Abstract
Background and Objective
Fesoterodine is a muscarinic receptor antagonist approved for the treatment of overactive bladder (OAB) in adults and neurogenic detrusor overactivity (NDO) in pediatric patients. This work aimed to characterize the population pharmacokinetics of 5-hydroxymethyl tolterodine (5-HMT, the active metabolite of fesoterodine) and its pharmacokinetic/pharmacodynamic relationship in pediatric patients with OAB or NDO following administration of fesoterodine.
Methods
5-HMT plasma concentrations from 142 participants of age ≥ 6 years were analyzed, and a nonlinear mixed-effects model was developed. Weight-based simulations of 5-HMT exposure and maximum cystometric capacity (MCC) were conducted using the final models.
Results
A one-compartment model with first-order absorption and a lag time, which included the effects of body weight, sex, cytochrome (CYP) 2D6 metabolizer status and fesoterodine formulation on pharmacokinetic parameters, best described the 5-HMT pharmacokinetics. An Emax model described the exposure–response relationship adequately. The median maximum concentration at steady state for pediatric patients weighing 25–35 kg and receiving 8 mg once daily (QD) was estimated to be 2.45 times greater than that in adults receiving 8 mg QD. Furthermore, simulation results showed dosing with fesoterodine 4 mg QD to pediatric patients weighing 25–35 kg and 8 mg QD to pediatric patients weighing >35 kg would achieve adequate exposure to demonstrate a clinically meaningful change from baseline (CFB) MCC.
Conclusions
Population models were developed for 5-HMT and MCC in pediatric patients. Weight-based simulations indicated that 4 mg QD for pediatric patients weighing 25–35 kg and 8 mg QD for those weighing > 35 kg provided similar exposures to those in adults following 8 mg QD and a clinically meaningful CFB MCC.
Clinical Trial Numbers
NCT00857896, NCT01557244
Plain Language Summary
Fesoterodine is a muscarinic receptor antagonist approved for the treatment of overactive bladder (OAB) in adults and neurogenic detrusor overactivity (NDO) in pediatric patients in the US. Population pharmacokinetic and pharmacokinetic/pharmacodynamic models were developed for 5-HMT based on data from two pediatric clinical trials that included 142 patients of age ≥ 6 years with OAB or NDO. Weight-based simulations of 5-HMT exposure and maximum cystometric capacity were conducted using the final models to examine the impact of covariates on 5-HMT exposure and the exposure–response profile. The results of these simulations indicate that 4 mg QD for pediatric patients weighing 25–35 kg and 8 mg QD for those weighing > 35 kg provide similar exposures to those in adults following 8 mg QD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
A one-compartment model with first-order absorption and elimination and a lag time, which included the effects of body weight, sex, CYP2D6 metabolizer and fesoterodine formulation on pharmacokinetic parameters, best described the 5-HMT pharmacokinetics. |
An Emax model which included the effect of age on maximum cystometric capacity (MCC) described the exposure–response relationship adequately. |
Weight-based simulations indicated that 4 mg QD for pediatric patients weighing 25–35 kg and 8 mg QD for those weighing > 35 kg provided similar exposures to those in adults following 8 mg QD and a clinically meaningful change from baseline (CFB) MCC. This proposed dosing regimen was different from that investigated in the pediatric phase 3 study and was optimized appropriately for the target pediatric population through the model-informed approach. |
1 Introduction
Neurogenic detrusor overactivity (NDO) is the involuntary contraction of the detrusor muscle as the bladder fills [1,2,3,4]. It may be necessary to perform clean intermittent catheterization for children with NDO to achieve continence and prevent renal deterioration from a high-pressure bladder [5]. Antimuscarinic medications can also be used in these patients to suppress detrusor contractions, increase bladder capacity and lower bladder pressure [6, 7].
Fesoterodine is a muscarinic receptor antagonist which is rapidly metabolized by nonspecific esterases to its active metabolite, 5-hydroxymethyl tolterodine (5-HMT), which is responsible for its antimuscarinic activity. 5-HMT is then further metabolized by CYP2D6 and CYP3A or excreted to urine as unchanged 5-HMT. Exposure to 5-HMT was increased (approximately doubled) in poor metabolizers (PM) of CYP2D6 [8].
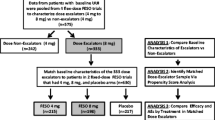
Fesoterodine tablet formulation (Toviaz®) is approved for the treatment of adults with overactive bladder (OAB) [9]. The fesoterodine pediatric NDO clinical development program included one phase II study (study 1066; NCT00857896) and one phase III study (study 1047; NCT01557244). Using clinical trial simulations based on pharmacokinetic data from study 1066, fesoterodine 4 and 8 mg once-daily (QD) doses administered as tablets were selected for study 1047 cohort 1 patients (> 25 kg), and fesoterodine 2 and 4 mg QD doses administered as beads in capsule (BIC) were selected for cohort 2 patients (≤ 25 kg) to target 5-HMT exposures similar to those in adults following administration of fesoterodine 4 and 8 mg tablets QD, respectively. Based on the results from pediatric clinical trials, fesoterodine is approved by the US FDA for the treatment of pediatric patients with NDO who are 6 years of age and older and weigh greater than 25 kg [9].
The present work established the population pharmacokinetic and pharmacokinetic/pharmacodynamic models for fesoterodine in pediatric patients and utilized them to inform the pediatric dose regimen through exposure matching and an exposure–response evaluation. To our knowledge, this is the first published report of population pharmacokinetic and pharmacokinetic/pharmacodynamic models describing (1) the pharmacokinetics of 5-HMT for pediatric patients and (2) the pharmacokinetic/pharmacodynamic relationship of antimuscarinics in OAB/NDO patients. The objectives of this analysis were (1) to develop population pharmacokinetic and pharmacokinetic/pharmacodynamic models for fesoterodine among pediatric patients with NDO and (2) to determine the appropriate dosing for pediatric NDO patients.
2 Methods
2.1 Study Population, Dosing and Sampling Schedule
2.1.1 Study 1066
This was an 8-week, open-label, dose-escalating, uncontrolled phase II study (ClinicalTrials.gov: NCT00857896) to evaluate the pharmacokinetics, safety and tolerability of fesoterodine in pediatric OAB patients aged 8–17 years, with approximately 50% of the population to include patients with NDO [10]. All patients were treated with fesoterodine 4 mg tablet QD during weeks 1 through 4, and all except for one patient were treated with fesoterodine 8 mg tablet QD during weeks 5 through 8. At visit 3 (week 4), pre-dose pharmacokinetic samples and 0.5–2, 2–4 and 4–6 h post-dose pharmacokinetic samples were collected. At visit 4 (week 8), 8–10, 10–14, 14–16 and 16–20 h post-dose pharmacokinetic samples were collected.
2.1.2 Study 1047
This was a 24-week, open-label, randomized phase III study (ClinicalTrials.gov: NCT01557244) to evaluate the efficacy, safety and pharmacokinetics of fesoterodine in patients aged 6–17 years with symptoms of NDO [11]. The study included two cohorts with different doses and formulations based on a body weight cutoff point (cohort 1 included patients > 25 kg randomly assigned to be dosed with fesoterodine 4 and 8 mg tablets or oxybutynin extended release (XL) tablets; cohort 2 included patients ≤ 25 kg randomly assigned to be dosed with fesoterodine 2 and 4 mg BIC) that were analyzed separately for efficacy and safety endpoints. The study design is shown in Online Resource 1 in the Supplementary Information. The primary endpoint was change from baseline (CFB) to week 12 in maximum cystometric capacity (MCC) [12, 13]. At visit 3 (week 4), up to three pharmacokinetic samples were collected from each patient assigned to fesoterodine treatment.
2.2 Analytical Methods
2.2.1 Bioanalytical Methods
Plasma concentrations of 5-HMT were determined using validated and sensitive liquid chromatography–tandem mass spectrometric methods by Q2 Solutions (NY, USA) that were conducted in compliance with Pfizer standard operating procedures, as summarized in Online Resource 2 [10].
2.2.2 Data Analysis Methods
NONMEM version VII, level 4.3 (ICON Development Solutions, Hanover, MD, USA), was used for model development [14]. Fixed- and random-effect parameters were estimated using the first-order conditional estimation with interaction (FOCE-I) method, and individual values for model parameters were obtained from empirical Bayes estimates (EBE). Perl-speaks-NONMEM (version 4.9.0) was used for bootstrap analyses. Statistical and graphical outputs were generated using the R programming and statistical language (R version 3.2.2) [15]. See Online Resource 3 for data exclusion or manipulation.
2.2.3 Urodynamic Assessment
MCC at baseline and visit 5 (week 12) in study 1047 was evaluated until voiding or leaking began, or at a pressure of \(\ge\) 40 cm H2O. Typically, a multichannel technique through a dual-lumen urodynamic catheter was used. The bladder would be filled at a constant fill rate with test medium warmed to body temperature until the MCC reached the expected bladder capacity (EBC) as defined below [4] or until, in the judgment of the investigator, filling should be stopped. EBC as a function of age was estimated as follows: EBC (age) = [30 + (child’s age in years) · 30] mL up to the age of 12 years. From the age of 12 years onwards, EBC is relatively constant at 390 mL.
2.3 Population Pharmacokinetic Model Development
This population pharmacokinetic model was developed using 5-HMT pharmacokinetic data from one phase II (study 1066) and one phase III study (study 1047) (Online Resource 4). Structural model selection was guided by objective function value (OFV; − 2 × loglikelihood) [16], standard goodness-of-fit diagnostic plots, precision of parameter estimates, plausibility of parameter estimates and \(\upeta\) shrinkage. One- and two-compartment disposition models with first-order oral absorption and elimination were evaluated as candidate structural models. Covariates known to be highly influential a priori were built into the structural model (e.g., allometric scaling of pharmacokinetic parameters using body weight [exponents of 0.75 and 1 for clearance (CL/F) and volume of distribution (Vd/F), respectively, referenced to 35 kg]). Since a relative bioavailability (BA) study of fesoterodine for a 4-mg dose of tablet and BIC (NCT02160158) suggested a possible effect of the formulation on the pharmacokinetics, the effect of drug formulation on some parameters related to absorption, such as F and Ka, was also tested in the structure model.
Inter-individual variance was added to structural model parameters to account for differences between individuals in the population and was assumed to be log-normally distributed. Models with and without covariance between random effects were investigated. Residual unexplained variability (RUV) in log-transformed 5-HMT concentration was modeled as an additive error.
A covariate modeling approach emphasizing parameter estimation rather than stepwise hypothesis testing was implemented. Predefined covariate-parameter relationships (i.e., the effects of sex and CYP2D6 metabolizer status as predictors of CL/F and Vd/F) were identified based on exploratory graphics. These covariates were selected from those which were found in the prior adult population analysis [17]. However, age was not included in this full model because age was considered to be a potential confounding factor in the relationship between body weight and pharmacokinetic parameters. Continuous covariates (xi) were included using a power function centered on a reference value as (xi /reference value)θx. Categorical covariates were introduced into the model (Eqs. 1 and 2):
where \({\theta }_{x, cov={X}_{1}}\) is the effect of the covariate belonging to category X1, X0 represents the most common category and X1 represents the minor category.
2.4 Population Pharmacokinetic/Pharmacodynamic Model Development
This population pharmacokinetic/pharmacodynamic model was developed using 5-HMT pharmacokinetic and MCC data from one phase III study (study 1047) (Online Resource 5). MCC values at baseline and week 12 were included in the population pharmacokinetic/pharmacodynamic modeling. Estimates of individual average concentration at steady state (Cavg,ss) as predictors for the response (MCC) were derived from the population pharmacokinetic model using clearance based on individual EBEs.
Structural model selection was guided by OFV, standard goodness-of-fit diagnostic plots, precision of parameter estimates, plausibility of parameter estimates and \(\upeta\) shrinkage. The following Emax model was identified as a structural model (Eq. 3):
where BASE and MCC represent the MCC at baseline and week 12, respectively, EC50 represents the Cavg,ss needed to reach half the maximum response, and Cavg,ss was calculated for each individual (\(\tau\) means the dose interval) (Eq. 4):
In this modeling approach, Emax was assumed to be the expected bladder capacity (EBC) [2] because Emax can be interpreted as the bladder capacity when individuals completely improve their symptom. Therefore, Emax was assumed to be described as follows (Eqs. 5 and 6):
where AGE represents age in years for an individual. Inter-individual variance was added to structural model parameters to account for differences between individuals in the population and was assumed to be log-normally distributed, as with the population pharmacokinetic model. Covariance between \({\upeta }_{i}\) for different parameters (i.e., BASE and Emax) was also estimated. A residual error model with a combination of additive and proportional effects was used to describe the RUV in MCC.
In this analysis, the relationship between age and MCC was of interest such that maximum MCC (Emax) was interpreted as bladder capacity using a linear function for age. Similarly, a linear relationship between age and baseline MCC was incorporated (Eqs. 7 and 8):
where \({\theta }_{\mathrm{base}}\) represents the typical baseline MCC for an individual above 12 years old in the population.
2.5 Model Evaluation
Plots of observations versus population predictions (PRED) and observations versus individual predictions (IPRED) were evaluated for randomness around the line of unity (the latter only if the \(\varepsilon\) shrinkage was sufficiently low, i.e., < 20%). Nonparametric bootstrap analysis (stratified by study source) of the final model was performed to calculate the median and 95% confidence intervals of parameter estimates from 1000 bootstrapped datasets. The predictive performance of the final model was evaluated by prediction-corrected visual predictive checks (pcVPC) [18] stratified by treatment group based on 1000 Monte Carlo simulations of the study dataset.
2.6 Model Application
The impact of body weight on secondary pharmacokinetic parameters, such as area under the plasma concentration–time curve over the dosing interval at steady state (AUCtau,ss) and maximum concentration at steady state (Cmax,ss), was evaluated using the final population pharmacokinetic model to support the rationale of weight-based dosage and administration. Quantitative descriptions of differences in exposure owing to weight (10–15 kg, 15–20 kg, 20–25 kg, 25–35 kg, 35–45 kg, 45–55 kg, and over 55 kg) were evaluated based on the EBEs for all participants in the analysis population.
The predicted Cavg,ss based on the population pharmacokinetic model was also used for the population pharmacokinetic/pharmacodynamic model, and then the impact of body weight on CFB MCC at week 12 was also evaluated. Stochastic simulations were implemented to assess the performance of specific dosing regimens given the final population pharmacokinetic/pharmacodynamic model. Virtual patients were extracted from pediatric patients enrolled in study 1047 without parameter uncertainty. Inter-individual variance and residual unexplained variability were simulated parametrically through the resampling of individual random effects from the final population models. The sequence of simulation tasks included the simulation of individual Cavg,ss followed by the simulation of individual MCC at baseline and week 12. The R code used for the simulations is presented in Online Resource 6.
3 Results
3.1 Population Pharmacokinetic Model
The pharmacokinetic dataset consisted of 142 pediatric patients who contributed 428 evaluable 5-HMT concentration measurements (Online Resource 7). Characteristics of the individuals in the 5-HMT pharmacokinetic analysis population are presented in Table 1. As a result of the population pharmacokinetic analysis, the final structural model was a one-compartment model with first-order absorption and elimination coupled with a first-order absorption lag for fesoterodine formulations, inter-individual variance in CL/F, Vd/F and ka along with covariance between the variance parameters, and an additive log-transformed scale error model. Two-compartment models did not offer clear superiority in terms of an improvement in the precision of parameter estimates. The model included the effect of fesoterodine formulation on the extent of absorption (i.e., F) (BIC vs. tablet), which showed improvements in OFV, the diagnostic plots and the precision of the pharmacokinetic parameter estimates. The inclusion of the effect of fesoterodine formulation on the absorption rate constant (BIC vs. tablet), instead of that on the extent of absorption, led to an increase in OFV without precise estimation [i.e., high relative standard errors (RSEs) of parameter estimates], so this covariate effect was not incorporated into the model. The final forms of the equation for the population pharmacokinetic model are described in Online Resource 8.
The parameter estimates from the final population pharmacokinetic model are presented in Table 2, with the median and 95% confidence interval (CI) of each parameter estimated from bootstrap analysis. The median values of the estimates from the bootstrapping were very similar to the population estimates in the final model. Time to reach Cmax at steady state (Tmax,ss) and the half-life of 5-HMT for the typical patient were 2.55 h and 7.73 h, respectively. CYP2D6 PM resulted in a 0.53-fold decrease in CL/F compared with the reference scenario of CYP2D6 extensive metabolizer (EM), and BIC dosing resulted in a 0.65-fold decrease in F compared with the reference scenario of tablet dosing, with neither of the 95% CIs including the null covariate effect value of 1.0. Female pediatric patients exhibited 86% and 64% of the CL/F and Vd/F, respectively, compared with the reference scenario of male pediatric patients, with both of the 95% CIs including the null covariate effect value of 1.0. Final model diagnostic plots of observed versus predicted concentrations stratified by dose are shown in Online Resource 9 and indicate that the population pharmacokinetic model fitted the observed data well. The final model pcVPC demonstrated good agreement between observed and predicted data (Fig. 1). Cmax,ss, AUCtau,ss and Cavg,ss based on individual empirical Bayesian estimates (EBE) for pediatric patients in study 1047 are summarized in Online Resource 10. As shown in the table (Online Resource 10), median 5-HMT exposures (i.e., Cmax,ss, AUCtau,ss and Cavg,ss) following administration of fesoterodine 4 and 8 mg tablets QD and those following fesoterodine 2 and 4 mg BIC QD increased proportionally, which is consistent with the increment in dose. The data showed that 5-HMT exposures in cohort 2 (patients ≤ 25 kg) following fesoterodine 2 and 4 mg BIC QD doses were considerably lower than those in cohort 1 (patients > 25 kg) following fesoterodine 4 and 8 mg QD doses, respectively.
Prediction-corrected visual predictive check. The prediction-corrected observed 5-HMT concentration data (open circles and solid lines; red for median, blue for the 5th and 95th percentiles) are plotted against time after the dose (a) and the observed MCC (open circles and solid lines; red for median, blue for the 5th and 95th percentiles) are plotted against individual predicted Cavg,ss values (b). The prediction-corrected simulated 5-HMT concentrations based on the index population (N = 1000 simulations) are represented by the red dashed lines and red areas (median and 95% prediction interval of the median, respectively) and the blue dashed lines and blue areas (median and 95% prediction intervals of the 5th and 95th percentiles, respectively). BIC fesoterodine beads in capsule, Cavg,ss average concentration at steady state, MCC maximum cystometric capacity, Tab fesoterodine tablet
3.2 Population Pharmacokinetic/Pharmacodynamic Model
The dataset for the population pharmacokinetic/pharmacodynamic analysis consisted of 121 pediatric patients who contributed 242 evaluable MCC observations. Characteristics of the individuals in the 5-HMT pharmacokinetic analysis population are presented in Online Resource 11. Tests of the relationship between individual predicted Cavg,ss and observed MCC, stratified by each treatment group, revealed that CFB MCC tends to increase with Cavg,ss for 5-HMT increases (Fig. 2). As a result of the population pharmacokinetic/pharmacodynamic analysis, an Emax model was fitted to describe the exposure–response trend adequately. The model included the effect of age on baseline MCC using a linear function for age, which is similar to the effect of age on Emax. The final forms of the equation for the population pharmacokinetic/pharmacodynamic model are described in Online Resource 8.
Relationship between observed MCC and predicted Cavg,ss for pediatric patients on fesoterodine 4 and 8 mg tablet QD or on fesoterodine 2 and 4 mg BIC QD in study 1047. In the upper panel, each open circle indicates the relationship between observed MCC (baseline and week 12) and predicted Cavg,ss for an individual. The red line represents a smooth logical regression calculated using the LOESS function in R software. In the lower panel, the closed circles indicate individual predicted Cavg,ss values, which were jittered to avoid overlaps within each treatment group. The bold black lines in the boxes represent medians for individual predicted Cavg,ss values in each treatment group, while the box edges on the left and right are the 25th and 75th percentiles, respectively. The whiskers extend up to 1.5 IQR. BIC fesoterodine beads in capsule, Cavg,ss average concentration at steady state, IQR interquartile range, MCC maximum cystometric capacity, QD once daily, Tab fesoterodine tablet
The parameter estimates with the bootstrap results for the final population pharmacokinetic/pharmacodynamic model are presented in Table 3. Predicted exposure–response profiles show consistency with the observed measurements across the strata (Fig. 1), and basic diagnostic plots (Online Resource 12) suggest that the final population pharmacokinetic/pharmacodynamic model is useful to predict median MCC trends across the whole Cavg,ss range in the entire body weight range of pediatric NDO patients.
3.3 Model Application
The predicted steady-state AUCtau,ss and Cmax,ss for pediatric patients weighing 10–15 kg, 15–20 kg, 20–25 kg, 25–35 kg, 35–45 kg, 45–55 kg, and over 55 kg after fesoterodine 4 mg or 8 mg tablet QD dosing were simulated using the final population pharmacokinetic model and then compared with adult exposures following the administration of fesoterodine 8 mg tablet QD as estimated by the adult final population pharmacokinetic model (Fig. 3). Simulations following the administration of fesoterodine 4 mg or 8 mg tablet QD showed a gradual decrease in exposure as body weight increased. When fesoterodine 4 mg or 8 mg tablet QD was administered with a body weight of 25 kg as the threshold (Fig. 3a), the median Cmax,ss value for pediatric patients weighing 25–35 kg and receiving 8 mg tablet QD was estimated to be 11.7 ng/mL, which is 2.45 times greater than the median Cmax,ss value estimated in adults receiving 8 mg tablet QD (4.79 ng/mL). A change in the threshold from 25 kg to 35 kg (i.e., pediatric patients weighing 25–35 kg received 4 mg tablet QD instead of 8 mg tablet QD) was accompanied by a decrease in 5-HMT exposure to the point at which it was comparable to the range of exposure (Cmax,ss and AUCtau,ss) estimated in adults receiving the 8 mg tablet (Fig. 3b).
Weight-based simulations of AUCtau,ss and Cmax,ss for pediatric patients on fesoterodine 4 and 8 mg tablet QD with a 25 kg threshold (a) and a 35 kg threshold (b). For each box, the median value for each metric (AUCtau,ss or Cmax,ss) is designated by a black line in the center of the box. Boxes indicate the IQR. Whiskers extend up to 1.5 IQR. Jitter dots are the EBEs used to visualize the distribution of each metric in each group. The stars in each box represent the geometric mean value for each metric. Solid (dotted) red lines represent median (range) values for each metric estimated for adults in the fesoterodine program, whereas red stars at the right and left ends of each figure represent the corresponding geometric mean values. AUCtau,ss area under the plasma concentration–time curve over the dosing interval at steady state, Cmax,ss maximal concentration at steady state, EBE empirical Bayes estimates, IQR interquartile range, QD once daily, Tab fesoterodine tablet
The predicted MCCs for pediatric patients with various body weights after fesoterodine 4 mg or 8 mg tablet QD dosing were simulated using the final population pharmacokinetic/pharmacodynamic model (Fig. 4). Simulation results showed that dosing patients weighing ≥ 25 to ≤ 35 kg with fesoterodine 4 mg tablet QD and patients weighing > 35 kg with 8 mg tablet QD would achieve a median [95% prediction interval] Cavg,ss of 2.83 ng/mL [1.13, 7.14] and 4.06 ng/mL [1.54, 11.4], respectively, and a median [95% prediction interval] CFB MCC of 47.8 mL [− 50.8, 211] and 68.8 mL [− 51.4, 286], respectively.
Weight-based simulations of CFB MCC in pediatric patients on fesoterodine 4 and 8 mg tablet QD with a 35 kg threshold. Virtual patients were extracted from patients enrolled in study 1047 (121 patients in each simulation) and then the Cavg,ss and MCC for each individual were simulated 1000 times. The solid red line shows the model-predicted population median following administration of the fesoterodine 4 mg tablet to patients with weight ≤ 35kg, the solid blue line shows the model-predicted population median following administration of the fesoterodine 8 mg tablet to patients with weight > 35 kg, and the blue areas represent model-predicted 95% prediction intervals (2.5th and 97.5th percentiles) for each dose level, respectively. CFB change from baseline, MCC maximum cystometric capacity, QD once daily; Tab fesoterodine tablet
4 Discussion
This is the first analysis of pooled pediatric studies which provides an understanding of the pharmacokinetic properties of 5-HMT and the exposure–response relationship for MCC after the administration of fesoterodine in pediatric NDO patients.
The pharmacokinetics of 5-HMT following multiple dosing of fesoterodine were adequately described by a one-compartment model with first-order absorption and elimination and a lag time, which included the effects of body weight on CL/F and Vd/F, sex on CL/F and Vd/F, CYP2D6 metabolizer on CL/F, and fesoterodine formulation on F. Diagnostic plots revealed no apparent time or concentration dependencies in the 5-HMT pharmacokinetics, and pcVPC stratified by treatment group indicated good agreement of the final model predictions with observed data. All fixed-effect parameters were estimated with reasonable precision (RSE ≤ approximately 30%), except for lag time. However, the sensitivity analysis for lag time revealed that there was no impact on the prediction of pharmacokinetic parameters such as individual AUCtau,ss and Cmax,ss, so this relatively high RSE for lag time seems to be acceptable. Inter-individual variance for CL/F, Vd/F and Ka was estimated with reasonable precision (RSE ≤ 20%). Moderate \(\upeta\) shrinkage was observed for Vd/F and Ka, which may be caused by the sparseness of sampling times and the lack of information to describe the absorption phase. Thus, the rather low reliability of EBE-based diagnostic plots and EBE-based Cmax,ss may require attention.
The model-predicted relative BA for BIC compared to tablets in pediatric patients was 64.8%, which is lower than the observed mean [90% CI] relative bioavailability for AUClast under fasting and fed conditions in a study in healthy adult subjects (87.9% [81.3–94.9%] and 79.9% [73.7–86.6%], respectively) (ClinicalTrials.gov: NCT02160158). It should be noted that in study 1047, it was not known if pediatric patients took the dose in the fed or fasted state. The design and analysis of the relative BA study in adults were different compared with study 1047 in many different aspects (e.g., adults versus pediatric participants, crossover versus parallel-group study design, intense versus sparse pharmacokinetic sampling, noncompartmental versus population pharmacokinetic analysis). Therefore, the effect of drug formulation on BA in the present population pharmacokinetic analysis may not provide results consistent with noncompartmental pharmacokinetic results from the fasted or fed state study in adults.
An Emax model was fitted to MCC data and was found to describe the exposure–response relationship adequately when age was included as main covariate. EC50 was estimated to be 6.22 ng/mL [95% CI 4.11, 10.1 ng/mL], which was within the expected range, though relatively large parameter uncertainty was observed (wide 95% CI). Since the number of observations over the EC50 was limited, the relationship between Cavg,ss and MCC appeared to be linear across the range of predicted Cavg,ss. Baseline MCC was estimated with good precision, and typical baseline MCC at median age (9 years) was estimated to be 146 mL, which was close to the observed median (152 mL). The inter-individual variance for BASE and Emax was estimated with good precision (RSE ≤ 20%). A mild \(\upeta\) shrinkage for both BASE and Emax was observed (< 25%). It is noteworthy that there was no significant trend between age and \(\eta\) s for both Emax and BASE, indicating that the model assumptions—that Emax was associated with age-based normal bladder capacity and BASE depended on age—were adequate and justified in this analysis.
The final population pharmacokinetic model provides a comprehensive evaluation of the effects of covariates, including body weight, on 5-HMT exposure in pediatric NDO patients. Results from the population pharmacokinetic analysis clearly showed suboptimal exposure in pediatric participants receiving 2 mg BIC compared to that initially expected (i.e., not comparable with adult exposures following the administration of fesoterodine 4 mg tablet QD). Results from the population pharmacokinetic/pharmacodynamic analysis also suggested that 2 mg BIC for pediatric NDO patients ≤ 25 kg had suboptimal exposure and efficacy compared to other treatment groups (i.e., the exposure was considerably lower than EC50), which is supported by the clinical efficacy data from study 1047 (ClinicalTrials.gov: NCT01557244). Based on the simulation using the population pharmacokinetic model, the median value of Cmax,ss among patients weighing 25–35 kg receiving fesoterodine 8 mg tablet QD was 11.7 ng/mL, which was approximately 2.5-fold greater than the median Cmax,ss value estimated in adults receiving 8 mg tablet QD (4.79 ng/mL). On the other hand, the exposure–response relationship for MCC and the simulation based on the population pharmacokinetic/pharmacodynamic model indicated that the exposures in patients weighing 25–35 kg following the administration of fesoterodine 4 mg tablet QD and those weighing >35 kg following the administration of fesoterodine 8 mg tablet QD were adequate to demonstrate a CFB MCC of approximately 40 mL. A post-hoc statistical subgroup analysis of CFB MCC at week 12 in study 1047 showed that pediatric patients weighing 25–35 kg for the fesoterodine 4 mg treatment group and those weighing > 35 kg for the fesoterodine 8 mg treatment group achieved a LS mean [95% confidence interval] of 30.30 mL [− 15.83, 76.42] and 99.49 mL [64.82,134.16], respectively, which generally supported the results of the pharmacokinetic/pharmacodynamic simulation. This level of exposure and response may be considered adequate and clinically relevant for the treatment of NDO patients based on their age and bladder capacity [19, 20]. The wide 95% CI of MCC can be explained by large variability of urodynamic endpoints [21]. For pediatric patients with body weights of 25–35 kg, a higher efficacy would be expected from the observed and predicted CFB MCC with fesoterodine 8 mg tablet QD than with 4 mg QD. In the meanwhile, the predicted exposure (Cmax,ss) in some of the patients at 8 mg QD would become relatively high compared with the reference adult exposure. Considering the balance between efficacy and exposure, the recommended dosing for pediatric patients weighing 25‒35 kg is to begin with 4 mg tablet QD and increase to 8 mg tablet QD if needed. For pediatric NDO patients weighing > 35 kg, the recommended dosing is to begin with 4 mg tablet QD and increase to 8 mg tablet QD after 1 week, which is supported by the efficacy and safety data reported in study 1047 and consistent with how all patients randomized to the 8 mg tablet QD treatment group were dosed during the study.
The study design, including the sparse pharmacokinetic sampling, the lack of placebo control, and the small sample size, limits the ability to make formal efficacy comparisons and detect rare adverse reactions [11].
5 Conclusion
Population pharmacokinetic and pharmacokinetic/pharmacodynamic models were developed for fesoterodine and could be used to predict 5-HMT exposure, its relationship with fesoterodine dose, effects of extrinsic and intrinsic factors on the exposure, and the exposure–response relationships for MCC in pediatric NDO patients. The pharmacokinetics of 5-HMT were best described by a one-compartment model with first-order absorption and elimination and a lag time; an Emax model best described the observed MCC data. Based on the results from the population analyses and the clinical efficacy data, recommended dosing for pediatric patients weighing 25‒35 kg is initially 4 mg tablet QD, which should be increased to 8 mg tablet QD if needed. For pediatric NDO patients weighing >35 kg, the recommended dosing should begin with 4 mg tablet QD and increase to 8 mg tablet QD after 1 week, which is supported by the clinical data reported in study 1047 and is consistent with how patients randomized to the 8 mg tablet QD treatment group were dosed during the study.
References
Neveus T, von Gontard A, Hoebeke P, et al. The standardization of terminology of lower urinary tract function in children and adolescents: report from the Standardisation Committee of the International Children’s Continence Society. J Urol. 2006;176:314–24.
Stein R, Bogaert G, Dogan HS, et al. EAU/ESPU guidelines on the management of neurogenic bladder in children and adolescent part I diagnostics and conservative treatment. Neurourol Urodyn. 2020;39:45–57.
Kroll P. Pharmacotherapy for pediatric neurogenic bladder. Paediatr Drugs. 2017;19:463–78.
Austin PF, Bauer SB, Bower W, et al. The standardization of terminology of lower urinary tract function in children and adolescents: update report from the standardization committee of the International Children’s Continence Society. Neurourol Urodyn. 2016;35:471–81.
Bauer SB. Special considerations of the overactive bladder in children. Urology. 2002;60:43–8.
Verpoorten C, Buyse GM. The neurogenic bladder: medical treatment. Pediatr Nephrol. 2008;23:717–25.
de Jong TP, Chrzan R, Klijn AJ, et al. Treatment of the neurogenic bladder in spina bifida. Pediatr Nephrol. 2008;23:889–96.
Malhotra B, Guan Z, Wood N, et al. Pharmacokinetic profile of fesoterodine. Int J Clin Pharmacol Ther. 2008;46:556–63.
Pfizer Inc. Toviaz® (fesoterodine fumarate). Full prescribing information. New York: Pfizer Inc.; 2021.
Malhotra B, El-Tahtawy A, Wang E, et al. Dose-escalating study of the pharmacokinetics and tolerability of fesoterodine in children with overactive bladder. J Pediatr Urol. 2012;8:336–42.
Kitta T, Darekar A, Malhotra B, et al. Fesoterodine treatment of pediatric patients with neurogenic detrusor overactivity: a 24-week, randomized, open-label, phase 3 study. J Pediatr Urol. 2022;22:S1477-5131. https://doi.org/10.1016/j.jpurol.2022.11.020.
McGuire EJ, Woodside JR, Borden TA, et al. Prognostic value of urodynamic testing in myelodysplastic patients. J Urol. 1981;126:205–9.
Galloway NT, Mekras JA, Helms M, et al. An objective score to predict upper tract deterioration in myelodysplasia. J Urol. 1991;145:535–7.
Beal SL, Sheiner LB, Boeckmann AJ, et al. NONMEM 7.4 user’s guides (1989–2018). https://nonmem.iconplc.com/nonmem743/guides. Accessed 26 July 2022.
R Core Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2021. https://www.R-project.org/. Accessed 26 July 2022.
Lindbom L, Pihlgren P, Jonsson EN. PsN-Toolkit—a collection of computer intensive statistical methods for non-linear mixed effect modeling using NONMEM. Comput Methods Progr Biomed. 2005;79:241–57.
Oishi M, Tomono Y, Yamagami H, et al. Population pharmacokinetics of the 5-hydroxymethyl metabolite of tolterodine after administration of fesoterodine sustained release tablet in Western and East Asian populations. J Clin Pharmacol. 2014;54:928–36.
Bergstrand M, Hooker AC, Wallin JE, et al. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J. 2011;13:143–51.
Franco I, Hoebeke P, Baka-Ostrowska M, et al. Long-term efficacy and safety of solifenacin in pediatric patients aged 6 months to 18 years with neurogenic detrusor overactivity: results from two phase 3 prospective open-label studies. J Pediatr Urol. 2020;16(180):e1-8.
Franco I, Horowitz M, Grady R, et al. Efficacy and safety of oxybutynin in children with detrusor hyperreflexia secondary to neurogenic bladder dysfunction. J Urol. 2005;173:221–5.
Frenkl T, Railkar R, Palcza J, et al. Variability of urodynamic parameters in patients with overactive bladder. Neurourol Urodyn. 2011;30:1565–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by Pfizer Inc. All authors are employees and shareholders of Pfizer Inc.
Conflict of Interest
All authors are employees and shareholders of Pfizer Inc.
Availability of Data and Material
Upon request, and subject to certain criteria, conditions and exceptions (see https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information), Pfizer will provide access to individual de-identified participant data from Pfizer-sponsored global interventional clinical studies conducted for medicines, vaccines and medical devices (1) for indications that have been approved in the US and/or EU or (2) in programs that have been terminated (i.e., development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary, and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The de-identified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer.
Code Availability
The NONMEM code is in Online Resource 4 and Online Resource 5. The R code for the simulation describing the assessment of the impact of body weight on MCC is in Online Resource 6. Other R codes used to generate the graphics can be obtained from the corresponding author on reasonable request.
Author Contributions
YS analyzed the data. All authors made substantial contributions to the interpretation of data and participated in drafting and critically revising the work. All authors approved the final version to be published and agree to be accountable for all aspects of the work.
Ethics Approval
The study protocols were approved by the institutional review boards/ethics committees of the study sites, and all participants provided written informed consent before participation in the studies. The studies were conducted in compliance with the ethical principles originating in or derived from the Declaration of Helsinki and conducted according to the International Conference on Harmonization Guidelines for Good Clinical Practice.
Consent to Participate
All participants provided written informed consent before participation in the studies.
Consent for Publication
Not applicable.
Trial Registration
These analyses included data from the following studies: NCT00857896 and NCT01557244
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Sano, Y., Shoji, S., Shahin, M. et al. Population Pharmacokinetic and Pharmacodynamic Modeling of Fesoterodine in Pediatric Patients with Neurogenic Detrusor Overactivity. Eur J Drug Metab Pharmacokinet 48, 257–269 (2023). https://doi.org/10.1007/s13318-023-00818-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-023-00818-8