Abstract
Background and Objective
Underdosing of adalimumab can result in non-response and poor disease control in patients with rheumatic disease or inflammatory bowel disease. In this pilot study we aimed to predict adalimumab concentrations with population pharmacokinetic model-based Bayesian forecasting early in therapy.
Methods
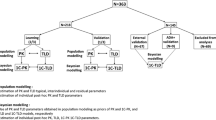
Adalimumab pharmacokinetic models were identified with a literature search. A fit-for-purpose evaluation of the model was performed for rheumatologic and inflammatory bowel disease (IBD) patients with adalimumab peak (first dose) and trough samples (first and seventh dose) obtained by a volumetric absorptive microsampling technique. Steady state adalimumab concentrations were predicted after the first adalimumab administration. Predictive performance was calculated with mean prediction error (MPE) and normalised root mean square error (RMSE).
Results
Thirty-six patients (22 rheumatologic and 14 IBD) were analysed in our study. After stratification for absence of anti-adalimumab antibodies, the calculated MPE was −2.6% and normalised RMSE 24.0%. Concordance between predicted and measured adalimumab serum concentrations falling within or outside the therapeutic window was 75%. Three patients (8.3%) developed detectable concentrations of anti-adalimumab antibodies.
Conclusion
This prospective study demonstrates that adalimumab concentrations at steady state can be predicted from early samples during the induction phase.
Clinical Trial Registration
The trial was registered in the Netherlands Trial Register with trial registry number NTR 7692 (www.trialregister.nl).


Similar content being viewed by others
References
Humira. https://www.ema.europa.eu/en/medicines/human/EPAR/humira#product-information-section. https://www.ema.europa.eu/en/medicines/human/EPAR/humira#product-information-section.
Derijks LJJ, Wong DR, Hommes DW, van Bodegraven AA. Clinical pharmacokinetic and pharmacodynamic considerations in the treatment of inflammatory bowel disease. Clin Pharmacokinet. 2018;57(9):1075–106. https://doi.org/10.1007/s40262-018-0639-4.
Roda G, Jharap B, Neeraj N, Colombel JF. Loss of response to Anti-TNFs: definition, epidemiology, and management. Clin Transl Gastroenterol. 2016;7:e135. https://doi.org/10.1038/ctg.2015.63.
Favalli EG, Raimondo MG, Becciolini A, Crotti C, Biggioggero M, Caporali R. The management of first-line biologic therapy failures in rheumatoid arthritis: current practice and future perspectives. Autoimmun Rev. 2017;16(12):1185–95.
van Mulligen E, Ahmed S, Weel AEAM, Hazes JMW, van der Helm-van Mil AHM, de Jong PHP. Factors that influence biological survival in rheumatoid arthritis: results of a real-world academic cohort from the Netherlands. Clin Rheumatol. 2021;40(6):2177–83. https://doi.org/10.1007/s10067-020-05567-6.
Roblin X, Marotte H, Rinaudo M, Del Tedesco E, Moreau A, Phelip JM, Genin C, Peyrin-Biroulet L, Paul S. Association between pharmacokinetics of adalimumab and mucosal healing in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2014;12(1):80-84.e2. https://doi.org/10.1016/j.cgh.2013.07.010.
Pouw MF, Krieckaert CL, Nurmohamed MT, van der Kleij D, Aarden L, Rispens T, Wolbink G. Key findings towards optimising adalimumab treatment: the concentration-effect curve. Ann Rheum Dis. 2015;74(3):513–8. https://doi.org/10.1136/annrheumdis-2013-204172.
Ungar B, Levy I, Yavne Y, Yavzori M, Picard O, Fudim E, Loebstein R, Chowers Y, Eliakim R, Kopylov U, Ben-Horin S. Optimizing anti-TNF-alpha therapy: serum levels of infliximab and adalimumab are associated with mucosal healing in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2016;14(4):550-557.e2. https://doi.org/10.1016/j.cgh.2015.10.025.
Baert F, Vande Casteele N, Tops S, Noman M, Van Assche G, Rutgeerts P, Gils A, Vermeire S, Ferrante M. Prior response to infliximab and early serum drug concentrations predict effects of adalimumab in ulcerative colitis. Aliment Pharmacol Ther. 2014;40(11–12):1324–32. https://doi.org/10.1111/apt.12968.
Baert F, Kondragunta V, Lockton S, Vande Casteele N, Hauenstein S, Singh S, Karmiris K, Ferrante M, Gils A, Vermeire S. Antibodies to adalimumab are associated with future inflammation in Crohn’s patients receiving maintenance adalimumab therapy: a post hoc analysis of the Karmiris trial. Gut. 2016;65(7):1126–31. https://doi.org/10.1136/gutjnl-2014-307882.
Frederiksen MT, Ainsworth MA, Brynskov J, Thomsen OO, Bendtzen K, Steenholdt C. Antibodies against infliximab are associated with de novo development of antibodies to adalimumab and therapeutic failure in infliximab-to-adalimumab switchers with IBD. Inflamm Bowel Dis. 2014;20(10):1714–21. https://doi.org/10.1097/MIB.0000000000000138.
Mazor Y, Almog R, Kopylov U, Ben Hur D, Blatt A, Dahan A, Waterman M, Ben-Horin S, Chowers Y. Adalimumab drug and antibody levels as predictors of clinical and laboratory response in patients with Crohn’s disease. Aliment Pharmacol Ther. 2014;40(6):620–8. https://doi.org/10.1111/apt.12869.
Imaeda H, Takahashi K, Fujimoto T, Bamba S, Tsujikawa T, Sasaki M, Fujiyama Y, Andoh A. Clinical utility of newly developed immunoassays for serum concentrations of adalimumab and anti-adalimumab antibodies in patients with Crohn’s disease. J Gastroenterol. 2014;49(1):100–9. https://doi.org/10.1007/s00535-013-0803-4.
Han ZM, Elodie WH, Yan LH, Xu PC, Zhao XM, Zhi FC. Correlation between ultrasonographic response and anti-tumor necrosis factor drug levels in Crohn’s disease. Ther Drug Monit. 2022. https://doi.org/10.1097/FTD.0000000000000988.
Ternant D, Karmiris K, Vermeire S, Desvignes C, Azzopardi N, Bejan-Angoulvant T, van Assche G, Paintaud G. Pharmacokinetics of adalimumab in Crohn’s disease. Eur J Clin Pharmacol. 2015;71(9):1155–7. https://doi.org/10.1007/s00228-015-1892-1.
Berends SE, Strik AS, Van Selm JC, Lowenberg M, Ponsioen CY, D’Haens GR, Mathot RA. Explaining interpatient variability in adalimumab pharmacokinetics in patients with Crohn’s disease. Ther Drug Monit. 2018;40(2):202–11. https://doi.org/10.1097/FTD.0000000000000494.
Ternant D, Ducourau E, Fuzibet P, Vignault C, Watier H, Lequerre T, Le Loet X, Vittecoq O, Goupille P, Mulleman D, Paintaud G. Pharmacokinetics and concentration-effect relationship of adalimumab in rheumatoid arthritis. Br J Clin Pharmacol. 2015;79(2):286–97. https://doi.org/10.1111/bcp.12509.
Ter Heine R, Keizer RJ, van Steeg K, Smolders EJ, van Luin M, Derijks HJ, de Jager CPC, Frenzel T, Bruggemann R. Prospective validation of a model-informed precision dosing tool for vancomycin in intensive care patients. Br J Clin Pharmacol. 2020;86(12):2497–506. https://doi.org/10.1111/bcp.14360.
Kylstra AH, Benoy S, Traksel RAM, Broeren MAC, Derijks LJJ. Therapeutic drug monitoring of adalimumab in rheumatic patients. Int J Clin Pharm. 2019;154(6):25–8.
Keizer RJ, Karlsson MO, Hooker A. Modeling and simulation workbench for NONMEM: tutorial on Pirana, PsN, and Xpose. CPT Pharmacometrics Syst Pharmacol. 2013;2:e50. https://doi.org/10.1038/psp.2013.24.
van Beek SW, Ter Heine R, Keizer RJ, Magis-Escurra C, Aarnoutse RE, Svensson EM. Personalized tuberculosis treatment through model-informed dosing of rifampicin. Clin Pharmacokinet. 2019;58(6):815–26. https://doi.org/10.1007/s40262-018-00732-2.
Schultheiss JPD, Altena S, Clevers MR, Baas D, Jharap B, Fidder HH. Adherence to adalimumab was not improved by a reminder-based intervention with an electronic needle container. Dig Dis Sci. 2021;66(5):1477–87. https://doi.org/10.1007/s10620-020-06395-z.
Berends SE, Bloem K, de Vries A, Schaap T, Rispens T, Strik AS, Talwar R, Lowenberg M, D’Haens GR, Mathot RA. Monitoring of adalimumab concentrations at home in patients with inflammatory bowel disease using dried blood samples. Ther Drug Monit. 2020;42(2):289–94. https://doi.org/10.1097/FTD.0000000000000686.
Bloem K, Schaap T, Boshuizen R, Kneepkens EL, Wolbink GJ, Vries A, Rispens T. Capillary blood microsampling to determine serum biopharmaceutical concentration: Mitra((R)) microsampler vs dried blood spot. Bioanalysis. 2018;10(11):815–23. https://doi.org/10.4155/bio-2018-0010.
Kneepkens EL, Pouw MF, Wolbink GJ, Schaap T, Nurmohamed MT, de Vries A, Rispens T, Bloem K. Dried blood spots from finger prick facilitate therapeutic drug monitoring of adalimumab and anti-adalimumab in patients with inflammatory diseases. Br J Clin Pharmacol. 2017;83(11):2474–84. https://doi.org/10.1111/bcp.13371.
Bartelds GM, Krieckaert CL, Nurmohamed MT, van Schouwenburg PA, Lems WF, Twisk JW, Dijkmans BA, Aarden L, Wolbink GJ. Development of antidrug antibodies against adalimumab and association with disease activity and treatment failure during long-term follow-up. JAMA. 2011;305(14):1460–8. https://doi.org/10.1001/jama.2011.406.
Faber NM. Estimating the uncertainty in estimates of root mean square error of prediction: application to determining the size of an adequate test set in multivariate calibration. Chemometrics Intellig Lab Syst. 1999;49(1):79–89. https://doi.org/10.1016/S0169-7439(99)00027-1.
Sheiner LB, Beal SL. Some suggestions for measuring predictive performance. J Pharmacokinet Biopharm. 1981;9(4):503–12.
Lindbom L, Ribbing J, Jonsson EN. Perl-speaks-NONMEM (PsN)—a Perl module for NONMEM related programming. Comput Methods Programs Biomed. 2004;75(2):85–94. https://doi.org/10.1016/j.cmpb.2003.11.003.
Blache C, Lequerré T, Roucheux A, Beutheu S, Dedreux I, Jacquot S, Le Loët X, Boyer O, Vittecoq O. Number and phenotype of rheumatoid arthritis patients’ CD4+CD25hi regulatory T cells are not affected by adalimumab or etanercept. Rheumatology (Oxford). 2011;50(10):1814–22. https://doi.org/10.1093/rheumatology/ker183.
Bloem K, van Leeuwen A, Verbeek G, Nurmohamed MT, Wolbink GJ, van der Kleij D, Rispens T. Systematic comparison of drug-tolerant assays for anti-drug antibodies in a cohort of adalimumab-treated rheumatoid arthritis patients. J Immunol Methods. 2015;418:29–38.
Bloem K, Hernández-Breijo B, Martínez-Feito A, Rispens T. Immunogenicity of therapeutic antibodies: monitoring antidrug antibodies in a clinical context. Ther Drug Monit. 2017;39(4):327–32. https://doi.org/10.1097/FTD.0000000000000404.
Yanai H, Lichtenstein L, Assa A, Mazor Y, Weiss B, Levine A, Ron Y, Kopylov U, Bujanover Y, Rosenbach Y, Ungar B, Eliakim R, Chowers Y, Shamir R, Fraser G, Dotan I, Ben-Horin S. Levels of drug and antidrug antibodies are associated with outcome of interventions after loss of response to infliximab or adalimumab. Clin Gastroenterol Hepatol. 2015;13(3):522-530.e2. https://doi.org/10.1016/j.cgh.2014.07.029.
Acknowledgements
We thank Wil Adriaans, Louise Merry-Meier and Antoinette Piepenbrock-van Schooten for their efforts for the inclusion of patients in this trial.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received to conduct this study.
Author contributions
Conceptualization: P.K., R.K., R.H. and L.D., methodology: P.K., R.K., R.H. and L.D., software: R.H. and R.K., formal analysis: A.V., T.S., K.B. and T.R., investigation: P.K., P.B., I.K., F.H. and L.S., data curation: P.K., R.K. and R.H., writing—original draft preparation: P.K., writing—review and editing: R.K., R.H., F.H., P.B., I.K., T.S., A.V., K.B., T.R., L.S. and L.D., supervision: P.K. and L.D.
Conflict of Interest
F.H.: speaker for Abbvie, Janssen–Cilag, MSD, Takeda, Celltrion, Teva, Sandoz and Dr Falk. Funding (grants/honoraria): Dr Falk, Janssen–Cilag, Abbvie and Takeda. Consulting fees: Celgene. P.K., R.K., R.H., L.D., A.V., T.S., K.B., T.R., P.B., I.K. and L.S. declare that they have no conflicts of interest.
Ethics Approval
The study was approved by the local ethics committee. The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of Máxima Medisch Centrum (W18.147, 22-01-2019).
Patient Consent
Informed consent was obtained from all subjects involved in the study.
Consent for publication
Not applicable.
Data Availability
Raw data were generated at Máxima Medical Center and Radboud University Medical Center. Derived data supporting the findings of this study are available from the corresponding author (P.K.) on request.
Code Availability
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Klaver, P.A.G., Keizer, R.J., ter Heine, R. et al. Early At-Home Measurement of Adalimumab Concentrations to Guide Anti-TNF Precision Dosing: A Pilot Study. Eur J Drug Metab Pharmacokinet 48, 377–385 (2023). https://doi.org/10.1007/s13318-023-00835-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-023-00835-7