Abstract
Background
Adherence to adalimumab in inflammatory bowel disease (IBD) patients is reported to be below par. Non-adherence may result in loss-of-response and increased hospitalization. We analyzed the effect of an electronic needle container (ENC) on adherence to adalimumab.
Methods
In this multicenter, 12-months observational study, we included adalimumab treated IBD patients. All patients were invited to receive an ENC. Patients who declined or did not complete the registration for an ENC served as controls. Primary endpoint was whether an ENC increased adherence, calculated from pharmacy refills as proportion of days covered (PDC). Secondary endpoints were clinical outcomes, including loss-of-response, identification of predictors of adherence and correlation between different modalities for measuring adherence. Loss-of-response was defined as a disease flare, dose-escalation or IBD-related hospitalization or surgery.
Results
The pharmacies’ records identified 198 eligible patients, of whom 32 were excluded. The ENC was supplied to 69 patients, the remaining 97 patient formed the control group. Median baseline PDC (98.4% vs. 96.1%, p = 0.047) and the proportion of adherent (PDC ≥ 86%) patients (87.0% vs. 74.2%, p = 0.045) was higher for the ENC group. The ENC did not improve the adherence of patients during follow-up (odds ratio 1.26, 95% CI 0.55–2.86). During follow-up, five (7.2%) patients in the ENC group and 13 (13.4%) in the control group discontinued adalimumab (log-rank p = 0.22). Loss-of-response occurred in 12 (17.4%) and 14 (14.4%) patients, respectively (log-rank p = 0.66).
Conclusions
Our results show no beneficial effect of a reminder-based intervention on adherence or treatment outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The introduction of anti-tumor necrosis factor-α (anti-TNF) treatment has greatly improved the treatment arsenal for IBD patients. Both infliximab and adalimumab are effective in inducing and maintaining remission and have shown to reduce hospitalization and surgery rates [1,2,3,4].
Unfortunately, the effectiveness of these therapies is partly hampered by non-adherence. Adherence rates between 55 and 83% to TNF-α inhibitor treatment have been reported in IBD [5,6,7]. These rates are mostly based on a cut-off of ≥ 80% adherence. This cut-off is acceptable for most daily medications but might be too low for biologics [8, 9]. Indeed, it has recently been demonstrated that the most optimal cut-off for adalimumab adherence is ≥ 86%. Reported adherence rates for adalimumab with this new cut-off were 69% and 79%. [8, 10]
It has been reported that lower adherence to TNF-α inhibitors is associated with loss-of-response, flares and an increased risk of hospitalization [8, 11]. Additionally, non-adherence to infliximab has been shown to result in an estimated increase of up to 90% in overall medical expenses [12,13,14].
Predicting, measuring and improving adherence is therefore of interest both from a clinical and economic perspective. This is, however, challenging [15]. Objective methods, such as measurements of blood or urine drug levels, are expensive, time-consuming and the outcomes depend on individual metabolic pathway differences [16, 17]. A noninvasive approach is the calculation of the proportion of days covered (PDC) in a prescription period from pharmacy’ refills, which is currently considered a suitable gold standard [18]. Nonetheless, the PDC only measures patients’ medication possession and not whether the medication is actually used.
There are many interventions possible that might improve adherence in patients. One such intervention is a reminder-based intervention, e.g., a text message or phone calls. In several diseases, this approach has been shown to increase adherence to treatment regimens [19, 20]. In this study, we applied such an approach by supplying patients through usual clinical care with an electronic needle container (ENC). The ENC informs and reminds patients when to inject their next adalimumab injection. Additionally, it measures and informs patients of their real-time medication adherence by registering deposited used adalimumab pens.
The aim of this study is therefore twofold. First, to evaluate the effect of an ENC as an instrument to increase adherence to adalimumab in IBD patients. Second, to investigate the effect on clinical outcomes and how well indirect measures of adherence correlates with real-time medication usage.
Methods
Design and Patients
The study was conducted in two centers, one tertiary care hospital, the University Medical Centre Utrecht, and one general hospital, Meander Medical Centre, in the Netherlands. Between December 2016 and August 2017, all adult outpatient IBD patients treated with adalimumab were invited to participate in an ENC program. The ENC was supplied by a pharmaceutical company with a pharmacy as intermediary to protect patients’ privacy. As the ENC was supplied through the pharmaceutical company’s supporting care program, patients could not be randomized. Thus, receiving the ENC was based on patients’ preferences.
Patients who did participate in the ENC program were requested to fill out several additional questionnaires. Self-reported medication adherence was evaluated by the Visual Analogue Scale (VAS) [16]. The VAS is a single question tool ranging from 0 to 100% to assess patients’ adherence. A VAS of ≥ 86% was considered adherent [8, 16].
Patients’ illness perceptions were assessed using the Brief Illness Perception Questionnaire (IPQ). The IPQ uses a scale 11-point Likert scale (0–10) to assess cognitive and emotional representations of illness across 8 dimensions: Consequences, Timeline, Personal Control, Treatment Control, Identity, Concerns, Understanding, and Emotional Response [21].
Treatment beliefs were evaluated with the Beliefs about Medicines Questionnaire specific (BMQ-s). Two subscales measure patients’ beliefs about the necessity of their medication (8 items) and the concerns about potential adverse consequences of taking their medication (9 items) on a 5-point Likert scale. After calculating mean scores of both scales, patients can be categorized into four attitudinal groups: accepting (high necessity, low concerns), ambivalent (high necessity, high concerns), indifferent (low necessity, low concerns) and skeptical (low necessity, high concerns) [22, 23].
A physician global assessment was used to assess disease activity (remission, mild, moderate or severe) at baseline. The physician’s global assessment was based on the treating physician’s clinical acumen along with, if available, recent colonoscopy results or laboratory markers.
Adherence Measures
The PDC was calculated from refill data supplied by the hospitals’ pharmacies. The PDC was calculated by dividing the number of days in a period covered by adalimumab treatment by the number of days in the period between refills. To correct for premature refills, the start of a new refill period was moved forward until no covered days were left from the previous period. The PDC is expressed as a percentage [18]. The PDC was calculated for up to 2 years before start of the ENC program and during the follow-up period in which the ENC was used with a maximum of 1 year.
In patients participating in the ENC program, adherence was also measured by the ENC (HealthBeacon Injection Care Management System™). A deposit of a used adalimumab pen at exactly the intended date according to the prescribed regimen was recorded as on-time, 1–2 days before the intended date as ‘early’, and until 2 days after the intended date as ‘late’. All other moments of deposits or no deposit were registered as missed. This led to an adherence percentage calculated by the ENC. This percentage was displayed on the ENC and visible to patients. Physicians would receive a report of their patients’ adherence every 3 months. Additionally, the ENC reminded patients when to administer the next adalimumab pen by two modalities. First, the ENC’s display would light up on the correct day. Second, on the scheduled day a text message was sent to patients to remind them to inject adalimumab.
Adherence is usually defined as taking medication at least 80% of the time. However, as it has recently been demonstrated that the optimal cut-off value for adalimumab was an adherence rate of ≥ 86%, we considered patient adherent if their adherence was ≥ 86% [8].
Outcome Measures
Primary outcome of interest was whether the use of an ENC led to an improvement of adherence. Additionally, we were interested in whether the ENC had any effect on clinical outcomes at 1 year follow-up, such as loss-of-response and drug survival and the correlation between real-time adherence measures and an indirect measure such as the PDC. Loss-of-response was defined as clinical worsening leading to either adalimumab discontinuation, dose escalation (i.e., shortening of dosing interval to less than every other week), IBD-related abdominal surgery or IBD-related hospitalization without surgery. Drug discontinuation was defined as cessation of adalimumab therapy for any reason (i.e., loss-of-response, side effects, remission or miscellaneous [patient’s wish, etc.]).
Statistical Analysis
Baseline characteristics are displayed for continuous variables as medians with interquartile range and for categorical variables as counts with percentages. Baseline differences for categorical variables were compared using the χ2 test or Fisher’s exact test when appropriate. Normality was tested for all continuous variables. The Mann–Whitney U test was used for comparing continuous variables, as all continuous variables were nonparametric distributed. McNemar’s test was used to compare between proportions at baseline and follow-up. We analyzed the “corrective” and “maintaining” potential of the ENC by subsetting on baseline adherence status and conducting a two proportion Z-test. To estimate a treatment effect of the ENC and adjust for baseline adherence status a generalized estimating equation model with a exchangeable correlation matrix was built.
Association between adherence measures was assessed by means of Kendall rank correlation coefficient. Diagnostic test characteristics for adherence measures were calculated from 2 × 2 tables. Receiver operated characteristics (ROC) curves were constructed to calculate the area under the curve (AUC) for the diagnostic tests. Factors associated with non-adherence were identified by univariate logistic regression analyses with demographic and disease characteristics with additional analyses performed in the ENC group for behavioral characteristics.
Cox proportional hazards regression with ENC use as time-varying covariate, to correct for premature ENC discontinuation, was performed to determine the association between ENC use and time to loss-of-response or drug discontinuation. Multivariable Cox proportional hazards modeling was performed with statistically significant covariates and factors.
Apart from ENC use, all models were also were also analyzed for the following factors and covariates: sex, educational level, time on adalimumab therapy, prior anti-TNF use, employment, marital status, smoking, IBD diagnosis, physician global assessment and age.
As it has been reported that up to 95% adherence patients still had better clinical outcomes, sensitivity analyses with cut-off values for adherence of ≥ 90% and ≥ 95% were conducted if applicable [8].
A two‐sided p value < 0.05 or a 95% confidence interval (CI) excluding 1.0 were considered statistically significant. R version 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria) was used for statistical analysis.
Results
Patient Population
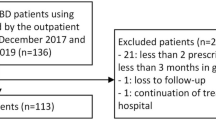
Between December 2016 and August 2017, according to the pharmacies’ records 198 adult IBD patients were being treated with adalimumab at the two participating hospitals. We excluded 31 patients because of recent or planned discontinuation (n = 19), upcoming drug holiday for pregnancy (n = 4), long travels abroad (n = 3), not having started adalimumab yet (n = 2), no informed consent (n = 2) and a diagnosis different from IBD (n = 1). Thus, 167 patients were included in the study. A total of 70 patients agreed to participate in the ENC program. One patient was excluded during follow-up as his diagnosis of Crohn’s disease was revoked during the follow-up period. The control group consisted of 97 patients: 61 patients that declined to use the ENC and 36 patients who initially agreed but did submit the necessary registration forms and thus could not receive the ENC (Fig. 1).
There were no significant differences in demographic or clinical characteristics between the ENC and control group, except for the disease location. CD was more often located in the colon (Montreal L2) in the control group compared to the ENC group (42.0% vs. 18.8%). The baseline characteristics for both groups are depicted in Table 1.
ENC Use
In total, 38 (55.1%) patients terminated the use of the ENC before completion of the 1 year follow-up. In five patients, this was due to adalimumab discontinuation. The remaining 33 patients stopped mainly because they regarded the device as impractical and/or unnecessary. The median time after which these patients discontinued the ENC was 6.9 months (IQR 4.1–8.8).
Adherence
Pharmacy refill data were available for all patients. Median baseline PDC was 98.4% (IQR 91.7–100%) for the ENC group and 96.1% (IQR 85.9–99.5%) for the control group (p = 0.047). More patients were adherent in the ENC group (n = 60 [87%]) compared to the control group [n = 72 (74.2%)] (p = 0.045).
During follow-up, there was no difference in median PDC between the ENC and control group, 94.8% (IQR 88.1–98.9%) and 92.8% (IQR 86.2–99.1%), respectively (p = 0.404). However, in both the ENC as well as the control group, a small but significant decrease of the PDC was observed during follow-up compared with baseline PDC of − 3.6% (p = 0.038) and −3.4% (p = 0.021), respectively. Additionally, the proportion of patients with 100% adherence did change during follow-up (p = 0.029). This difference was attributed to a decrease in the proportion of patients with 100% adherence in the ENC group during follow-up (21 [30.4%) to 8 [11.6%), p = 0.009). (Table 2)
No difference in the rate of correcting non-adherent patients by the ENC was observed compared to the control group (18.67%, 95% CI − 17.8 to 55.2%, p = 0.336) nor for the rate of maintaining patients adherent (0.28%, 95% CI − 12.00 to 12.55%, p = 0.965). This was confirmed by the generalized estimating equations model which observed no effect as well [Odds Ratio (OR) 1.26, 95% CI 0.55–2.86]. No other factors and covariates were predictive either, except, as expected, being adherent at baseline (OR 4.79, 95% CI 2.09–10.94). The same outcome was observed for the sensitivity analyses.
Predictors of Adherence
Univariable logistic regression was used to identify potential predictors of adherence at baseline. The aforementioned factors and covariates were analyzed. Within the ENC group, additional univariable logistic regression was performed for the necessity and concern score of the BMQ as well as the 8 questions of the IPQ. No factors were predictive of adherence. Sensitivity analyses with a cut-off value of ≥ 90% and ≥ 95% yielded the same results, except for female sex. The odds for females of being ≥ 95% adherent were lower compared to males (OR 0.51, 95% CI 0.27–0.95).
Additionally, linear regression was performed to see whether there were factors that predicted a higher PDC at baseline. No factors were found to be predictive of a higher PDC at baseline nor in the sub-analysis of the ENC group for the BMQ scales and IPQ questions.
Correlation of Adherence Measures
The correlation between several adherence measures was investigated. Within the ENC group, the PDC according to the ENC was weakly but significantly correlated with the PDC during follow-up (Kendall’s tau 0.25, p = 0.003). The VAS was also weakly but significantly correlated with both PDC at baseline (Kendall’s tau 0.25, p = 0.013) and PDC during follow-up (Kendall’s tau 0.25, p = 0.01) but not with the ENC (Kendall’s tau 0.16, p = 0.10).
However, more relevant is how accurately the PDC calculated from pharmacy refills correctly identifies adherent patients. Therefore, we evaluated the test characteristics of the PDC calculated from pharmacy refills during follow-up with real-time adherence from the ENC. Using a cut-off value of ≥ 86% for both measurements the sensitivity was 88.89% (95% CI 77.37–95.81%), specificity 40.00% (95% CI 16.34–67.71%), a positive predictive value (PPV) of 84.21% (95% CI 77.73–89.07%), a negative predictive value (NPV) of 50.00% (95% CI 27.36–72.64%),an accuracy of 78.26% (95% CI 66.69–87.29%) and an AUC of 0.64.
Additionally, to evaluate the clinical applicability of the VAS we determined how accurately patients score themselves as being adherent. With cut-off values of ≥ 86% for adherent and considering the ENC as golden standard, the VAS had a sensitivity of 94.00% (95% CI 83.45–98.75%), specificity of 15.38% (95% CI 1.92–45.45%), a PPV of 81.03% (95% CI 77.03–84.48%), a NPV of 40.00% (95% CI 11.03–78.19%), an overall accuracy of 77.78% (95% CI 65.54–87.28%) and an AUC of 0.55 when a cut-off value of ≥ 86% for the VAS was applied as well.
Clinical Outcomes
During the 12-month follow-up period, two patients (2.9%) in the ENC group and 11 (11.3%) in the control group started with additional IBD therapy (p = 0.046). Thiopurines were most commonly initiated as additional medication.
Within 1 year, 18 (10.8%) patients discontinued adalimumab. Five (7.2%) patients in the ENC group and 13 (13.4%) in the control group (p = 0.209). Reasons for discontinuation in both groups were similar (p = 0.230). (Table 3) Overall adalimumab drug survival was comparable between the ENC and control group (log rank p = 0.22) (Fig. 2a)
During follow-up, 26 (15.7%) patients lost response to adalimumab therapy, 12 (17.4%) patients in the intervention group and 14 (14.4%) patients in the control group, respectively (p = 0.605). Reasons for loss-of-response to adalimumab were comparable between the groups (p = 0.517). (Table 3) There were no differences between the groups regarding time till loss-of-response (log rank p = 0.66) (Fig. 2b)
Univariable Cox proportional hazards modeling was performed to analyze the effect of ENC use on drug survival and loss-of-response. As ENC use was terminated before the end of follow-up by 38 (55.1%) patients, time-varying covariates were used therefore. Moderate disease activity according to the physician’s global assessment [adjusted Hazard Ratio (aHR) 9.12, 95% CI 2.03–41.07] and a longer disease duration (aHR 1.04, 95% CI 1.00–1.09) were associated with an increased risk of adalimumab discontinuation. For loss-of-response both mild (aHR 3.27, 95% CI 1.40–7.66) and moderate disease activity (aHR 14.7, 95% CI 3.31–65.66) according to the physician’s global assessment were associated with an increased risk of loss-of-response, while a longer adalimumab treatment was protective (aHR 0.81, 95% CI 0.69–0.95). (Table 4)
Discussion
In this cohort study, we evaluated the effect of a reminder-based intervention with an ENC on adalimumab adherence in IBD patients. No differences were observed in the proportion of adherent patients between the intervention and control group at baseline or at 1-year follow-up. Although the ENC did not lead to an absolute decrease in adherence, there was a significantly lower proportion of 100% adherent patients in the ENC group during follow-up. There was no effect of the ENC on clinical outcomes.
The overall rate of adherence in our cohort was 79.5%. This is equal to the recently reported adherence rate of 79.5% for adalimumab, based on the same cut-off value (i.e., ≥ 86%) [8]. However, overall, adherence estimates from the literature are lower. The pooled adherence rate to adalimumab in IBD patients in a large systematic review was 71% [6]. Another systematic review reported that only 55% of adalimumab treated CD patients were adherent [5]. Similarly, a low adherence rate of 57% was reported by two prospective studies [7, 11]. Additionally, an adherence rate of 69% for subcutaneous anti-TNF treatment was reported for patients from a tertiary care center [10]. The large differences in reported adherence rates may be explained by the large heterogeneity in the applied definition of adherence.
Non-adherence is a universal dilemma encountered in medical conditions. Many randomized controlled trials (RCT) on adherence have been conducted in several diseases. A large Cochrane review on interventions for enhancing adherence did not identify any effective and easily implementable intervention that increased adherence and/or had a positive effect on treatment outcomes [24]. Only one RCT in ulcerative colitis was included in the review. In this RCT, a multifaceted intervention consisting of an educational and motivational talk with a free choice of up to three additional interventions which could be changed over time, such as (non)electronic pill boxes, reminder charts and a simplified dosing regimen. In both groups there was a decline in adherence to 5-aminosalicates during follow-up. However, this decline was lower in the intervention group. In this study, no treatment outcomes were investigated [25].
In our study, a reminder-based intervention was used. Such an intervention might be useful when employed in a group where forgetfulness is the major reason for unintentional non-adherence [19]. However, research suggests that unintentional non-adherence is more complicated than just forgetfulness. It may be associated with beliefs about medicines. Additionally, intentional non-adherence may play a pivotal role. Factors contributing to intentional non-adherence are complex and can be within patients’ control (e.g., concerns about adverse effects or necessity) or outside (e.g., inadequate healthcare provider’ communication) [20]. Our results also imply that improving adherence is more complicated than just reminding people to take their medication. Furthermore, 55.1% of ENC users terminated the ENC before the end of follow-up because they felt the device was impractical and unnecessary. Of note, most of the patients with an ENC were already using adalimumab for several years and most already established their own routine. This might have contributed to a more negative disposition toward this intervention.
The intricacies of adherence are reported throughout related literature. Previous studies on therapy adherence in IBD have identified several demographic and clinical factors as predictors for non-adherence. These include female gender, younger patients, employment, unmarried status, shorter disease duration, concomitant medications, adalimumab use and constraints related to treatment [5, 6, 26,27,28,29,30]. However, for all studies reporting a significant relationship between these predictors and non-adherence, there were at least as many studies reporting no significant relationship between the same predictors and non-adherence [31]. Recently, several cumulative risk factors (current narcotic use, psychiatric history, prior biologic use, and smoking) were identified as predictors for non-adherence. The more of these risk factors, the higher the probability of non-adherence [10]. In our study, no risk factors for non-adherence were identified. However, data on several of the more recently identified factors, such as psychiatric history, could not be reliably investigated.
It has also been reported that having a shorter timeline perception (i.e., viewing IBD as an acute episodic disease) and a stronger negative emotional response according to the IPQ are related to non-adherence, while doubts about personal necessity and fear for adverse effects identified by BMQ were also associated with non-adherence [11, 23]. Interventions focusing on illness perceptions and treatment beliefs could thus possibly contribute more effectively to decreasing intentional non-adherence. We did not find any associations between the IPQ or BMQ regarding non-adherence. However, due to the small sample size in which this was assessed, our study lacks the power for these analyses.
The ENC also provided us with an opportunity to investigate tools to assess adherence. The PDC (or variants thereof such as the medication possession ratio) has been validated for other drugs in different chronic disorders and is considered a suitable gold standard [18]. Yet, it has never been directly compared to real-time medication use in IBD. Furthermore, no useful clinical tool has been validated to assess adherence to anti-TNF agents in IBD at present. The only validated tool in IBD is the Modified Morisky Adherence Scale-8, which does not correlate with anti-TNF agents adherence as this scale was developed for daily medication intake [32]. The VAS has been proposed as an appropriate tool in clinical practice to quantify medication adherence [16]. However, it has never been evaluated against a gold standard such as the PDC.
Our results show that there is a weak, but significant, correlation between all investigated adherence measures. However, how well these adherence measures correctly identify adherent patients is clinically more relevant. When applying a cut-off value of ≥ 86%, all tools accurately identified adherent patients. The identification of non-adherent patients was below par, though. This led to low specificity and NPV of especially the VAS.
Not only did we investigate adherence, we also evaluated direct clinical outcomes. Overall, in our study we noted drug discontinuation in 10.8% and loss-of-response (with or without drug discontinuation) in 15.7% of patients. Our results are comparable to previously reported data [33]. In addition, we compared the groups with and without ENC. The ENC did not have an effect on drug survival nor loss-of-response rates. This is not surprising, since no effect on adherence was observed either.
This study has several strengths. First, patients were included from both a university and general hospital, thus more accurately representing the average IBD patient’s population. Second, we used a suitable gold standard and even compared this gold standard with real-time adherence. Last, not only did we investigate whether adherence improved but also whether there was an effect on clinical outcomes.
Some limitations of this study need to be discussed as well. First, it was not possible to randomize patients to receive the ENC since this was supplied through a pharmaceutical’s additional care program, thus increasing the risk of bias and confounding. Second, clinical outcomes were derived from patient’ electronic charts and were not always objectified by for example, colonoscopy or calprotectin levels. Third, in all studies investigating adherence, the Hawthorne effect should be considered. This effect suggests that study participants might modify their behavior due to being aware of being observed [34]. However, as we did not see an increase in the proportion of adherent patients during follow-up, we doubt the Hawthorne effect played a meaningful role, if at all. Fourth, although we considered the ENC the golden standard, it is still, however unlikely, possible to ‘cheat’. Additionally, patients might forget depositing an adalimumab pen, which might have given an underestimation of the adherence rates.
In conclusion, our results show there was no beneficial effect of a reminder-based intervention on either adherence or treatment outcomes. We believe that adherence is more complicated than just forgetfulness. More complex factors, such as beliefs about treatment and disease, need to be addressed to improve adherence in patients.
References
Ford AC, Sandborn WJ, Khan KJ, Hanauer SB, Talley NJ, Moayyedi P. Efficacy of biological therapies in inflammatory bowel disease: systematic review and meta-analysis. Am J Gastroenterol. 2011;106:644–659. https://doi.org/10.1038/ajg.2011.73.
Behm BW, Bickston J. Tumor necrosis factor-alpha antibody for maintenance of remission in Crohn’ s disease (Cochrane review). Cochrane Database Syst Rev. 2009;. https://doi.org/10.1002/14651858.CD006893.www.cochranelibrary.com.
Costa J, Magro F, Caldeira D, Alarcão J, Sousa R, Vaz-Carneiro A. Infliximab reduces hospitalizations and surgery interventions in patients with inflammatory bowel disease: a systematic review and meta-analysis. Inflamm Bowel Dis. 2013;19:2098–2110. https://doi.org/10.1097/MIB.0b013e31829936c2.
Feagan BG, Panaccione R, Sandborn WJ, et al. Effects of adalimumab therapy on incidence of hospitalization and surgery in Crohn’s disease: results from the CHARM study. Gastroenterology. 2008;135:1493–1499. https://doi.org/10.1053/j.gastro.2008.07.069.
Fidder HH, Singendonk MMJ, van der Have M, Oldenburg B, van Oijen MGH. Low rates of adherence for tumor necrosis factor-α inhibitors in Crohn’s disease and rheumatoid arthritis: results of a systematic review. World J Gastroenterol. 2013;19:4344–4350. https://doi.org/10.3748/wjg.v19.i27.4344.
Lopez A, Billioud V, Peyrin-Biroulet C, Peyrin-Biroulet L. Adherence to anti-TNF therapy in inflammatory bowel diseases: a systematic review. Inflamm Bowel Dis. 2013;19:1528–1533. https://doi.org/10.1097/MIB.0b013e31828132cb.
Wentworth BJ, Buerlein RCD, Tuskey AG, Overby MA, Smolkin ME, Behm BW. Nonadherence to biologic therapies in inflammatory bowel disease. Inflamm Bowel Dis. 2018;24:2053–2061. https://doi.org/10.1093/ibd/izy102.
Govani SM, Noureldin M, Higgins PDR, et al. Defining an optimal adherence threshold for patients taking subcutaneous anti-TNFs for inflammatory bowel diseases. Am J Gastroenterol. 2018;113:276–286. https://doi.org/10.1038/ajg.2017.438.
Karve S, Cleves MA, Helm M, Hudson TJ, West DS, Martin BC. Good and poor adherence: optimal cut-point for adherence measures using administrative claims data. Curr Med Res Opin. 2009;25:2303–2310. https://doi.org/10.1185/03007990903126833.
Shah NB, Haydek J, Slaughter J, et al. Risk factors for medication nonadherence to self-injectable biologic therapy in adult patients with inflammatory bowel disease. Inflamm Bowel Dis. 2020;26:314–320. https://doi.org/10.1093/ibd/izz253.
van der Have M, Oldenburg B, Kaptein AA, et al. Non-adherence to anti-TNF therapy is associated with illness perceptions and clinical outcomes in outpatients with inflammatory bowel disease: Results from a prospective multicentre study. J Crohn’s Colitis. 2016;10:549–555. https://doi.org/10.1093/ecco-jcc/jjw002.
Kane SV, Chao J, Mulani PM. Adherence to infliximab maintenance therapy and health care utilization and costs by Crohn’s disease patients. Adv Ther. 2009;26:936–946. https://doi.org/10.1007/s12325-009-0069-7.
Carter CT, Waters HC, Smith DB. Impact of infliximab adherence on crohn’s disease-related healthcare utilization and inpatient costs. Adv Ther. 2011;28:671–683. https://doi.org/10.1007/s12325-011-0048-7.
Wan GJ, Kozma CM, Slaton TL, Olson WH, Feagan BG. Inflammatory bowel disease: healthcare costs for patients who are adherent or non-adherent with infliximab therapy. J Med Econ. 2014;17:384–393. https://doi.org/10.3111/13696998.2014.909436.
Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;5:487–497.
Severs M, Zuithoff PNPA, Mangen MJJ, et al. Assessing self-reported medication adherence in inflammatory bowel disease: a comparison of tools. Inflamm Bowel Dis. 2016;22:2158–2164. https://doi.org/10.1097/MIB.0000000000000853.
Herman ML, Kane SV. Treatment nonadherence in inflammatory bowel disease: identification, scope, and management strategies. Inflamm Bowel Dis. 2015;21:2979–2984. https://doi.org/10.1097/MIB.0000000000000581.
Hess LM, Raebel MA, Conner DA, Malone DC. Measurement of adherence in pharmacy administrative databases: a proposal for standard definitions and preferred measures. Ann Pharmacother. 2006;40:1280–1288. https://doi.org/10.1345/aph.1H018.
Fenerty SD, West C, Davis SA, Kaplan SG, Feldman SR. The effect of reminder systems on patients’ adherence to treatment. Patient Prefer Adherence. 2012;6:127–135. https://doi.org/10.2147/PPA.S26314.
Ryan R, Santesso N, Lowe D, Hill S, Grimshaw J, Prictor M. Interventions to improve safe and effective medicines use by consumers. Cochrane Database Syst Rev. 2014;. https://doi.org/10.1002/14651858.CD007768.pub3.www.cochranelibrary.com.
Broadbent E, Petrie KJ, Main J, Weinman J. The brief illness perception questionnaire. J Psychosom Res. 2006;60:631–637. https://doi.org/10.1016/j.jpsychores.2005.10.020.
Horne R, Weinman J, Hankins M. The beliefs about medicines questionnaire: the development and evaluation of a new method for assessing the cognitive representation of medication. Psychol Health. 1999;14:1–24. https://doi.org/10.1080/08870449908407311.
Horne R, Parham R, Driscoll R, Robinson A. Patient’s attitudes to medicines and adherence to maintenance treatment in inflammatory bowel disease. Inflamm Bowel Dis. 2009;15:837–844. https://doi.org/10.1002/ibd.20846.
Nieuwlaat R, Wilczynski N, Navarro T, et al. Interventions for enhancing medication adherence (review). Cochrane Database Syst Rev. 2014;. https://doi.org/10.1002/14651858.CD000011.pub4.
Moshkovska T, Stone MA, Smith RM, Bankart J, Baker R, Mayberry JF. Impact of a tailored patient preference intervention in adherence to 5-aminosalicylic acid medication in ulcerative colitis: Results from an exploratory randomized controlled trial. Inflamm Bowel Dis. 2011;17:1874–1881. https://doi.org/10.1002/ibd.21570.
Coenen S, Weyts E, Ballet V, et al. Identifying predictors of low adherence in patients with inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2016;28:503–507. https://doi.org/10.1097/MEG.0000000000000570.
D’Incà R, Bertomoro P, Mazzocco K, Vettorato MG, Rumiati R, Sturniolo GC. Risk factors for non-adherence to medication in inflammatory bowel disease patients. Aliment Pharmacol Ther. 2008;27:166–172. https://doi.org/10.1111/j.1365-2036.2007.03555.x.
Severs M, Mangen MJJ, Fidder HH, et al. Clinical predictors of future nonadherence in inflammatory bowel disease. Inflamm Bowel Dis.. 2017;23:1568–1576. https://doi.org/10.1097/MIB.0000000000001201.
Shale MJ, Riley SA. Studies of compliance with delayed-release mesalazine therapy in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2003;18:191–198. https://doi.org/10.1046/j.1365-2036.2003.01648.x.
Ediger JP, Walker JR, Graff L, et al. Predictors of medication adherence in inflammatory bowel disease. Am J Gastroenterol. 2007;102:1417–1426. https://doi.org/10.1111/j.1572-0241.2007.01212.x.
Jackson CA, Clatworthy J, Robinson A, Horne R. Factors associated with non-adherence to oral medication for inflammatory bowel disease: a systematic review. Am J Gastroenterol. 2010;105:525–539. https://doi.org/10.1038/ajg.2009.685.
Trindade AJ, Ehrlich A, Kornbluth A, Ullman TA. Are your patients taking their medicine? Validation of a new adherence scale in patients with inflammatory bowel disease and comparison with physician perception of adherence. Inflamm Bowel Dis. 2011;17:599–604. https://doi.org/10.1002/ibd.21310.
Roda G, Jharap B, Neeraj N, Colombel JF. Loss of response to anti-TNFs: definition, epidemiology, and management. Clin Transl Gastroenterol. 2016;7:e135-5. https://doi.org/10.1038/ctg.2015.63.
McCarney R, Warner J, Iliffe S, Van Haselen R, Griffin M, Fisher P. The Hawthorne effect: a randomised, controlled trial. BMC Med Res Methodol. 2007;7:1–8. https://doi.org/10.1186/1471-2288-7-30.
Funding
The electronic needle container (HealthBeacon Injection Care Management System™) was supplied to patients through the AbbVie Care health program. Neither AbbVie Inc. nor AbbVie B.V. were involved in the design or reporting of this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Herma H. Fidder has done consultation for Abbvie BV, Janssen BV, Ferring BV and Takeda BV. The remaining authors disclose no conflicts of interest.
Ethical approval
This study was carried out with the approval of and in accordance with the ethical guidelines of the Institutional Review Board (IRB) of both participating centers. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Schultheiss, J.P.D., Altena, S., Clevers, M.R. et al. Adherence to Adalimumab Was Not Improved by a Reminder-Based Intervention with an Electronic Needle Container. Dig Dis Sci 66, 1477–1487 (2021). https://doi.org/10.1007/s10620-020-06395-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-020-06395-z