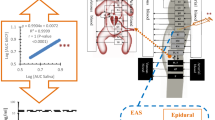
Abstract
Background and Objective
Pethidine (meperidine) can decrease labor pain-associated mother’s hyperventilation and high cortisol-induced newborn complications. However, prenatal transplacentally acquired pethidine can cause side effects in newborns. High pethidine concentrations in the newborn brain extracellular fluid (bECF) can cause a serotonin crisis. Therapeutic drug monitoring (TDM) in newborns' blood distresses them and increases infection incidence, which can be overcome by using salivary TDM. Physiologically based pharmacokinetic (PBPK) modeling can predict drug concentrations in newborn plasma, saliva, and bECF after intrauterine pethidine exposure.
Methods
A healthy adult PBPK model was constructed, verified, and scaled to newborn and pregnant populations after intravenous and intramuscular pethidine administration. The pregnancy PBPK model was used to predict the newborn dose received transplacentally at birth, which was used as input to the newborn PBPK model to predict newborn plasma, saliva, and bECF pethidine concentrations and set correlation equations between them.
Results
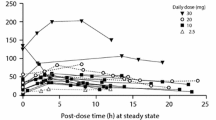
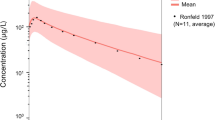
Pethidine can be classified as a Salivary Excretion Classification System class II drug. The developed PBPK model predicted that, after maternal pethidine intramuscular doses of 100 mg and 150 mg, the newborn plasma and bECF concentrations were below the toxicity thresholds. Moreover, it was estimated that newborn saliva concentrations of 4.7 µM, 11.4 µM, and 57.7 µM can be used as salivary threshold concentrations for pethidine analgesic effects, side effects, and the risk for serotonin crisis, respectively, in newborns.
Conclusion
It was shown that saliva can be used for pethidine TDM in newborns during the first few days after delivery to mothers receiving pethidine.
Graphical Abstract





Similar content being viewed by others
References
Lee MC, Abrahams M. Pain and analgesics. 11th ed. London: Elsevier; 2012.
Huch R. Maternal hyperventilation and the fetus. J Perinat Med. 1986;14:3–17. https://doi.org/10.1515/jpme.1986.14.1.3.
Motoyama E, Acheson F, Rivard G, Cook C. Adverse effect of maternal hyperventilation on the fetus. Lancet. 1966;287:286–8. https://doi.org/10.1016/S0140-6736(66)90639-8.
Thalme B, Belfrage P, Raabe N. Lumbar epidural analgesia in labour. Acta Obstet Gynecol Scand. 1974;53:27–35. https://doi.org/10.3109/00016347409156885.
Segal S, Wang SY. The effect of maternal catecholamines on the caliber of gravid uterine microvessels. Anesth Analg. 2008;106:888–92. https://doi.org/10.1213/ane.0b013e3181617451.
Reynolds F. The effects of maternal labour analgesia on the fetus. Best Pract Res Clin Obstet Gynaecol. 2010;24:289–302. https://doi.org/10.1016/j.bpobgyn.2009.11.003.
Garimella V, Cellini C. Postoperative pain control. Clin Colon Rectal Surg. 2013;26:191–6. https://doi.org/10.1055/s-0033-1351138.
Kee WN. Intrathecal pethidine: pharmacology and clinical applications. Anaesth Intensive Care. 1998;26:137–46. https://doi.org/10.1177/0310057X9802600202.
Cooper LV, Stephen GW, Aggett PJ. Elimination of pethidine and bupivacaine in the newborn. Arch Dis Child. 1977;52:638–41. https://doi.org/10.1136/adc.52.8.638.
Freeborn SF, Calvert RT, Black P, Macfarlane T, D’Souza SW. Saliva and blood pethidine concentrations in the mother and the newborn baby. BJOG. 1980;87:966–9. https://doi.org/10.1111/j.1471-0528.1980.tb04459.x.
Belfrage P, Boréus LO, Hartvig P, Irestedt L, Raabe N. Neonatal depression after obstetrical analgesia with pethidine. The role of the injection-delivery time interval and of the plasma concentrations of pethidine and norpethidine. Acta Obstet Gynecol Scand. 1981;60:43–9. https://doi.org/10.3109/00016348109154108.
Rickli A, Liakoni E, Hoener MC, Liechti ME. Opioid-induced inhibition of the human 5-HT and noradrenaline transporters in vitro: link to clinical reports of serotonin syndrome. Br J Pharmacol. 2018;175:532–43. https://doi.org/10.1111/bph.14105.
Olive J, Masana L, Gonzalez J. Meperidine and reversible parkinsonism. Mov Disord. 1994;9:115–6. https://doi.org/10.1002/mds.870090126.
Tortella FC, Cowan A, Adler MW. Studies on the excitatory and inhibitory influence of intracerebroventricularly injected opioids on seizure thresholds in rats. Neuropharmacology. 1984;23:749–54. https://doi.org/10.1016/0028-3908(84)90107-2.
Latta KS, Ginsberg B, Barkin RL. Meperidine: a critical review. Am J Ther. 2002;9:53–68.
Seifert CF, Kennedy S. Meperidine is alive and well in the new millennium: evaluation of meperidine usage patterns and frequency of adverse drug reactions. Pharmacotherapy. 2004;24:776–83. https://doi.org/10.1592/phco.24.8.776.36066.
World Health Organization. WHO recommendation on opioid analgesia for pain relief during labour. 2021. https://srhr.org/rhl/article/who-recommendation-on-opioid-analgesia-for-pain-relief-during-labour. Accessed 24 Feb 2023.
Alsmadi MM, Idkaidek N. Optimization of drugs pharmacotherapy during pregnancy using physiologically based pharmacokinetic models-an update. Curr Drug Metab. 2018;19:972–8. https://doi.org/10.2174/1389200219666180702104034.
Food U, Administration D. Guidance for industry. Pharmacokinetics in pregnancy—study design, data analysis, and impact on dosing and labeling. 2004. 2017. Accessed 21 Feb 2023.
Illamola SM, Bucci-Rechtweg C, Costantine MM, Tsilou E, Sherwin CM, Zajicek A. Inclusion of pregnant and breastfeeding women in research–efforts and initiatives. Br J Clin Pharmacol. 2018;84:215–22. https://doi.org/10.1111/bcp.13438.
Kearns GL, Abdel-Rahman SM, Alander SW, Blowey DL, Leeder JS, Kauffman RE. Developmental pharmacology—drug disposition, action, and therapy in infants and children. N Engl J Med. 2003;349:1157–67. https://doi.org/10.1056/NEJMra035092.
Abduljalil K, Jamei M, Johnson TN. Fetal physiologically based pharmacokinetic models: systems information on fetal blood components and binding proteins. Clin Pharmacokinet. 2020;59:629–42. https://doi.org/10.1007/s40262-019-00836-3.
Jong GW, Vulto AG, de Hoog M, Schimmel KJ, Tibboel D, van den Anker JN. Unapproved and off-label use of drugs in a children’s hospital. N Engl J Med. 2000;343:1125. https://doi.org/10.1056/NEJM200010123431515.
Conroy S, Choonara I, Impicciatore P, Mohn A, Arnell H, Rane A, et al. Survey of unlicensed and off label drug use in paediatric wards in European countries. Br Med J. 2000;320:79–82. https://doi.org/10.1136/bmj.320.7227.79.
Zhao W, Jacqz-Aigrain E. Principles of therapeutic drug monitoring. In: Seyberth HW, Rane A, Schwab M, editors. Pediatric clinical pharmacology. Berlin: Springer; 2011. p. 77–90.
Morselli PL, Rovei V. Placental transfer of pethidine and norpethidine and their pharmacokinetics in the newborn. Eur J Clin Pharmacol. 1980;18:25–30. https://doi.org/10.1007/BF00561475.
Hawcutt D, Rose A, Fuerst-Recktenwald SNT, Turner M. Points to consider when planning the collection of blood or tissue samples in clinical trials of investigational medicinal products in children, infants and neonates. In: Van den Anker J, Rose K, editors. Guide to paediatric drug development and clinical research. Washington D.C: Karger Publishers; 2010. p. 97–110.
Gorodischer R, Koren G. Salivary excretion of drugs in children: theoretical and practical issues in therapeutic drug monitoring. Dev Pharmacol Ther. 1992;19:161–77. https://doi.org/10.1159/000457481.
Cook CE, Amerson E, Poole WK, Lesser P, O’Tuama L. Phenytoin and phenobarbital concentrations in saliva and plasma measured by radioimmunoassay. Clin Pharmacol Ther. 1975;18:742–7. https://doi.org/10.1002/cpt1975186742.
Tal A, Aviram M, Gorodischer R. Variations in theophylline concentrations detected by 24-hour saliva concentration profiles in ambulatory children with asthma. J Allergy Clin Immunol. 1990;86:238–43.
Tsiropoulos I, Kristensen O, Klitgaard NA. Saliva and serum concentration of lamotrigine in patients with epilepsy. Ther Drug Monit. 2000;22:517–21. https://doi.org/10.1097/00007691-200010000-00003.
Gordi T, Hai TN, Hoai NM, Thyberg M, Ashton M. Use of saliva and capillary blood samples as substitutes for venous blood sampling in pharmacokinetic investigations of artemisinin. Eur J Clin Pharmacol. 2000;56:561–6. https://doi.org/10.1007/s002280000179.
García-Robles A, Solaz-García Á, Verdú-Andrés J, Poveda-Andrés JL, Cháfer-Pericás C, Ponce-Rodriguez HD, et al. The usefulness of saliva in therapeutic drug monitoring of caffeine in preterm infants. Ther Drug Monit. 2021;23:250–4. https://doi.org/10.21203/rs.3.rs-236907/v1.
Idkaidek N, Hamadi S, Bani-Domi R, Al-Adham I, Alsmadi M, Awaysheh F, et al. Saliva versus Plasma Therapeutic Drug Monitoring of Gentamicin in Jordanian Preterm Infants. Development of a Physiologically-Based Pharmacokinetic (PBPK) Model and Validation of Class II Drugs of Salivary Excretion Classification System. Drug Res. 2020;70:455–62. https://doi.org/10.1055/a-1233-3582.
Hutchinson L, Sinclair M, Reid B, Burnett K, Callan B. A descriptive systematic review of salivary therapeutic drug monitoring in neonates and infants. Br J Clin Pharmacol. 2018;84:1089–108. https://doi.org/10.1111/bcp.13553.
Drobitch RK, Svensson CK. Therapeutic drug monitoring in saliva. Clin Pharmacokinet. 1992;23:365–79. https://doi.org/10.2165/00003088-199223050-00003.
Idkaidek N, Arafat T. Saliva versus plasma pharmacokinetics: theory and application of a salivary excretion classification system. Mol Pharm. 2012;9:2358–63. https://doi.org/10.1021/mp300250r.
Idkaidek NM. Interplay of biopharmaceutics, biopharmaceutics drug disposition and salivary excretion classification systems. Saudi Pharm J. 2014;22:79–81. https://doi.org/10.1016/j.jsps.2013.02.002.
Dobson NR, Liu X, Rhein LM, Darnall RA, Corwin MJ, McEntire BL, et al. Salivary caffeine concentrations are comparable to plasma concentrations in preterm infants receiving extended caffeine therapy. Br J Clin Pharmacol. 2016;82:754–61. https://doi.org/10.1111/bcp.13001.
Chang H-P, Anderson GC, Wood CE. Feasible and valid saliva collection for cortisol in transitional newborn infants. Nurs Res. 1995;44:117–9.
Gabrielsson JL, Johansson P, Bondesson U, Karlsson M, Paalzow LK. Analysis of pethidine disposition in the pregnant rat by means of a physiological flow model. J Pharmacokinet Biopharm. 1986;14:381–95. https://doi.org/10.1007/BF01059198.
Kaiko RF, Foley KM, Grabinski PY, Heidrich G, Rogers AG, Inturrisi CE, et al. Central nervous system excitatory effects of meperidine in cancer patients. Ann Neurol. 1983;13:180–5. https://doi.org/10.1002/ana.410130213.
Mather LE, Meffin PJ. Clinical pharmacokinetics pethidine. Clin Pharmacokinet. 1978;3:352–68. https://doi.org/10.2165/00003088-197803050-00002.
Pokela M-L, Olkkola KT, Koivisto M, Ryhänen P. Pharmacokinetics and pharmacodynamics of intravenous meperidine in neonates and infants. Clin Pharmacol Ther. 1992;52:342–9. https://doi.org/10.1038/clpt.1992.153.
Guay D, Meatherall R, Chalmers J, Grahame G. Cimetidine alters pethidine disposition in man. Br J Clin Pharmacol. 1984;18:907–14. https://doi.org/10.1111/j.1365-2125.1984.tb02563.x.
Lazebnik N, Kuhnert BR, Carr PC, Brashear WT, Syracuse CD, Mann LI. Intravenous, deltoid, or gluteus administration of meperidine during labor? Am J Obstet Gynecol. 1989;160:1184–9. https://doi.org/10.1016/0002-9378(89)90185-3.
Allegaert K, van den Anker J. Ontogeny of phase I metabolism of drugs. J Clin Pharmacol. 2019;59:S33–41. https://doi.org/10.1002/jcph.1483.
Bayer Technology Services GmbH. Open Systems Pharmacology Suite. 2019. https://docs.open-systems-pharmacology.org/working-with-pk-sim/pk-sim-documentation. Accessed 10 Jun 2021.
Dallmann A, Ince I, Coboeken K, Eissing T, Hempel G. A physiologically based pharmacokinetic model for pregnant women to predict the pharmacokinetics of drugs metabolized via several enzymatic pathways. Clin Pharmacokinet. 2018;57:749–68. https://doi.org/10.1007/s40262-017-0594-5.
Nation RL. Meperidine binding in maternal and fetal plasma. Clin Pharmacol Ther. 1981;29:472–9. https://doi.org/10.1038/clpt.1981.65.
Chan K, Tse J, Jennings F, Orme MLE. Pharmacokinetics of low-dose intravenous pethidine in patients with renal dysfunction. J Clin Pharmacol. 1987;27:516–22. https://doi.org/10.1002/j.1552-4604.1987.tb03059.x.
Thelen K, Coboeken K, Willmann S, Burghaus R, Dressman JB, Lippert J. Evolution of a detailed physiological model to simulate the gastrointestinal transit and absorption process in humans, part 1: oral solutions. J Pharm Sci. 2011;100:5324–45. https://doi.org/10.1002/jps.22726.
Cao J, Du Y, Wang YJ, Wu B, Jia J, Wei ZW, et al. Pharmacokinetics of meperidine (pethidine) in rabbit oral fluid: correlation with plasma concentrations after controlled administration. Pharmazie. 2018;73:324–8. https://doi.org/10.1691/ph.2018.8014.
Björkman S. Reduction and lumping of physiologically based pharmacokinetic models: prediction of the disposition of fentanyl and pethidine in humans by successively simplified models. J Pharmacokinet Pharmacodyn. 2003;30:285–307. https://doi.org/10.1023/A:1026194618660.
Davis NR, Mapleson WW. A physiological model for the distribution of injected agents, with special reference to pethidine. Br J Anaesth. 1993;70:248–58. https://doi.org/10.1093/bja/70.3.248.
Tomson G, Garle R, Thalme B, Nisell H, Nylund L, Rane A. Maternal kinetics and transplacental passage of pethidine during labour. Br J Clin Pharmacol. 1982;13:653–9. https://doi.org/10.1111/j.1365-2125.1982.tb01432.x.
Nestorov I. Whole-body physiologically based pharmacokinetic models. Expert Opin Drug Metab Toxicol. 2007;3:235–49. https://doi.org/10.1517/17425255.3.2.235.
Abduljalil K, Pan X, Pansari A, Jamei M, Johnson TN. Preterm physiologically based pharmacokinetic model. Part II: applications of the model to predict drug pharmacokinetics in the preterm population. Clin Pharmacokinet. 2019. https://doi.org/10.1007/s40262-019-00827-4.
Abduljalil K, Pan X, Pansari A, Jamei M, Johnson TN. A preterm physiologically based pharmacokinetic model. Part I: physiological parameters and model building. Clin Pharmacokinet. 2020;59:485–500. https://doi.org/10.1007/s40262-019-00825-6.
Claassen K, Thelen K, Coboeken K, Gaub T, Lippert J, Allegaert K, et al. Development of a physiologically-based pharmacokinetic model for preterm neonates: evaluation with in vivo data. Curr Pharm Des. 2015;21:5688–98. https://doi.org/10.2174/1381612821666150901110533.
Dallmann A, Solodenko J, Ince I, Eissing T. Applied concepts in PBPK modeling: how to extend an open systems pharmacology model to the special population of pregnant women. CPT Pharmacometrics Syst Pharmacol. 2018;7:419–31. https://doi.org/10.1002/psp4.12300.
Dallmann A, Ince I, Meyer M, Willmann S, Eissing T, Hempel G. Gestation-specific changes in the anatomy and physiology of healthy pregnant women: an extended repository of model parameters for physiologically based pharmacokinetic modeling in pregnancy. Clin Pharmacokinet. 2017;56:1303–30. https://doi.org/10.1007/s40262-017-0539-z.
Liu XI, Momper JD, Rakhmanina NY, Green DJ, Burckart GJ, Cressey TR, et al. Physiologically based pharmacokinetic modeling framework to predict neonatal pharmacokinetics of transplacentally acquired emtricitabine, dolutegravir, and raltegravir. Clin Pharmacokinet. 2021;60:795–809. https://doi.org/10.1007/s40262-020-00977-w.
Willmann S, Höhn K, Edginton A, Sevestre M, Solodenko J, Weiss W, et al. Development of a physiology-based whole-body population model for assessing the influence of individual variability on the pharmacokinetics of drugs. J Pharmacokinet Pharmacodyn. 2007;34:401–31. https://doi.org/10.1007/s10928-007-9053-5.
Szeto HH, Inturrisi CE, Houde R, Saal S, Cheigh J, Reidenberg MM. Accumulation of normeperidine, an active metabolite of meperidine, in patients with renal failure or cancer. Ann Intern Med. 1977;86:738–41. https://doi.org/10.7326/0003-4819-86-6-738.
Stone P, Macintyre P, Jarvis D. Norpethidine toxicity and patient controlled analgesia. BJA Br J Anaesthesia. 1993;71:738–40. https://doi.org/10.1093/bja/71.5.738.
Hagmeyer KO, Mauro LS, Mauro VF. Meperidine-related seizures associated with patient-controlled analgesia pumps. Ann Pharmacother. 1993;27:29–32. https://doi.org/10.1177/106002809302700106.
Hamad NI, Awad R, Salem AF, Arafat T. Pethidine level in Jordanian women and their newborns during labor after a single intravenous dose. Int J Clin Anesthesiol. 2014;2:1032.
Husemeyer R, Cummings A, Rosankiewicz J, Davenport H. A study of pethidine kinetics and analgesia in women in labour following intravenous, intramuscular and epidural administration. Br J Clin Pharmacol. 1982;13:171–6. https://doi.org/10.1111/j.1365-2125.1982.tb01352.x.
Holmberg L, Odar-Cederlöf I, Boréus LO, Heyner L, Ehrnebo M. Comparative disposition of pethidine and norpethidine in old and young patients. Eur J Clin Pharmacol. 1982;22:175–9. https://doi.org/10.1007/BF00542464.
Payne K. Epidural and intramuscular pethidine-a pharmacokinetic study. S Afr Med J. 1983;63:193–6.
Boréus LO, Sköldefors E, Ehrnebo M. Appearance of pethidine and norpethidine in cerebrospinal fluid of man following intramuscular injection of pethidine. Acta Anaesthesiol Scand. 1983;27:222–5. https://doi.org/10.1111/j.1399-6576.1983.tb01939.x.
Ramírez J, Innocenti F, Schuetz EG, Flockhart DA, Relling MV, Santucci R, et al. CYP2B6, CYP3A4, and CYP2C19 are responsible for the in vitro N-demethylation of meperidine in human liver microsomes. Drug Metab Dispos. 2004;32:930–6.
Diestelhorst C, Boos J, McCune JS, Russell J, Kangarloo SB, Hempel G. Physiologically based pharmacokinetic modelling of Busulfan: a new approach to describe and predict the pharmacokinetics in adults. Cancer Chemother Pharmacol. 2013;72:991–1000. https://doi.org/10.1007/s00280-013-2275-x.
Hughes JH, Upton RN, Reuter SE, Rozewski DM, Phelps MA, Foster DJ. Development of a physiologically based pharmacokinetic model for intravenous lenalidomide in mice. Cancer Chemother Pharmacol. 2019;84:1073–87. https://doi.org/10.1007/s00280-019-03941-z.
Alsmadi MM, Al Eitan LN, Idkaidek NM, Alzoubi KH. The development of a PBPK model for atomoxetine using levels in plasma, saliva and brain extracellular fluid in patients with normal and deteriorated kidney function. CNSNDDT. 2022;21:704–16. https://doi.org/10.2174/1871527320666210621102437.
Alsmadi MM, Al-Daoud NM, Jaradat MM, Alzughoul SB, Abu Kwiak AD, Abu Laila SS, et al. Physiologically-based pharmacokinetic model for alectinib, ruxolitinib, and panobinostat in the presence of cancer, renal impairment, and hepatic impairment. Biopharm Drug Disposition. 2021;42:263–84. https://doi.org/10.1002/bdd.2282.
Wong YC, Centanni M, de Lange EC. Physiologically based modeling approach to predict dopamine D2 receptor occupancy of antipsychotics in brain: translation from rat to human. J Clin Pharmacol. 2019;59:731–47. https://doi.org/10.1002/jcph.1365.
Hassan HE, Mercer SL, Cunningham CW, Coop A, Eddington ND. Evaluation of the P-glycoprotein (Abcb1) affinity status of a series of morphine analogs: comparative study with meperidine analogs to identify opioids with minimal P-glycoprotein interactions. Int J Pharm. 2009;375:48–54. https://doi.org/10.1016/j.ijpharm.2009.03.037.
Kramer WG, Gross DR, Medlock C. Contribution of the lung to total body clearance of meperidine in the dog. J Pharm Sci. 1985;74:569–71. https://doi.org/10.1002/jps.2600740517.
Rodgers T, Rowland M. Physiologically based pharmacokinetic modelling 2: predicting the tissue distribution of acids, very weak bases, neutrals and zwitterions. J Pharm Sci. 2006;95:1238–57. https://doi.org/10.1002/jps.20502.
Alsmadi MM. Physiologically based pharmacokinetic (PBPK) model of ivermectin (IVM). 2014. https://www.proquest.com/openview/6b2ac6600b76fb8b784476e77e0a5a61/1.pdf?pq-origsite=gscholar&cbl=18750. Accessed 26 Feb 2023.
Sweeney RE, Langenberg JP, Maxwell DM. A physiologically based pharmacokinetic (PB/PK) model for multiple exposure routes of soman in multiple species. Arch Toxicol. 2006;80:719–31. https://doi.org/10.1007/s00204-006-0114-0.
Willmann S, Thelen K, Becker C, Dressman JB, Lippert J. Mechanism-based prediction of particle size-dependent dissolution and absorption: cilostazol pharmacokinetics in dogs. Eur J Pharm Biopharm. 2010;76:83–94. https://doi.org/10.1016/j.ejpb.2010.06.003.
Hebert MF, Easterling T, Kirby B, Carr D, Buchanan M, Rutherford T, et al. Effects of pregnancy on CYP3A and P-glycoprotein activities as measured by disposition of midazolam and digoxin: a University of Washington specialized center of research study. Clin Pharmacol Ther. 2008;84:248–53. https://doi.org/10.1038/clpt.2008.1.
Ganguly S, Edginton AN, Gerhart JG, Cohen-Wolkowiez M, Greenberg RG, Gonzalez D, et al. Physiologically based pharmacokinetic modeling of meropenem in preterm and term infants. Clin Pharmacokinet. 2021;60:1591–604. https://doi.org/10.1007/s40262-021-01046-6.
Maharaj AR, Gonzalez D, Cohen-Wolkowiez M, Hornik CP, Edginton AN. Improving pediatric protein binding estimates: an evaluation of α1-acid glycoprotein maturation in healthy and infected subjects. Clin Pharmacokinet. 2018;57:577–89. https://doi.org/10.1007/s40262-017-0576-7.
Patterson KB, Dumond JB, Prince HA, Jenkins AJ, Scarsi KK, Wang R, et al. Protein binding of lopinavir and ritonavir during four phases of pregnancy: implications for treatment guidelines. J Acquir Immune Defic Syndr. 2013;63:51. https://doi.org/10.1097/QAI.0b013e31827fd47e.
La Rosa C, Mather L, Morgan D. Pethidine binding in plasma: effects of methodological variables. Br J Clin Pharmacol. 1984;17:411–5. https://doi.org/10.1111/j.1365-2125.1984.tb02365.x.
Morgan D, Moore G, Thomas J, Triggs E. Disposition of meperidine in pregnancy. Clin Pharmacol Ther. 1978;23:288–95. https://doi.org/10.1002/cpt1978233288.
Rowland M, Tozer TN. Clinical pharmacokinetics and pharmacodynamics: concepts and applications. 5th ed. Philadelphia: Lippincott Williams and Wilkins; 2005.
Couto N, Al-Majdoub ZM, Achour B, Wright PC, Rostami-Hodjegan A, Barber J. Quantification of proteins involved in drug metabolism and disposition in the human liver using label-free global proteomics. Mol Pharm. 2019;16:632–47. https://doi.org/10.1021/acs.molpharmaceut.8b00941.
Soetaert K, Petzoldt T. Inverse modelling, sensitivity and monte Carlo analysis in R using package FME. J Stat Softw. 2010;33:1–28. https://doi.org/10.18637/jss.v033.i03.
Hornik CP, Wu H, Edginton AN, Watt K, Cohen-Wolkowiez M, Gonzalez D. Development of a pediatric physiologically-based pharmacokinetic model of clindamycin using opportunistic pharmacokinetic data. Clin Pharmacokinet. 2017;56:1343–53. https://doi.org/10.1007/s40262-017-0525-5.
Do Jones R, Jones HM, Rowland M, Gibson CR, Yates JW, Chien JY, et al. PhRMA CPCDC initiative on predictive models of human pharmacokinetics, part 2: comparative assessment of prediction methods of human volume of distribution. J Pharm Sci. 2011;100:4074–89. https://doi.org/10.1002/9780470921920.edm049.
Sampson MR, Frymoyer A, Rattray B, Cotten CM, Smith B, Capparelli E, et al. Predictive performance of a gentamicin population pharmacokinetic model in neonates receiving full-body hypothermia. Ther Drug Monit. 2014;36:584–9. https://doi.org/10.1097/FTD.0000000000000056.
Heiskanen T, Langel K, Gunnar T, Lillsunde P, Kalso EA. Opioid concentrations in oral fluid and plasma in cancer patients with pain. J Pain Symptom Manage. 2015;50:524–32. https://doi.org/10.1016/j.jpainsymman.2014.09.004.
Bista SR, Haywood A, Norris R, Good P, Tapuni A, Lobb M, et al. Saliva versus plasma for pharmacokinetic and pharmacodynamic studies of fentanyl in patients with cancer. Clin Ther. 2015;37:2468–75. https://doi.org/10.1016/j.clinthera.2015.09.002.
Gesseck AM, Poklis JL, Wolf CE, Xu J, Bashir A, Hendricks-Muñoz KD, et al. A case study evaluating the efficacy of an ad hoc hospital collection device for fentanyl in infant oral fluid. J Anal Toxicol. 2020;44:741–6. https://doi.org/10.1093/jat/bkaa069.
Alsmadi MM, Alfarah MQ, Albderat J, Alsalaita G, AlMardini R, Hamadi S, et al. The development of a population physiologically based pharmacokinetic model for mycophenolic mofetil and mycophenolic acid in humans using data from plasma, saliva, and kidney tissue. Biopharm Drug Disposition. 2019;40:325–40. https://doi.org/10.1002/bdd.2206.
Wiener PC, Hogg MI, Rosen M. Neonatal respiration, feeding and neurobehavioural state. Effects of intrapartum bupivacaine, pethidine and pethidine reversed by naloxone. Anaesthesia. 1979;34:996–1004. https://doi.org/10.1111/j.1365-2044.1979.tb06247.x.
Nissen E, Lilja G, Matthiesen A-S, Ransjo-Arvidsson A-B, Uvnas-Moberg K, Widstrom A-M. Effects of maternal pethidine on infants’ developing breast feeding behaviour. Acta Paediatr. 1995;84:140–5. https://doi.org/10.1111/j.1651-2227.1995.tb13596.x.
O’Connor A, Schug SA, Cardwell H. A comparison of the efficacy and safety of morphine and pethidine as analgesia for suspected renal colic in the emergency setting. Emerg Med J. 2000;17:261–4. https://doi.org/10.1136/emj.17.4.261.
Dallmann A, Himstedt A, Solodenko J, Ince I, Hempel G, Eissing T. Integration of physiological changes during the postpartum period into a PBPK framework and prediction of amoxicillin disposition before and shortly after delivery. J Pharmacokinet Pharmacodyn. 2020;47:341–59. https://doi.org/10.1007/s10928-020-09706-z.
Pavek P, Ceckova M, Staud F. Variation of drug kinetics in pregnancy. Curr Drug Metab. 2009;10:520–9. https://doi.org/10.2174/138920009788897993.
Edwards D, Svensson CK, Visco JP, Lalka D. Clinical Pharmacokinetics of Pethidine: 1982. Clin Pharmacokinet. 1982;7:421–33. https://doi.org/10.2165/00003088-198207050-00003.
Austin K, Stapleton J, Mather L. Pethidine clearance during continuous intravenous infusions in postoperative patients. Br J Clin Pharmacol. 1981;11:25–30. https://doi.org/10.1111/j.1365-2125.1981.tb01097.x.
Kiem S, Schentag JJ. Interpretation of antibiotic concentration ratios measured in epithelial lining fluid. Antimicrob Agents Chemother. 2008;52:24–36. https://doi.org/10.1128/AAC.00133-06.
Aljayyoussi G, Rajoli R, Pertinez H, Pennington S, Hong WD, O’Neill P, et al. Modelling of systemic versus pulmonary chloroquine exposure in man for COVID-19 dose selection. medRxiv. 2020. https://doi.org/10.1101/2020.04.24.20078741.
Schüller M, Tran KTT, Øiestad EL, Pedersen-Bjergaard S. Membrane-based liquid-phase microextraction of basic pharmaceuticals—a study on the optimal extraction window. J Chromatogr A. 2022;1664: 462769. https://doi.org/10.1016/j.chroma.2021.462769.
DrugBank. Meperidine- Compound summary. 1992. https://go.drugbank.com/drugs/DB00454. Accessed 26 May 2022.
Barter ZE, Chowdry JE, Harlow JR, Snawder JE, Lipscomb JC, Rostami-Hodjegan A. Covariation of human microsomal protein per gram of liver with age: absence of influence of operator and sample storage may justify interlaboratory data pooling. Drug Metab Dispos. 2008;36:2405–9. https://doi.org/10.1124/dmd.108.021311.
Acknowledgements
The authors acknowledge Jordan University of Science and Technology (Irbid, Jordan) and the University of Petra (Amman, Jordan) for all the facilities and support provided.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study received no funding.
Conflicts of interest
Mo’tasem M. Alsmadi and Nasir Idkaidek declare that they have no conflict of interest
Availability of data and material
All of the used data in this work were included in the article.
Code availability
The PK-Sim files used in this work can be provided upon request via email.
Author contributions
MMA had substantial contributions to the conception and design of the work; the acquisition, analysis, and interpretation of data, and the drafting of the work. MMA and NI were involved in revising the work critically for important intellectual content. All authors provided final approval of the version to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alsmadi, M.M., Idkaidek, N. The Analysis of Pethidine Pharmacokinetics in Newborn Saliva, Plasma, and Brain Extracellular Fluid After Prenatal Intrauterine Exposure from Pregnant Mothers Receiving Intramuscular Dose Using PBPK Modeling. Eur J Drug Metab Pharmacokinet 48, 281–300 (2023). https://doi.org/10.1007/s13318-023-00823-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-023-00823-x