Abstract
Background and Objective
Clinical evidence suggests no clinically relevant pharmacokinetic interactions between indacaterol (IND), glycopyrronium (GLY) and mometasone furoate (MF). A population pharmacokinetic (popPK) analysis was conducted to identify structural models describing systemic pharmacokinetic profiles of IND, GLY and MF, and estimate the effect of covariates on their pharmacokinetics following inhalation as IND/GLY/MF.
Methods
Pharmacokinetic data from 698 patients with asthma were pooled from two Phase III studies that evaluated IND/MF medium- (150/160 µg) and high-dose (150/320 µg), IND/GLY/MF medium- (150/50/80 μg) and high-dose (150/50/160 μg), and a device bridging Phase II study with MF. One popPK model was developed each for IND, GLY and MF using a nonlinear mixed-effect modelling approach. Maximal and trough plasma concentrations were compared across formulations and studies, including data for IND/GLY from chronic obstructive pulmonary disease (COPD) patients. The effect of predefined covariates on the pharmacokinetics of components was evaluated using a full covariate modelling approach.
Results
The final pharmacokinetic models were two-compartment disposition models with first-order elimination and sequential zero-order/first-order absorption (IND), with bolus administration and first-order elimination (GLY), and with mixed zero-order/first-order absorption and first-order elimination (MF). All model parameters were estimated with good precision (% relative standard error: IND and MF ≤25%; GLY <10%). No clinically relevant covariate effect was observed on the pharmacokinetics of IND, GLY and MF. IND and GLY pharmacokinetic profiles were similar across different formulations.
Conclusion
Two-compartment popPK models adequately described the pharmacokinetics of IND, GLY and MF. The effect of covariates was not clinically relevant. The pharmacokinetic profiles of MF were comparable for combination products at corresponding medium- or high-dose inhaled corticosteroids. On a population level, the pharmacokinetics of IND and GLY were comparable between patients with asthma and COPD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Two-compartment popPK models adequately described the pharmacokinetics of indacaterol (IND), glycopyrronium (GLY) and mometasone furoate (MF). The effects of covariates were not clinically relevant |
The pharmacokinetic profiles of IND, GLY and MF were comparable when administered as different fixed-dose combination products (IND/MF vs IND/GLY/MF with medium- or high-dose MF, respectively) |
The pharmacokinetic profiles for IND and GLY in patients with asthma when administered as IND/MF and IND/GLY/MF were similar to pharmacokinetic profiles observed in patients with chronic obstructive pulmonary disease |
1 Introduction
The World Health Organization estimated that 339 million people suffered from asthma globally in 2016 and recognises asthma to be of major public health importance [1]. Inhaled corticosteroids (ICS) are the cornerstone in asthma management. The Global Initiative for Asthma (GINA) recommends treatment with ICS-containing therapy, either as-needed (mild asthma) or daily, to reduce the risk of severe exacerbations and to control symptoms [2].
For patients with persistent symptoms and/or exacerbations, a dose ‘step-up’, i.e., a higher dose of ICS and/or use of ICS with a long-acting β2-agonist (ICS/LABA) is recommended [2]. Emerging data support the use of a long-acting muscarinic antagonist (LAMA) as add-on therapy for patients who continue to suffer despite the ICS/LABA combination [3]. Nevertheless, despite the inhaled treatments, asthma remains uncontrolled in many patients, partly due to poor adherence to treatment regimens [4]. An important step in simplifying management and improving adherence with prescribed therapy is to reduce the dose frequency (i.e., have a fixed-dose combination [FDC]) as necessary to maintain disease control [5]. The FDC of LABA/LAMA/ICS in a single inhaler will allow the three components to work on diverse targets [6], and can potentially contribute to enhanced patient medication adherence [7, 8]. An orally inhaled FDC of indacaterol acetate/glycopyrronium bromide/mometasone furoate (IND/GLY/MF, a LABA/LAMA/ICS) is approved as a once-daily (o.d.) maintenance treatment for patients with inadequately controlled asthma. Likewise, IND/MF o.d. is approved for maintenance treatment of patients with asthma. Both FDCs are administered via the Breezhaler® device.
The pharmacokinetics of IND, GLY and MF have been characterised in patients with chronic obstructive pulmonary disease (COPD) and asthma when administered as a monotherapy or an FDC (IND/GLY [in COPD] [9], IND/MF [in asthma] [10], and IND/GLY/MF [in asthma] [11]). Clinical evidence suggests no clinically relevant pharmacokinetic interactions between IND, GLY and MF when administered as IND/GLY, IND/MF or IND/GLY/MF [9,10,11,12,13,14,15].
Population pharmacokinetic (popPK) models describe the between-subject variability (BSV) of exposure to a drug and aim to identify and quantify sources of variability in the patient population such as body weight [16]. Prior popPK modelling has not been reported for FDCs of IND/MF or IND/GLY/MF in patients with asthma. However, popPK models for both IND and GLY have been established when administered as IND/GLY to COPD patients [17]. The present analysis is based on 3 studies (PALLADIUM [18], IRIDIUM [19] and E2201 [20]) conducted in patients with asthma for which key design elements are provided in Table 1. The current analysis, utilising different FDC studies, aimed to identify the structural popPK models describing the pharmacokinetics of IND, GLY and MF, and to estimate the effect of covariates on the pharmacokinetics of IND, GLY and MF, including the effect of Japanese ethnicity in patients with asthma.
2 Methods
2.1 Study Design
The popPK analysis was based on data from multicentre, randomised studies in patients with asthma including two Phase III studies (PALLADIUM [18] and IRIDIUM [19]) and a device bridging Phase II study (E2201 [20] [NCT01555151]). Data were supplemented by a Phase III study (QUARTZ [21]) for comparison only (Table 1). Data from a Phase III study (SHINE [22], in patients with COPD) with similar sampling time points, and doses of IND (150 µg), GLY (50 µg) and IND/GLY (110/50 µg) were also used to compare the pharmacokinetics of IND and GLY between patients with COPD and asthma. Data from the two populations were used for a direct comparison of maximal plasma concentrations (Cmax) and trough concentrations (Ctrough). Based on in vitro performance data, the dose of each monocomponent of IND/GLY 110/50 μg has been developed to provide lung deposition to match that of the monotherapy treatments—indacaterol 150 μg (Onbrez® Breezhaler®) and glycopyrronium 50 μg (Seebri® Breezhaler®) [17].
The Phase II E2201 study investigated corresponding high- and low-dose MF monotherapies delivered via the Twisthaler® and Breezhaler® devices (Table 2). A 3-step bridging approach identified doses of MF in the Breezhaler® device that were comparable to the corresponding doses of MF in the Twisthaler® device. The approach has been described previously [20]. Table 2 outlines comparable MF doses in the Twisthaler® device, and IND/MF and IND/GLY/MF combinations delivered via the Breezhaler® device.
2.2 Pharmacokinetic Sample Collection and Bioanalysis
The Phase III studies collected sparse pharmacokinetic data up to 1 h post dose. A device bridging Phase II study (E2201) included MF pharmacokinetic sampling (6 samples over 24 h) up to 24 h post dose, which was included in the popPK analysis to complement the Phase III data, allowing for estimation of distribution parameters for MF. The pharmacokinetic profiling subset for all studies included all randomised patients who consented to participate for additional pharmacokinetic sampling and had at least one evaluable pharmacokinetic measurement. All blood samples were taken by either direct venepuncture or an indwelling catheter inserted in a forearm vein. At specified time points, 4 mL of blood was collected in lithium heparin tubes for determination of IND and GLY, and 6–9 mL of blood was collected in K2-EDTA tubes for determination of MF. The plasma concentrations for IND, GLY and MF were determined using three separate validated LC-MS/MS assays with lower limits of quantification (LLOQs) of 5.00 pg/mL, 0.250 pg/mL and 1.00 pg/mL, respectively. Concentrations below LLOQ were included in the popPK analysis and contributed to the likelihood used for the parameter estimation. For summary statistics, concentrations below LLOQ were set equal to the LLOQ. Pharmacokinetic profiles which were considered implausible (e.g., unexpected decrease in systemic concentrations post dosing or flat exposure profile, unusual exposure profile with unexpected high or below the limit of quantification plasma concentrations, and systemic exposures collected far outside the collection time window), were flagged for exclusion prior to the model fitting. A sensitivity analysis was performed with and without the excluded data.
2.3 popPK Modelling Strategy
One popPK model was developed each for IND, GLY and MF. The pharmacokinetic models were constructed using a nonlinear mixed-effect modelling approach and were implemented in Monolix version 2018R1 (Lixoft SAS, Antony, France) using the stochastic approximation of expectation maximisation algorithm. Pharmacokinetic data were explored using comparative plots of plasma concentration-time profiles. Summary statistics for Cmax and Ctrough were compared across formulations and studies. The popPK analysis was performed to estimate the population parameters (mean and BSV) and the effect of covariates.
Two-compartment disposition models with first-order absorption or bolus administration were adequate to describe the pharmacokinetics for IND or GLY in COPD patients [17]. These existing models guided the present model building. Model building for MF was based on the pooled dataset from the present analysis. For the popPK models of IND/GLY, an attempt was made to maintain the models comparable to the existing models established in COPD patients [17]. If a two-compartmental model could not be fitted, the apparent inter-compartmental clearance (Q/F) and/or the apparent peripheral volume (Vp/F) was fixed to the values identified previously in COPD patients [17].
The pharmacokinetic models were parameterised using apparent systemic clearance (CL/F), apparent central volume (Vc/F), Q/F, Vp/F, absorption rate constant (Ka), and relative bioavailability (F). For absorption, instantaneous bolus and mixed or sequential first/zero-order absorption were considered as alternatives. For MF, depending on the formulation, a different nominal dose was required to deliver the same lung dose (Table 2); a fixed multiplicative factor on the bioavailability for MF doses was introduced (2.5 in Twisthaler®, 1.0 as part of IND/MF FDC or when delivered with the Breezhaler® device, and 0.5 as part of IND/GLY/MF FDC). The multiplicative correction factors for bioavailability were introduced based on doses that were matched to give the same pharmacodynamic response. Subsequently when building the base model, we evaluated whether deviations from these factors were required to describe the pharmacokinetics. BSV in pharmacokinetic parameters was modelled using multiplicative exponential random effects. Residual variability was modelled using a combined additive/proportional error model. The additive component was set to zero if the estimate tended to go towards zero.
2.4 Covariate Analysis
Body weight was included as a default covariate on clearance terms (CL/F and Q/F) and on volumes (Vc/F and Vp/F) with an allometric scaling of 0.75 and 1, respectively. The impact of other predefined covariates (sex, age, race, smoking status, baseline forced expiratory volume in 1 s [FEV1] and estimated glomerular filtration rate [eGFR] on CL/F and race on CL/F and Vc/F) was explored using a full covariate modelling approach (Table 3) [23].
Study effects and effects of different formulations were evaluated as part of base model building. Continuous covariates were modelled as a power function after normalising the covariates to their population medians. For example, the effect of body weight on apparent clearance was modelled as Eq. 1:
where WTi is the body weight of the subject i, θ is the effect of weight on clearance, WTref is the reference value listed in Table 3, and . . . denotes other covariate effects multiplied together. Categorical covariates were modelled as a ratio to the population mean and exponent coefficients were estimated for selected categories and set to zero for the reference category as in Table 3. For example, the effect of sex on apparent clearance was modelled as Eq. 2:
where sexi is the sex of the subject i and θ is the effect of female sex on the apparent clearance.
The covariate modelling approach consisted of three steps:
-
include simultaneously all predefined covariates according to Table 3
-
remove non-significant covariates at the significance level p < 0.01 in the Wald test
-
include additional covariate relations if trends were detected in plots of random effect against covariates and if there remained a significant correlation between the random effects and the covariate as judged by Pearson’s correlation test at a significance level of p < 0.01
2.5 Model Evaluation
At all stages of model development, model adequacy was assessed based on a combination of judgement of model plausibility and robustness, objective function value, goodness-of-fit diagnostics, and simulation-based diagnostics (visual predictive check [VPC] and normalised prediction distribution errors [NPDEs]). Predictive performance of the models was assessed by the NPDE and VPC. The NPDE was calculated in Monolix. The VPC was stratified by treatment group and by study using 500 simulations of pharmacokinetic profiles from the final model including BSV and using covariate values and dosing records from the modelling dataset plus residual variability (but not parameter uncertainty). For each simulation, profiles of the simulated study population were summarised as 5%, 50% (median), and 95% percentiles; the percentiles characterise the overall BSV.
Diagnostic plots were examined to assess model adequacy, possible lack of fit or violation of assumptions. Plots of observations versus predicted value (PRED) and versus individual predicted value (IPRED) were evaluated for randomness around the line of unity. Plots of individual weighted residual (IWRES) versus time, time after last dose and IPRED were evaluated for randomness around the zero line. These diagnostic plots were also stratified by study and treatment to ensure adequacy of fit across these design factors (not shown).
2.6 Simulations for Model Properties
Properties of models were illustrated by simulating rich steady-state profiles over 24 h with samples taken every 6 min for a set of virtual patients. A reference virtual patient was defined as a patient with covariate values of a typical patient in the pooled data set. Simulation results were summarised by plotting pharmacokinetic profiles and by tabulating the area under the concentration-time curve over 24 h (AUC0–24h), Cmax and Ctrough values as a function of covariate values.
Variants of the virtual reference patient were generated for different treatments and with values of the covariates illustrating the range of values occurring in the pooled patient population. Two hundred pharmacokinetic profiles were simulated for each covariate effect using BSV and without residual variability.
3 Results
3.1 Observed Data
Pharmacokinetic data from 698 patients with asthma were included in the primary analysis. The majority of patients were Caucasian (n = 523) followed by Asians (n = 115), of which the majority were Japanese (n = 107). Baseline characteristics of patients in the pharmacokinetic main analyses are summarised in Table 4. Pharmacokinetic samples of IND (n = 14), GLY (n = 2) and MF (n = 35) were below LLOQ.
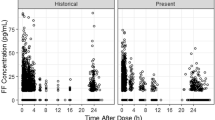
Different pharmacokinetic profiles of IND were comparable with each other and did not depend on whether IND was administered as monotherapy or in combination (Online Resource 1). Similarly, GLY profiles were comparable independent of whether GLY was administered as part of the IND/GLY/MF 150/50/80 μg (medium-dose ICS) or 150/50/160 μg (high-dose ICS) (Online Resource 1). The pharmacokinetic profiles of MF showed some differences depending on whether MF was administered as a monocomponent via the Twisthaler® device (o.d. or twice daily [b.i.d.] regimen) or via the Breezhaler® device (o.d. regimen), as a FDC (at a medium or high dose) (Online Resource 1 and Fig. 1). Comparing the pharmacokinetics of IND across studies (Fig. 1) indicated a possible study effect with higher Cmax concentrations in the PALLADIUM study. Comparing the pharmacokinetics of IND and GLY in patients with asthma with the pharmacokinetics in COPD patients (Fig. 1) indicated that on a population level, the pharmacokinetics in these two populations are comparable (Online Resource 2). For the high-dose MF formulations, both Cmax and Ctrough were similar when MF was administered as part of IND/MF 150/320 μg o.d. or IND/GLY/MF 150/50/160 μg o.d. delivered via the Breezhaler® (Fig. 1; IRIDIUM). Administration of MF Twisthaler® 400 μg b.i.d. resulted in lower Cmax but comparable Ctrough with that observed with the FDCs (Fig. 1; PALLADIUM). For medium-dose MF formulations, both Cmax and Ctrough were similar when MF was administered as part of IND/MF 150/160 μg o.d. or IND/GLY/MF 150/50/80 μg o.d. (Fig. 1; IRIDIUM). Administration of MF Twisthaler® 400 μg o.d. resulted in lower Cmax but comparable Ctrough with that observed with the FDCs (Fig. 1; PALLADIUM).
Observed Cmax and Ctrough concentrations across studies and indications for a indacaterol, b glycopyrronium and c mometsaone furoate. For indacaterol: steady-state concentrations on Day 86 or Day 85 or Day 84. For IND/MF medium dose (IND/MF 150/160 µg q.d. via the Breezhaler® device) and IND/MF high dose (IND/MF 150/320 µg q.d. via the Breezhaler® device), results from PALLADIUM [18] are shown to the left of results from the IRIDIUM study [19]. To ensure comparability between studies, calculation of Cmax and Ctrough (indicated by circles in graphs) was based on samples taken at nominal times of up to 1 h excluding later samples in the SHINE [22] study, and excluding the samples at 2 min, which were not available in all the studies. For glycopyrronium: steady-state concentrations on Day 86 or Day 85. To ensure comparability between studies, calculation of Cmax and Ctrough (indicated by circles in graphs) was based on samples taken at nominal times of up to 1 h excluding later samples in the SHINE [22] study. For mometasone furoate: steady-state concentrations on Day 86, Day 85 or Day 28, respectively. Formulations are grouped according to their MF dose as high, high*, medium and low, with administration in all groups being q.d. except the high* group with b.i.d. For IND/MF, results from the PALLADIUM study [18] are shown to the left of results from the IRIDIUM study [19]; For MF low-dose (MF 200 µg q.d. via the Twisthaler® device) results from the E2201 study [20] are shown to the left of results from the QUARTZ study [21]. To ensure comparability between studies, calculation of Cmax and Ctrough (indicated by circles in graphs) was based on samples taken at nominal times of up to 1 h excluding later samples in the E2201 study [20], and excluding the samples at 2 min, which were not available in all the studies. Cmax maximum concentration, Ctrough trough concentration, b.i.d. twice daily, GLY glycopyrronium, IND indacaterol, IQR interquartile range, MF mometasone furoate, q.d. once daily. The centre line of the interquartile box represents median value, top and bottom borders of the box represent the 75th and 25th percentile, respectively, whiskers represent range (±1.5xIQR)
3.2 Base Models
The parameter estimates for the IND, GLY and MF base pharmacokinetic models are shown in Online Resource 4. The initial two-compartment model with first-order absorption and a study effect on central volume described the data for IND. The VPC showed some deviations between simulations and observations at early time points. Furthermore, using a short zero-order absorption of a fraction of the drug followed by a rapid first-order absorption of the rest of the drug improved the model diagnostics. For IND, the BSV was estimated on CL/F, Vc/F, Q/F and Vp/F, and the fraction of drug absorbed rapidly. The correlation between the random effects of CL/F and Vc/F was estimated.
Similar to IND, the initial two-compartment model with first-order absorption and a study effect on central volume described the data for GLY. A simpler model with bolus administration described the data and did not require estimation of the absorption rate. The BSV for GLY was estimated on CL/F and Vc/F including their correlation.
MF had a similar initial model to IND and GLY with treatment effects on bioavailability and central volume that described the data. For MF, the VPC showed some deviations between simulations and observations at early time points, particularly in the IRIDIUM study with samples at 2 min. For MF, using a mixed zero-order/first-order absorption improved the model diagnostics and the mixed absorption model described the absorption as an initial very rapid absorption of a fraction of the drug, overlaid by a slower first-order absorption. BSV was estimated on CL/F, Vc/F, Q/F and Vp/F and the fraction of drug absorbed rapidly, and included a term describing the correlation between the random effects of CL/F and Vc/F. For MF, formulation effects were initially tested differentiating all formulation for Vc/F and F. The resulting model was simplified by grouping together formulations from the PALLADIUM and IRIDIUM studies that showed similar kinetics. Effects remained on the following:
-
Vc/F and F for MF Twisthaler® 400 μg administered o.d. or b.i.d. relative to the IND/MF Breezhaler® 150/320 μg (high-dose MF) formulation
-
Vc/F for IND/GLY/MF versus IND/MF
-
Bioavailability for medium-dose MF formulations of IND/GLY/MF 150/50/80 μg and IND/MF 150/160 μg versus the corresponding high-dose MF formulations (IND/GLY/MF 150/50/160 μg and IND/MF 150/320 μg)
The resulting MF model described the pharmacokinetic data from the PALLADIUM and IRIDIUM studies adequately as judged by model diagnostics and VPCs. However, for the E2201 study, there were remaining differences between observed and predicted trough concentrations and a formulation effect on Vp/F had to be introduced. This formulation effect on Vp/F differentiated between treatments administered as puffs of 400 μg administered via the Twisthaler® device, and treatments administered as puffs of 200 µg with the Twisthaler® device.
3.3 Covariate Selection
All the predefined covariates according to Table 3 were included into the model. Additionally, the allometric scaling factors on the apparent clearance and the central volume were estimated for IND and MF, since after correcting for default allometric scaling factors, there remained a non-negligible correlation between the corresponding random effects and body weight. For IND, having added all the covariate effects, the correlation between CL/F and Vc/F could no longer be estimated, and was removed from the model. Then, non-significant covariates at the significance level p < 0.01 in the Wald test were removed (sex, age, smoking status, baseline FEV1, eGFR and grouped race on CL/F [for all three components], and grouped race on Vc/F [for MF]) to obtain the final model in which remaining covariates were tested for clinical relevance. For MF, after correcting for the pre-planned covariate relationships, there remained a non-negligible correlation between random effects of the central volume and FEV1 at baseline. To describe this observed correlation, the corresponding covariate was added to the model for MF.
3.4 Final popPK Models
The final pharmacokinetic models were two-compartment disposition models with first-order elimination and sequential zero-order/first-order absorption (IND) and with bolus administration and first-order elimination (GLY) and with mixed zero-order/first-order absorption and first-order elimination (MF). For mometasone, formulation and study effects were estimated on relative bioavailability on Vc/F and Vp/F. For IND and MF, the BSV was estimated on CL/F, Vc/F, Q/F and Vp/F, and the fraction of drug absorbed via zero-order absorption, while for GLY, the BSV was estimated on CL/F and Vc/F. For all three final models, the correlation between CL/F and Vc/F was estimated.
Covariates included in the final IND and MF model were body weight on CL/F, Vc/F, Q/F and Vp/F, and grouped race (Caucasian/White, Japanese, other) on Vc/F (IND), and baseline FEV1 on CL/F and Vc/F (MF). Covariates included in the final GLY model were body weight on CL/F, Vc/F, Q/F and Vp/F with fixed, default allometric scaling factors, and Caucasian/White, Japanese, and other grouped races on Vc/F. For IND and MF, the allometric scaling factors for body weight were fitted for CL/F and Vc/F.
The parameter estimates for the final popPK model of IND, GLY and MF are described in Table 5. All model parameters were estimated with good precision (IND and MF: % relative standard error (RSE) ≤25%; GLY: %RSE <10%), except the covariate effects for IND, which were estimated with precision (%RSE) of ≤35%. For IND and MF, the estimated shrinkage was low on CL/F, moderate on Vc/F and important on Q/F, Vp/F and Fr, while for GLY, the estimated shrinkage was low on CL/F and Vc/F. For GLY, introducing covariates in the model (effect of ethnicity on Vc/F) reduced the BSV on Vc/F by only 0.03, which represents a minor contribution versus estimated BSV term (0.53 for Vc/F). The covariate estimate (−0.65) for Japanese relative to Caucasian patients was estimated with precision (%RSE) of ~32%. The effect of non-Caucasian/Japanese patients was small with a large relative residual error (−0.063 [%RSE 320%]) and was maintained in the model only to enable estimation of the effect of the Japanese patients relative to Caucasian.
For MF, the BSV on Vc/F was reduced by 0.03, which is about 7% of the standard deviation of this BSV. Covariate effects and formulation effects were estimated with adequate precision (%RSE ~30%) with a few exceptions.
3.5 Final Model Evaluation
Simulation-based diagnostics, VPC evaluated the predictive performance of the models and indicated an overall satisfactory distribution across studies and the treatment groups (for IND and MF) and across the treatment groups (for GLY) (Fig. 2).
Visual predictive check for final popPK model for a indacaterol, b glycopyrronium and c mometsaone furoate. Dots, observed data; black lines, 5th, 50th and 95th percentiles of observed data; shaded areas with red and blue lines, model-based 95% prediction intervals for respective percentiles. GLY glycopyrronium, IND indacaterol, MF mometasone furoate, popPK population pharmacokinetics
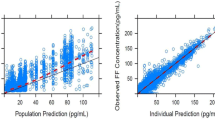
Goodness-of-fits plots showed, in general, an adequate fit of the model for IND and MF and a good fit of the model for GLY) (Fig. 3); both NPDE versus time and versus population prediction demonstrated only minor trends (Online Resource 3).
Goodness-of-fit diagnostics for popPK models for a indacaterol, b glycopyrronium and c mometsaone furoate. Individual IWRES values are indicated by circles. IWRES larger than 4 or smaller than -4 are shown at these limits and differentiated from other residuals using a triangle as a symbol for plotting. LOESS regression shown as thick dashed lines. IWRES individual weighted residual, LOESS locally estimated scatterplot smoothing, popPK population pharmacokinetics
3.6 Simulations Illustrating Covariate Effects
The impact of covariates on Cmax and AUC0–24h was assessed by simulations. For IND, the AUC0–24h in 55 kg and 105 kg patients was 10% higher and 10% lower, respectively, compared with the population mean AUC0–24h in patients with 75 kg body weight in the PALLADIUM study. The population mean AUC0–24h was the same for patients from the PALLADIUM and IRIDIUM studies. Japanese patients and those from other ethnicities had a similar mean AUC0–24h as that observed in Caucasian patients. Similar trends were noted for Ctrough. Compared with the population mean Cmax in patients with 75 kg body weight in the PALLADIUM study, the Cmax in 55 kg and 105 kg patients was 12% higher and 12% lower, respectively. Japanese patients had a 20% higher mean Cmax than Caucasians; patients of other ethnicities and races had a 5% higher mean Cmax than Caucasian patients.
Similarly, for GLY, the AUC0–24h in 55 kg and 105 kg patients was 26% higher and 22% lower, respectively, compared with the population mean AUC0–24h in patients with 75 kg body weight. Japanese patients and those from other ethnicities had a similar mean AUC0–24h to that observed in Caucasian patients. Compared with the population mean Cmax in patients with 75 kg body weight in the PALLADIUM study, the mean Cmax in 55 kg and 105 kg patients was 33% higher and 27% lower, respectively. The population mean Cmax values were 60% higher for Japanese than for Caucasians; patients of other ethnicities and races had a 5% higher mean Cmax than Caucasian patients. The Ctrough varied in a similar way as the simulated AUC0–24h with, in addition, a reduction of Ctrough by 8% for Japanese patients relative to Caucasians.
For MF, simulated AUC0–24h and Cmax varied with body weight and baseline FEV1. Compared with mean AUC0–24h in patients with 75 kg body weight and a baseline FEV1 of 2 L, the AUC0–24h in 55 kg and 105 kg patients was 11% higher and 11% lower, respectively; the AUC0–24h in a patient with baseline FEV1 of 1.2 L and 3 L was 8% lower and 7% higher, respectively. For high-dose MF formulations in the PALLADIUM and IRIDIUM studies, IND/MF and IND/GLY/MF had the same mean AUC0–24h, whereas MF Twisthaler® had 25% lower AUC0–24h compared with FDC treatments. For medium-dose MF formulations in the PALLADIUM and IRIDIUM studies, IND/MF and IND/GLY/MF had the same mean AUC0–24h, whereas MF Twisthaler® had 37% lower AUC0–24h than the FDC treatments. Similar trends were noted for Ctrough. Compared with the mean Cmax in patients with 75 kg body weight and a baseline FEV1 of 2 L, the Cmax in 55 kg and 105 kg patients was 11% higher and 11% lower, respectively. The Cmax in patients with baseline FEV1 of 1.2 L and 3 L was 10% lower and 9% higher, respectively. In addition, patients in the PALLADIUM study had 13% higher mean Cmax than those in the IRIDIUM study.
4 Discussion
The popPK models of IND, GLY and MF described the observed data well, and predictive checks confirmed the appropriateness of the models for simulations. For IND or GLY, no major previously unknown dependence of the pharmacokinetics on baseline covariates was identified. The pharmacokinetic profile for IND and GLY observed as part of the FDCs of IND/MF or IND/GLY/MF in asthma patients was quite similar to that observed with IND/GLY FDC in COPD patients.
The typical pharmacokinetic profiles of the FDCs of IND/MF or IND/GLY/MF are shown in Fig. 4. No difference was identified in the pharmacokinetics of IND when administered as part of FDCs of IND/MF or IND/GLY/MF with medium- or high-dose MF, respectively, and in the pharmacokinetics of GLY when administered as part of IND/GLY/MF (with medium- or high-dose MF), respectively. For the medium- and high-dose ICS formulations, when MF was administered as part of IND/MF or IND/GLY/MF, the pharmacokinetic profiles were similar with the IND/GLY/MF combination having slightly higher maximal and lower trough concentrations than IND/MF. When administered alone via the Twisthaler® device, MF reached lower maximal concentrations but had comparable or higher trough concentrations; these differences were more pronounced for high-dose MF, which was administered alone as b.i.d and in combination as an o.d. regimen.
Simulated typical pharmacokinetic profiles for a indacaterol, b glycopyrronium and c mometsaone furoate. Grey shaded area illustrates variability (5th and 95th percentile) due to unexplained between-subject variability. Simulated plasma concentrations-time profiles of indacaterol across all four treatments were identical. Simulated plasma concentrations-time profiles of glycopyrronium across all two treatments were identical
All monocomponents of IND/GLY/MF act locally in the lungs. We investigated the systemic pharmacokinetics which depend on the amount of drug absorbed from the lungs and from the gastrointestinal tract (for inhaled IND, GLY and MF, absorption occurs predominantly via the lungs [24,25,26,27,28]). For inhaled drug delivery, it is challenging to evaluate the relationship between plasma drug concentrations and pulmonary efficacy due to potentially different drug deposition patterns following inhaled delivery and difficulty in collecting samples to determine drug concentrations in the lung [29, 30]. Therefore, while plasma drug concentrations are relevant for systemic safety and are useful to provide an approximation of comparable doses between devices, they cannot be considered as a reliable surrogate for pulmonary efficacy. The effects of covariates on IND, GLY, or MF systemic pharmacokinetics following inhalation of IND/MF or IND/GLY/MF in patients with asthma were small in magnitude and were not associated with discernible safety or efficacy differences in the IRIDIUM and PALLADIUM studies [18, 19]. In the IRIDIUM study [19], there were no discernible differences in the incidence of the most commonly observed adverse events (AEs) that could potentially be attributable to IND, GLY or MF secondary pharmacology in specific subpopulations (e.g., subjects with a low body weight and/or of Japanese ethnicity). Similarly, in the PALLADIUM study [18], there were no discernible differences in the incidence of the most commonly observed AEs that could potentially be attributable to IND or MF secondary pharmacology in specific subpopulations.
4.1 popPK of IND
A two-compartment disposition model with sequential zero/first-order absorption and first-order elimination described the systemic exposure of inhaled IND in patients with asthma. The pharmacokinetics of IND remained linear over the entire sampling period (up to 85 days). Cmax and AUC0–24h were calculated by simulations based on the final popPK model as described in the methods section. Simulated AUC0–24h varied with body weight while the simulated Cmax values varied with body weight and race, and were different for the PALLADIUM and IRIDIUM studies. The mean AUC0–24h and Cmax were higher in patients with lower body weight; Japanese patients and those with other ethnicities had higher mean Cmax values. Overall, the differences in simulated mean exposures were small in magnitude and were not considered clinically relevant in view of the available safety data at higher doses of indacaterol [24]. A 4-fold higher dose (600 μg) of IND did not result in an increase in QTc duration in a thorough QT study in healthy volunteers [31]
4.2 popPK of GLY
A two-compartment disposition model with bolus administration and first-order elimination described the systemic exposure of inhaled GLY in patients with asthma. The pharmacokinetics of GLY remained linear over the entire period (up to 85 days). Simulated AUC0–24h varied with body weight while the simulated Cmax values varied with body weight and race. The trend in effect of covariates on simulated systemic exposures was similar to that observed for IND, with mean AUC0–24h, Cmax and Ctrough higher in patients with lower body weight. Japanese patients and those with other ethnicities had higher mean Cmax values compared with Caucasians while Ctrough was lower in Japanese patients. As GLY has a wide therapeutic index, the slightly higher simulated exposures in patients with low body weight or higher Cmax in Japanese patients does not raise any safety concerns. Multi-fold higher systemic concentrations of GLY were well tolerated by healthy subjects after intravenous (IV) administration. The mean Cmax after IV administration of 120 μg of GLY was 9720 pg/mL [27] which was more than 40-fold higher than the simulated mean Cmax (208.8 pg/mL) following administration of 50 μg of GLY as part of IND/GLY/MF 150/50/160 μg o.d. in Japanese patients. These exposure levels following IV administration were not associated with any safety concerns. In addition, an 8-fold higher dose (400 μg) compared with a single dose of 50 μg did not result in QTc prolongation or other cardiovascular parameters following inhaled administration [32].
4.3 popPK of MF
A two-compartment disposition model with mixed zero/first-order absorption and first-order elimination described the systemic exposure of inhaled MF in patients with asthma. The pharmacokinetics of MF remained linear over the entire period (up to 85 days). Simulated systemic exposure (AUC0–24h and Cmax) varied with body weight and baseline FEV1. Compared with mean AUC0–24h and Cmax observed in patients with 75 kg body weight and baseline FEV1 of 2 L, the AUC was higher in patients with 55 kg and lower in those with 105 kg weight; these were lower in patient with baseline FEV1 of 1.2 L and higher in patients with FEV1 3 L. There was no difference in mean AUC0–24h of high-dose MF between IND/MF and IND/GLY/MF formulations (IRIDIUM and PALLADIUM studies), and similarly for medium-dose MF. However, the mean AUC0–24h of MF was lower when administered as monotherapy using the Twisthaler® versus FDCs. The mean Cmax was slightly higher in patients in the PALLADIUM study compared with those in the IRIDIUM study. The systematic differences observed in MF plasma concentrations are small relative to the BSV, and are not considered to be clinically relevant in view of available safety data at total daily doses up to 1600 μg for MF administered via the Twisthaler® device in patients with asthma [33], and observed safety profile for IND/MF and IND/GLY/MF following administration via the Breezhaler® device in Phase III studies [18, 19].
The pharmacokinetics of IND and GLY in COPD patients after inhaled administration of IND/GLY has been assessed previously [17]. The dependence of pharmacokinetics on covariates was comparable between asthma and COPD population. For IND, weight was identified in both populations as a covariate, while for GLY, weight and Japanese/East Asian ethnicity were identified in both populations as a covariate. Although smoking status was considered as a covariate in models for both patients with asthma and COPD, current smokers were only included in the COPD studies, and the corresponding covariate effect has only been identified in COPD patients. For IND, a statistically significant decrease of CL/F for female patients and with age has been identified in COPD patients; in patients with asthma the effect of age was not statistically significant; the point estimate indicated a small decrease of CL/F with age (exponent of −0.15, see Eq. 1), and no effect of sex has been detected (exponent of 0.056; see Eq. 2). For GLY, a statistically significant decrease of CL/F with decreasing eGFR has been identified in COPD patients. In patients with asthma, the effect of eGFR was not statistically significant; the point estimate was smaller than the estimate in COPD patients. Overall exposure as assessed by Ctrough and Cmax was similar in the asthma and COPD patient population; patients with asthma in the PALLADIUM study having about 20% higher mean Cmax exposure compared with patients in other studies.
The study was strengthened with data from across monotherapy, LABA/ICS therapy and LABA/LAMA/ICS therapy combinations leveraged together to characterise systemic exposure for IND, GLY and MF as part of IND/MF, IND/GLY/MF development. A sparse pharmacokinetic sampling scheme selected for Phase III studies was adequate to allow characterisation of systemic exposure for IND, GLY and MF in the Phase III setting and allowed evaluation of covariate effects in asthma patients. Sensitivity analysis using all data from the study (including implausible pharmacokinetic profiles identified for exclusion) suggested no impact of outliers. For long-acting inhaled drug products, absorption may be rate limiting, and may exhibit multiphasic kinetics [26] which, with a two-compartment model, will be described by parameters commonly associated with distribution or elimination such as the central volume. For this reason, effects differentiating between treatments were considered for all parameters including those commonly associated with distribution or elimination. More specifically, effects on relative bioavailability were considered to adjust for differences in trough concentrations or differences in overall exposure and effects on apparent central volume (Vc/F) were considered to adjust for differences in maximal concentrations. A more detailed and mechanistic description of the absorption process requires rich pharmacokinetic profiles after inhalation and after IV administration [27].
5 Conclusions
Two-compartment population pharmacokinetic models were demonstrated to adequately describe the pharmacokinetics of IND, GLY and MF. The pharmacokinetic profiles of IND and GLY were similar, independent of whether IND was administered as part of the IND/MF or IND/GLY/MF medium- or high-dose formulations or whether GLY was administered as part of IND/GLY/MF medium- or high-dose formulations. There was no statistically significant covariate effect of age, sex, smoking status, baseline eGFR and FEV1 at baseline on the pharmacokinetics of IND and GLY. Similarly, there was no statistically significant covariate effect of age, sex, Japanese ethnicity, smoking status, and baseline eGFR on the pharmacokinetics of MF. The effects of covariates on the pharmacokinetics of IND, GLY or MF following inhalation of IND/MF or IND/GLY/MF in patients with asthma were small in magnitude and only explained a small fraction of the BSV and, as such, are not considered to be clinically relevant. The pharmacokinetic profiles for IND and GLY in patients with asthma were similar to those observed in COPD patients.
References
World Health Organization, News room, asthma fact sheet. 2020. https://www.who.int/news-room/fact-sheets/detail/asthma. Accessed 1 June 2020.
Global Initiative for Asthma. Global strategy for asthma management and prevention, 2020. https://www.ginasthma.org. Accessed 1 June 2020.
Lipworth BJ. Emerging role of long acting muscarinic antagonists for asthma. Br J Clin Pharmacol. 2014;77:55–62.
Rabe KF, Vermeire PA, Soriano JB, Maier WC. Clinical management of asthma in 1999: the Asthma Insights and Relief in Europe (AIRE) study. Eur Respir J. 2000;16:802–7.
Tamura G, Ohta K. Adherence to treatment by patients with asthma or COPD: comparison between inhaled drugs and transdermal patch. Respir Med. 2007;101:1895–902.
Cazzola M, Matera MG. Fixed-dose combination inhalers. In: Page CP, Barnes PJ editors Pharmacology and therapeutics of asthma and COPD. Handbook of experimental pharmacology, vol 237 (2016).
Chuchalin AG, Tsoi AN, Richter K, et al. Safety and tolerability of indacaterol in asthma: a randomized, placebo-controlled 28-day study. Respir Med. 2007;101:2065–75.
Stoloff SW, Stempel DA, Meyer J, et al. Improved refill persistence with fluticasone propionate and salmeterol in a single inhaler compared with other controller therapies. J Allergy Clin Immunol. 2004;113:245–51.
Ultibro Breezhaler: EPAR—Product Information. https://www.ema.europa.eu/en/documents/product-information/ultibro-breezhaler-epar-product-information_en.pdf. Accessed 15 Feb 2021.
Atectura Breezhaler: EPAR—Product Information. https://www.ema.europa.eu/en/documents/product-information/atectura-breezhaler-epar-product-information_en.pdf. Accessed 15 Feb 2021.
Enerzair Breezhaler: EPAR—Product Information. https://www.ema.europa.eu/en/documents/product-information/enerzair-breezhaler-epar-product-information_en.pdf. Accessed 15 Feb 2021.
Vaidya SS, Khindri S, Calder N, et al. Pharmacokinetics of indacaterol and mometasone furoate delivered alone or in a free or fixed dose combination in healthy subjects. Pulm Pharmacol Ther. 2016;37:30–6.
Vaidya SS, Jauernig J, Ethell B, et al. Pharmacokinetics of indacaterol, glycopyrronium and mometasone furoate following once daily inhalation as a combination in healthy subjects. Pulm Pharmacol Ther. 2020. https://doi.org/10.1016/j.pupt.2020.101964.
Santus P, Radovanovic D, Cristiano A, et al. Role of nebulized glycopyrrolate in the treatment of chronic obstructive pulmonary disease. Drug Des Dev Ther. 2017;11:3257–71.
Merck Sharp & Dohme. Asmanex Twisthaler (mometasone furoate) inhalation powder prescribing information. 2014. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/205641s000lbl.pdf. Accessed 1 June 2020
Bruce C. Population pharmacokinetics: an overview. Aust Prescr. 2014;37:210–3.
Demin I, Bartels C, Graham G, et al. Population pharmacokinetics of IND/GLY (indacaterol/glycopyrronium) in COPD patients. Int J Clin Pharmacol Ther. 2016;54:405.
van Zyl-Smit RN, Krull M, Gessner C, et al. Once-daily mometasone plus indacaterol versus mometasone or twice-daily fluticasone plus salmeterol in patients with inadequately controlled asthma (PALLADIUM): a randomised, double-blind, triple-dummy, controlled phase 3 study. Lancet Respir Med. 2020;8(10):987–99.
Kerstjens HAM, Maspero J, Chapman KR, et al. Once-daily, single-inhaler mometasone-indacaterol-glycopyrronium versus mometasone-indacaterol or twice-daily fluticasone-salmeterol in patients with inadequately controlled asthma (IRIDIUM): a randomised, double-blind, controlled phase 3 study. Lancet Respir Med. 2020;8(10):1000–12.
Buhl R, Tanase A-M, Hosoe M, et al. A randomized, double-blind study to compare the efficacy and safety of two doses of mometasone furoate delivered via Breezhaler® or Twisthaler® in patients with asthma. Pulm Pharmacol Ther. 2020;62:1–10.
Kornmann O, Mucsi J, Kolosa N, et al. Efficacy and safety of inhaled once-daily low-dose indacaterol acetate/mometasone furoate in patients with inadequately controlled asthma: phase III randomised QUARTZ study findings. Respir Med. 2020;161:105809.
Bateman ED, Ferguson ET, Barnes N, et al. Dual bronchodilation with QVA149 versus single bronchodilator therapy: the SHINE study. Eur Respir J. 2013;42(6):1484–94.
Gastonguay MR. Full covariate models as an alternative to methods relying on statistical significance for inferences about covariate effects: a review of methodology and 42 case studies. Population Approach Group Europe (PAGE). 2011;20 Abstr 2229.
Onbrez Breezhaler: EPAR—Product Information. https://www.ema.europa.eu/en/documents/product-information/onbrez-breezhaler-epar-product-information_en.pdf. Accessed 2 Sep 2020.
Weers JG, Clark AR, Rao N, et al. In vitro-in vivo correlations observed with indacaterol-based formulations delivered with the Breezhaler. J Aerosol Med Pulm Drug Deliv. 2015;28:1–13.
Seebri Breezhaler: EPAR—Product Information. https://www.ema.europa.eu/en/documents/product-information/seebri-breezhaler-epar-product-information_en.pdf. Accessed 23 Mar 2021.
Bartels C, Looby M, Sechaud R, Kaiser G. Determination of the pharmacokinetics of glycopyrronium in the lung using a population pharmacokinetic modelling approach. Br J Clin Pharmacol. 2013;76:868–79.
Vaidya S, Ziegler D, Tanase AM, et al. Pharmacokinetics of mometasone furoate delivered via two dry powder inhalers. Pulm Pharmacol Ther. 2021. https://doi.org/10.1016/j.pupt.2021.102019(in press).
Forbes B, Asgharian B, Dailey LA, et al. Challenges in inhaled product development and opportunities for open innovation. Adv Drug Deliv Rev. 2011;63(1–2):69–87.
Bäckman P, Adelmann H, Petersson G, Jones CB. Advances in inhaled technologies: understanding the therapeutic challenge, predicting clinical performance, and designing the optimal inhaled product. Clin Pharmacol Ther. 2014;95(5):509–20.
Khindri S, Sabo R, Harris S, et al. Cardiac safety of indacaterol in healthy subjects: a randomized, multidose, placebo- and positive-controlled, parallel-group thorough QT study. BMC Pulm Med. 2011;11:31.
Drollmann A, Sechaud R, Pal P, et al. Glycopyrronium does not affect QT interval in healthy subjects: a randomized, three-period, cross-over, placebo- and positive-controlled study. Int J Clin Pharmacol Ther. 2014;52(9):739–45.
Affrime MB, Cuss F, Padhi D, et al. Bioavailability and metabolism of mometasone furoate following administration by metered-dose and dry-powder inhalers in healthy human volunteers. J Clin Pharmacol. 2000;40:1227–36.
Acknowledgements
Under the direction of authors, Vatsal Vithlani and Najeeb Ashraf (professional medical writers; Novartis) assisted in the preparation of this article in accordance with the third edition of Good Publication Practice guidelines.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The studies were funded by Novartis. Writing support was funded by study sponsors.
Conflict of interest
CB, MJ, JY, HCT are employees of Novartis. SV was an employee of Novartis at the time of conduct of study.
Ethics approval
Not applicable
Consent to participate
Not applicable
Consent to publication
Not applicable
Availability of data and material
Novartis is committed to sharing access to patient-level data and supporting documents from eligible studies with qualified external researchers. These requests are reviewed and approved by an independent review panel on the basis of scientific merit. All data provided are anonymized to respect the privacy of patients who have participated in the trial in line with applicable laws and regulations.
Code availability
Not applicable.
Author contributions
CB, MJ, JY, HCT and SY contributed to planning, design and data collection of this analysis. CB and JY contributed to data analysis. CB, MJ, JY, HCT and SY contributed to interpretation of the results, drafting and revising the manuscript and agree to be accountable for all aspects of the work.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Bartels, C., Jain, M., Yu, J. et al. Population Pharmacokinetic Analysis of Indacaterol/Glycopyrronium/Mometasone Furoate After Administration of Combination Therapies Using the Breezhaler® Device in Patients with Asthma. Eur J Drug Metab Pharmacokinet 46, 487–504 (2021). https://doi.org/10.1007/s13318-021-00689-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-021-00689-x