubp J, White J, Borin M, Gambertoglio J. Comparative study of bioavailabilities and pharmacokinetics of clindamycin in healthy volunteers and patients with AIDS. Antimicrob Agents Chemother. 1993;37:1137.
Morgan ET, Dempsey JL, Mimche SM, Lamb TJ, Kulkarni S, Cui JY, et al. Physiological regulation of drug metabolism and transport: pregnancy, microbiome, inflammation, infection, and fasting. Drug Metab Dispos. 2018;46:503–13.
De Wildt SN, Kearns GL, Leeder JS, Van Den Anker JN. Cytochrome P450 3A. Clin Pharmacokinet. 2012;37:485–505.
Varsamidis K, Varsamidou E, Mavropoulos G. Doppler ultrasonographic evaluation of hepatic blood flow in clinical sepsis. Ultrasound Med Biol. 2003;29:1241–4.
Glen P. An investigation of liver blood flow in systemic inflammation. 2009. Available from https://eleanor.lib.gla.ac.uk/record=b2694398. Accessed 30 March 2023.
Vardakas KZ, Voulgaris GL, Maliaros A, Samonis G, Falagas ME. Prolonged versus short-term intravenous infusion of antipseudomonal β-lactams for patients with sepsis: a systematic review and meta-analysis of randomised trials. Lancet Infect Dis. 2018;18:108–20.
Rea RS, Capitano B, Bies R, Bigos KL, Smith R, Lee H. Suboptimal aminoglycoside dosing in critically ill patients. Ther Drug Monit. 2008;30:674–81.
Cobussen M, Stassen PM, Posthouwer D, van Tiel FH, Savelkoul PHM, Havenith T, et al. Improving peak concentrations of a single dose regime of gentamicin in patients with sepsis in the emergency department. PLoS One. 2019;14: e0210012.
Coles ME, Thompson GC, Newell M. Plasma protein changes in inflammatory states. Pathology. 1978;10(4):394–5.
Liu F, Aulin LBS, Kossen SSA, Cathalina J, Bremmer M, Foks AC, et al. A system pharmacology Boolean network model for the TLR4-mediated inflammatory response in early sepsis. J Pharmacokinet Pharmacodyn. 2022;49:645–55.
Gabay C. Interleukin-6 and chronic inflammation. Arthritis Res Ther. 2006;8:S3.
Acknowledgments
The authors acknowledge Mohammed Saleh for providing advice on implementing the blood plasma partition coefficient in this physiologically based modeling workflow.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Data Availability Statement
The R scripts supporting the findings of this study are available within the supplementary information files.
Code Availability
The modeling and simulation code is provided in the supplementary materials.
Authors’ Contributions
FL and JGCH designed the study; FL performed the analysis; LBSA, MM, EHJK, and JGCH supported the interpretation of the results; FL, LBSA, and JGCH wrote the manuscript; all authors critically reviewed the manuscript.
Funding
No funding was receveid to conduct this study.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Liu, F., Aulin, L.B.S., Manson, M.L. et al. Unraveling the Effects of Acute Inflammation on Pharmacokinetics: A Model-Based Analysis Focusing on Renal Glomerular Filtration Rate and Cytochrome P450 3A4-Mediated Metabolism. Eur J Drug Metab Pharmacokinet 48, 623–631 (2023). https://doi.org/10.1007/s13318-023-00852-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-023-00852-6