Abstract
Background and Objectives
Co-Crystal of Tramadol-Celecoxib (CTC) is a first-in-class active pharmaceutical ingredient (API–API) co-crystal of rac-tramadol.HCl and celecoxib in a 1:1 molecular ratio (100 mg CTC: 44 mg rac-tramadol.HCl and 56 mg celecoxib). Tramadol and celecoxib pharmacokinetics are modified after CTC administration versus administration of reference products. This randomised, open-label, crossover, phase 1 study assessed CTC pharmacokinetics, dose proportionality, safety and tolerability in Japanese and Caucasian subjects.
Methods
CTC (100, 150 and 200 mg) was administered orally to healthy Japanese/Caucasian subjects. Tramadol, O-desmethyltramadol and celecoxib plasma concentrations were determined pre-dose and up to 48 h post-dose. Maximum observed plasma concentration (Cmax), and area under the plasma concentration–time curve from dosing to last measurable concentration (AUCt) and from dosing extrapolated to infinity (AUC∞) were evaluated. Dose proportionality was assessed in a dose-adjusted bioavailability analysis of variance and in a power model. Inter-cohort comparability of pharmacokinetic exposure was confirmed if the ratio (Japanese cohort/Caucasian cohort) of geometric least-squares means and corresponding 90% confidence intervals were 80–125%. Post hoc weight-adjusted comparability analyses were performed. Safety was assessed throughout.
Results
Sixty subjects (21 males/9 females per cohort) were randomised; 57 completed the study. Cohorts were age and BMI matched; there were expected inter-cohort weight differences. Exposure to each analyte increased in both cohorts with increasing CTC dose. Tramadol’s pharmacokinetic exposure was comparable between cohorts after adjusting for body weight; the pharmacokinetic exposure of O-desmethyltramadol and celecoxib was increased in Japanese subjects.
Conclusions
Differences in pharmacokinetics were not sufficient to suggest that CTC dose adjustment is required in Japanese subjects.
Clinical Trial Registration
EudraCT: 2015-003071-29.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Co-Crystal of Tramadol-Celecoxib (CTC) is a first-in-class active pharmaceutical ingredient (API–API) co-crystal of rac-tramadol.HCl and celecoxib in a 1:1 molecular ratio (100 mg CTC: 44 mg rac-tramadol.HCl and 56 mg celecoxib). |
In this phase 1 study in Japanese and Caucasian subjects, the pharmacokinetic exposure of tramadol after CTC administration was comparable between the two cohorts (after adjusting for body weight). The pharmacokinetic exposure of O-desmethyltramadol and celecoxib was increased in Japanese subjects. CTC was well tolerated. |
Differences in pharmacokinetics following CTC administration were not sufficient to suggest that dose adjustment is required in Japanese subjects. |
1 Introduction
Co-crystallisation of an active pharmaceutical ingredient (API), with either a non-active coformer or another API, may provide improved physicochemical and pharmacokinetic characteristics, compared with commercially available single entity ‘reference’ products [1,2,3]. API–API co-crystals are novel drug formulations [1,2,3] and may be of particular value in the treatment of moderate to severe acute pain, where a multimodal approach to analgesia is recommended [4] and unmet needs, such as tolerability issues and limited efficacy, persist [4,5,6]. Co-Crystal of Tramadol-Celecoxib (CTC) is a first-in-class API–API co-crystal consisting of rac-tramadol.hydrochloride (HCl) and celecoxib in a 1:1 molecular ratio (so that 100 mg of CTC contains 44 mg rac-tramadol.HCl and 56 mg celecoxib). CTC combines four complementary mechanisms of action—cyclooxygenase-2 inhibition, µ-opioid receptor agonism, noradrenaline reuptake inhibition and serotonin reuptake inhibition—thereby providing a potential means of delivering multimodal analgesia [7].
CTC is in development for the treatment of moderate to severe acute pain. In vitro analytical studies demonstrated that physicochemical properties, such as intrinsic dissolution rates, of both tramadol and celecoxib are modified by co-crystallisation—the intrinsic dissolution rate of celecoxib is faster from the co-crystal than from celecoxib alone, and that of tramadol slower than from tramadol.HCl alone [7]. In preclinical in vivo studies, synergistic antinociceptive effects occurred without any such synergism in measures of adverse effects [8]. CTC (100, 150 and 200 mg) was efficacious in a phase 2 clinical trial of moderate to severe acute dental pain [9]. The clinical pharmacokinetics of CTC were established in two key phase 1 trials, in which administration of single or multiple doses of 200 mg CTC resulted in modified tramadol and celecoxib pharmacokinetic profiles, compared with administration of reference products alone or in free combination. For tramadol, a lower maximum observed plasma concentration (Cmax) and slightly prolonged time to Cmax (Tmax) were observed with CTC. The Cmax of celecoxib was lower than after administration of celecoxib alone and the Tmax faster than after either celecoxib alone or in free combination with tramadol [10, 11].
The majority of subjects in these phase 1 and 2 clinical trials were white or Caucasian [9,10,11]. The pharmacokinetics and metabolism of standard-formulation tramadol and celecoxib are affected by genetic polymorphisms of cytochrome P450 (CYP) 2D6 and CYP2C9, respectively [12, 13]. Certain polymorphisms are more common in some Asian populations, including in Japanese subjects [13,14,15,16,17]. Therefore, as part of the ongoing CTC clinical development programme, it was considered important to assess pharmacokinetics in Japanese subjects and to compare these with results obtained in Caucasian subjects. The primary objective of the current study was to assess the pharmacokinetics of three single doses of CTC (100, 150 and 200 mg) in both Japanese and Caucasian subjects. Secondary objectives were to assess the dose proportionality, safety and tolerability of these CTC doses.
2 Methods
2.1 Subjects
Subjects were male or female Japanese and Caucasian volunteers aged 20–45 years who were in good health according to medical history, physical examination, vital signs, electrocardiogram (ECG) and laboratory tests, and who had a body mass index (BMI) of 18.0–25.0 kg/m2 and body weight of 50–90 kg. Japanese and Caucasian cohorts were sex- and BMI matched, as closely as possible. Subjects were defined as Japanese if they were born in Japan, had two parents of Japanese descent and had not resided outside Japan for > 5 years. Key exclusion criteria included pregnancy and lactation; a history of significant gastrointestinal, liver or kidney disease, or other conditions that might affect drug pharmacokinetics; use of opioids or opioid antagonists within 30 days of enrolment; and use of any medication within 7 days of, or during, the study. Full inclusion and exclusion criteria are included in Online Resource 1.
2.2 Study Design
This phase 1 study (EudraCT No 2015-003071-29) was conducted between 20 October 2015 and 17 December 2015. The trial was a randomised, open-label, two-cohort, three-period, crossover, single-dose bioavailability study, conducted in a single centre in the UK by a contract research organisation (Richmond Pharmacology Ltd). Screening and subject eligibility assessments took place between days − 21 and − 1, and enrolment and randomisation took place between day − 1 and day 1. Subjects were randomised, via an automated system and central randomisation list, to receive each of three oral doses of CTC once over the course of three dosing periods, according to six pre-determined treatment sequences (Fig. 1). The treatments were 100 mg CTC (1 × 100 mg tablet consisting of 44 mg rac-tramadol.HCl and 56 mg celecoxib; Laboratorios del Dr. Esteve, S.A.U.), 150 mg CTC (1 × 150 mg tablet consisting of 66 mg rac-tramadol.HCl and 84 mg celecoxib) and 200 mg CTC (2 × 100 mg tablets, total dose consisting of 88 mg rac-tramadol.HCl and 112 mg celecoxib). Each dose was separated by a washout period of at least 10 days. For each dosing period, subjects entered the study clinic on day − 1 and remained until pharmacokinetic and safety assessments were completed at 48 h post-dose on day 3. Dosing occurred on day 1 of each period, under fasting conditions (subjects fasted from 10 h pre-dose until at least 4 h post-dose). Subjects received standardised meals and fluid intake during the remainder of each in-patient stay. During each in-patient stay and for 48 h before check-in to each study period subjects had to refrain from the consumption of grapefruit juice and any food or drinks containing alcohol, caffeine, poppy seeds or xanthine. Tobacco use was not permitted during the study or for 90 days before the first administration of CTC. A follow-up visit was conducted 7–10 days after final CTC administration. The maximum study length was 52 days per subject.
The study drug was manufactured and packaged according to Good Manufacturing Practice and supplied to the principal investigator in labelled containers by the manufacturer (Laboratorios del Dr. Esteve, S.A.U.).
The study protocol was approved by an ethics committee (South Central-Berkshire B Research Ethics Committee, UK; IRAS:188440; REC reference: 15/SC/0549). The study sponsor was Mundipharma Research Ltd. The study was performed in accordance with Good Clinical Practice guidelines, according to the principles of the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice guidelines. All participants provided written informed consent.
2.3 Blood Sampling
Blood samples were taken for pharmacokinetic analysis pre-dose and at 0.5, 0.75, 1, 1.25, 1.5, 1.75, 2, 2.5, 3, 3.5, 4, 5, 6, 8, 10, 12, 16, 24, 36 and 48 h after dosing in each of the three dosing periods. Methods for blood sample collection have been described in detail elsewhere [10]. In brief, blood samples were collected in K2-EDTA-containing tubes and centrifuged for 15 min at 1500 g and 4 °C, within 30 min of collection. Following centrifugation, plasma was separated and stored at −80 °C.
2.4 Bioanalytical Assay
Plasma concentrations of tramadol, O-desmethyltramadol (M1; tramadol’s primary metabolite) and celecoxib were measured using validated high-performance liquid chromatography with tandem mass spectrometry. Samples (0.05 mL) were extracted by solid-phase extraction and separated by reverse-phase liquid chromatography on a XBridge C18 column (3.5 μm 50 × 2.1 mm, Waters Corporation, Milford, MA, USA), using a gradient of water with 0.1% formic acid, and acetonitrile with 0.1% formic acid as a mobile phase. Detection was performed via tandem mass spectrometry on an API 4000 triple quadrupole mass spectrometer (Sciex, Ontario, Canada) using Turbo Ion spray ionisation. Propranolol and E-6087, a cyclooxygenase-2 inhibitor, were used as internal standards. Linearity and range; selectivity; specificity in the presence of concomitant medication; intra- and inter-run precision and accuracy; limits of quantification; dilution integrity; carry-over; recovery; matrix effect; and stability in an autosampler at room temperature, after freeze–thaw cycles and long-term, were evaluated during method validation. The lower and upper limits of quantification were 4.00 and 640.00, 1.00 and 160.00, and 2.50 and 1000.00 ng/mL, for tramadol, M1 and celecoxib, respectively. Samples below these limits were not quantified and were considered to be below the lower limit of quantification. Samples above the limit of quantification were reassayed after dilution, using a dilution factor equal to or lower than the maximum dilution factor tested during method validation (dilution factor of 10). The inter-run accuracy (nominal values) varied from 99.81 to 103.65% for tramadol, 96.21 to 103.69% for M1, and 105.84 to 108.68% for celecoxib, and 94.2, 92.7 and 109.5% for limits of quantification, respectively. The inter-run precision (coefficient of variation) varied from 4.77 to 8.67% for tramadol, 7.67 to 9.38% for M1 and 6.19 to 7.05% for celecoxib, and 15.5, 15.3 and 10.1% for limits of quantification, respectively. Assay specificity was assessed using six independent matrix sources from Caucasian and Japanese volunteers, verified for the absence of interference and compared with the respective limits of quantification at the retention times and mass transitions of analytes and internal standards. Absolute recovery was 97.9–99.2, 96.5–101.0 and 74.1–78.0% for tramadol, M1 and celecoxib, respectively. The stability of the samples at the bench was 25 h and samples were stable under three freeze–thaw cycles. The maximum sample storage duration was 76 days; long-term stability of frozen samples was 160 days.
2.5 Pharmacokinetic Parameters
The primary pharmacokinetic parameters were tramadol and celecoxib Cmax and area under the plasma concentration–time curve measured from the time of dosing to the last measurable concentration (AUCt). Other pharmacokinetic parameters included area under the plasma concentration–time curve measured from the time of dosing and extrapolated to infinity (AUC∞); time to Cmax (Tmax); and terminal phase half-life (T½Z).
2.6 Safety
Safety was evaluated via the assessment of adverse events (AEs), standard laboratory evaluations, physical examination, vital signs (including pulse oximetry) and 12-lead ECG. AEs were monitored throughout the study and severity was graded by the investigator. All other safety assessments were conducted during initial screening and at the end-of-study follow-up visit. In each dosing period, vital signs were also assessed pre-dose and at 1, 2, 4, 6, 8, 12, 16, 24, 36 and 48 h post-dose; physical examination was conducted pre-dose and at 48 h post-dose; and ECG at 2 h post-dose.
2.7 Data Analyses
The primary objective was not associated with any formal statistical hypotheses and therefore sample size was calculated based on the number of subjects required to assess the dose proportionality of CTC in each cohort (a secondary study objective). Using a dose-adjusted bioavailability analysis of variance (ANOVA) model, dose proportionality of two CTC doses would be demonstrated if the 90% confidence intervals (CI) for the geometric least-squares means of AUCt and Cmax fell entirely between 80.00 and 125.00%. The sample size calculation was performed using nQuery (Cork, Ireland) applying the t test of equivalence in ratio of means for crossover designs, assuming true ratios of 100% between CTC doses and a standard deviation of the period differences on the log scale of 0.330. No adjustment was made for multiple testing. Twenty-six subjects were calculated to be required per cohort to provide 90% power to determine dose proportionality. With an assumption that approximately 10% of randomised subjects would not be eligible for inclusion in the pharmacokinetic population or would not provide sufficient valid pharmacokinetic parameters to be included in the statistical analysis for a comparison, 30 subjects were randomised within each cohort.
The intention-to-treat (ITT) population consisted of all randomised subjects. The pharmacokinetic population consisted of all subjects who received one dose of CTC and from whom at least one valid pharmacokinetic parameter was recorded. The safety population consisted of all subjects who received at least one dose of CTC.
Pharmacokinetic parameters were summarised descriptively by CTC dose in each cohort. AUC∞ was calculated from the ratio of the final observed plasma concentration to the terminal phase rate constant; this was added to AUCt. (AUCt was calculated using the linear up/log down trapezoidal method). Pharmacokinetic calculations were conducted using Phoenix WinNonlin, Version 6.4.
Within-cohort dose proportionality for tramadol and celecoxib was calculated by evaluating AUCt, AUC∞ and Cmax using two methods; the dose-adjusted bioavailability ANOVA model and a power model. In the ANOVA, proportionality of two CTC doses was demonstrated if 90% CI values for the ratio of geometric least-squares means for AUCt, AUC∞ and Cmax fell between 80.00 and 125.00%. Dose proportionality was calculated using 100 mg CTC as a reference dose (i.e. 150 mg CTC and 200 mg CTC were each compared to 100 mg CTC). In the power model, dose proportionality was demonstrated if the 90% CIs of the slope estimates fell entirely between the critical region (0.6781–1.3219). The key modelling assumption in the power model was that the logarithm of each pharmacokinetic variable was linearly related to the logarithm of the dose.
For comparisons of pharmacokinetic exposure across cohorts, Cmax, AUCt and AUC∞ were analysed on a log scale using an ANOVA model with fixed effects for sequence, period and cohort. Test/reference (Japanese cohort/Caucasian cohort) ratios were calculated by transforming the difference between the natural log least-squares means back to the original scale and multiplying by 100. Comparability of tramadol, M1 or celecoxib pharmacokinetic exposure between cohorts was to be assumed if the 90% CIs for a test/reference ratio were > 80 and < 125%. A post hoc weight-adjusted inter-cohort comparability analysis was also performed. Statistical programming and analyses were performed using SAS® version 9.3 or higher (SAS Institute, Cary, USA).
3 Results
3.1 Subjects
One hundred and twenty-seven subjects were assessed for study eligibility; of these, 67 failed screening and 60 participated in the study. All 60 study subjects were randomised and included in the ITT, pharmacokinetic and safety analysis populations (30 in each cohort). Overall, 57 subjects completed the study [29 (96.7%) subjects in the Japanese cohort and 28 (93.3%) subjects in the Caucasian cohort; Fig. 1]. One subject from the Japanese cohort withdrew consent (and left the study and returned to Japan) prior to administration of 200 mg CTC in the third dosing period. Two Caucasian subjects discontinued prematurely, one due to a protocol violation [a positive test for cannabis at the second dosing period check-in (subject did not receive 100 or 150 mg CTC)] and one due to a non-serious tooth infection (subject did not receive 200 mg CTC in the third dosing period). Another Caucasian subject experienced regurgitation during the third dosing period (during which the subject had received 200 mg CTC), which invalidated the pharmacokinetic data obtained in this period. Subjects without valid data for a specific dosing period were excluded from the pharmacokinetic analyses for that dose. In the case of safety analyses, subjects were included for those doses that they received.
The mean age of subjects in the Japanese and Caucasian cohorts was 31.5 and 30.0 years, respectively. Both cohorts included 21 males and 9 females. Although cohorts were BMI matched—with mean BMI values of 21.6 and 22.1 in the Japanese and Caucasian cohorts, respectively—Japanese subjects were, on average, shorter and lighter than Caucasian subjects (Table 1).
3.2 Pharmacokinetics
3.2.1 Tramadol
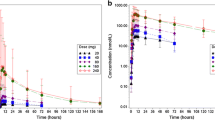
Tramadol plasma concentration–time curves obtained after administration of CTC to Japanese and Caucasian subjects are shown in Fig. 2a and b, respectively. In both cohorts, tramadol Cmax, AUCt and AUC∞ exhibited dose proportionality, as assessed by dose-adjusted bioavailability analysis and power model analysis (Tables 2 and 3, respectively).
Tmax values were between 2.75 and 3.50 h (Table 4). Comparability of primary pharmacokinetic parameters between the Japanese and Caucasian cohorts could not be formally demonstrated for tramadol as the upper 90% CI values for test/reference ratios exceeded 125% (Table 5). However, when repeating the analysis adjusting for subjects’ weight, Cmax for tramadol was comparable in the two cohorts for all three doses (estimated ratios of 105.56, 109.25 and 108.73% for 100, 150 and 200 mg CTC, respectively, with 90% CIs between 80 and 125%; Table 5). While weight-adjusted AUC ratios fell between 80 and 125%, most upper 90% CI values exceeded 125% (Table 5).
3.2.2 M1
Mean M1 plasma concentration–time curves in Japanese and Caucasian subjects are shown in Fig. 3a and b. In both cohorts, Cmax, AUCt and AUC∞ increased with increasing dose (Table 6).
Tmax was between 3.50 and 4.00 h (Table 6). In inter-cohort assessments, the majority of test/reference ratios and all upper 90% CI values for 100, 150 and 200 mg CTC were outside the pre-determined comparability range, including after adjustment for body weight (Table 7).
M1/tramadol ratios were similar between cohorts and across CTC doses. For mean AUCt, M1/tramadol ratios ranged from 0.1848 (200 mg CTC) to 0.2084 (100 mg) in the Japanese cohort and 0.1996 (200 mg CTC) to 0.2129 (150 mg) in the Caucasian cohort. For mean AUC∞, M1/tramadol ratios ranged from 0.1902 (200 mg CTC) to 0.2171 (100 mg) in the Japanese cohort and 0.2123 (200 mg CTC) to 0.2263 (150 mg) in the Caucasian cohort. One Caucasian subject consistently demonstrated high tramadol levels and low M1 levels, resulting in low M1/tramadol ratios (0.0146–0.0223).
3.2.3 Celecoxib
Mean celecoxib plasma concentration–time curves are shown in Fig. 4a and b. Celecoxib Cmax, AUCt and AUC∞ exhibited dose proportionality in both cohorts (Table 2 and Table 3), with the exception of Cmax in the Caucasian cohort, for which dose proportionality could not be demonstrated in the power model analysis (Table 3).
Tmax values were between 1.75 and 3.00 h (Table 8). Inter-cohort comparability was demonstrated for AUC parameters at a dose of 100 mg CTC and similar results were seen for AUC∞ at 150 mg. AUCt was increased in the Japanese cohort (upper 90% CI values exceeded 125%) after 150 and 200 mg CTC (Table 9). Inter-cohort differences were similar for Cmax and AUCt after body weight adjustment. In the case of AUC∞, inter-cohort differences were absent across all three doses after body weight adjustment (Table 9).
3.3 Safety
No severe AEs, serious AEs or deaths were reported and there were no clinically significant changes in laboratory parameters, vital signs or ECGs. The proportion of subjects experiencing at least one AE after administration of each CTC dose is shown in Table 10. Overall, a total of 19 AEs occurred in 10 (33.3%) Japanese subjects and 34 AEs occurred in 17 (56.7%) Caucasian subjects. Eleven AEs in 5 (16.7%) Japanese subjects were considered treatment-related, as were 19 AEs in 10 (33.3%) Caucasian subjects. Most AEs were mild and occurred only once per cohort or treatment group. Of those occurring in more than 5% of subjects after any treatment, nausea was reported in 2 (6.7%) Japanese and 5 (16.7%) Caucasian subjects, dizziness in 3 (10.0%) Japanese and 2 (6.7%) Caucasian subjects, fatigue in 2 (6.7%) Japanese subjects and hyperhidrosis in 2 (6.7%) Caucasian subjects (Table 10).
4 Discussion
In the current study, exposure to tramadol, M1 and celecoxib increased with the administration of increasing doses of CTC in both Japanese and Caucasian cohorts. The pharmacokinetic exposure of tramadol was comparable between these two cohorts after adjusting for body weight, while that of M1 and celecoxib was increased in Japanese subjects. M1/tramadol ratios were similar between cohorts and across CTC doses. CTC was well tolerated at all doses in both cohorts. Although the highest number of AEs occurred after administration of 200 mg CTC in both cohorts, the difference between dose groups was rather small overall and thus may not indicate that AEs were dose related. In summary, the study’s findings suggest that, relative to doses provided to Caucasian subjects, no dose adjustment is required before CTC is administered to Japanese subjects.
This open-label study was randomised in nature and used cohorts that were well matched with respect to sex and BMI and of an adequate size to address the research question under investigation. The data reported here add to those obtained from earlier key phase 1 studies of CTC, conducted primarily in white or Caucasian subjects [10, 11]. In an earlier single-dose (200 mg CTC) phase 1 study, Tmax values were slightly prolonged in the case of tramadol and were faster in the case of celecoxib, compared with those obtained after administration of reference products alone or in free combination. Cmax values for tramadol and celecoxib were lower than after administration of reference products alone, as were AUC values for celecoxib (those for tramadol were similar to reference products alone) [11]. In a multiple-dose study, tramadol Cmax and AUC were lower and Tmax delayed after administration of CTC compared with the individual reference product alone or in free combination with celecoxib. In the case of celecoxib, Cmax and AUC values were lower after CTC administration than after administration of celecoxib alone. Celecoxib’s Cmax was higher and Tmax faster than for celecoxib administered in free combination with tramadol [10]. These pharmacokinetic profiles are broadly in line with those seen in the present study. The most common AEs in the present study were consistent with those reported for standard-formulation tramadol and celecoxib [18, 19], and with those reported in earlier trials of CTC [9,10,11].
Tramadol is largely metabolised by the isoenzyme CYP2D6. Levels of CYP2D6 activity are known to affect the pharmacokinetics of both tramadol and M1, including in patients with pain [18, 20,21,22], and some studies report similar findings for CYP3A4 [18, 20,21,22]. CYP2D6 is subject to genetic polymorphisms that can have clinically relevant effects on analgesia [23], and tramadol pharmacogenomics is an area of growing importance in determining clinical treatment regimens [24]. Polymorphisms affecting tramadol pharmacokinetics, in particular CYP2D6*10, have been reported in some Asian populations [13, 14, 25, 26], including in Japanese subjects [15]. Differences in CYP3A4 expression levels between Japanese and Caucasian cohorts have also been reported [27]. CYP2D6*10 is associated with a low conversion of tramadol to M1 and is thought to have a high incidence in Japanese populations, occurring in approximately 20–45% of individuals [28]. In the present study, one subject (in the Caucasian cohort) appeared to have low M1/tramadol ratios and was therefore considered to possibly be a poor CYP2D6 metaboliser (although genotyping did not form part of the study). The influence of genetic polymorphisms on the metabolism of celecoxib is less clear. Celecoxib is primarily metabolised by CYP2C9 [19, 29], and while polymorphisms such as CYP2C9*3 have been reported to affect pharmacokinetics, including in Asian populations [16, 17], the clinical relevance of these findings is still subject to debate [29,30,31]. In the current study, some differences were seen in the pharmacokinetic exposure of tramadol and M1, although these were primarily accounted for by differences in body weight in the case of tramadol. Therefore, although genotyping was not conducted, these data suggest that CYP2D6 polymorphism status did not have a substantial impact on the pharmacokinetic exposure of tramadol after CTC administration in this study. Slight increases in celecoxib exposure were seen in Japanese versus Caucasian cohorts.
5 Conclusion
In conclusion, differences in tramadol, M1 or celecoxib pharmacokinetics between Japanese and Caucasian subjects following CTC administration were not sufficient to suggest that dose adjustment is required in Japanese subjects. In addition, exposure to each API within CTC increased with CTC dose in both populations and CTC was well tolerated in both cohorts. Following demonstration of efficacy in a phase 2 study of acute pain following an oral surgical procedure [9], CTC is now under evaluation in phase 3 studies of bunionectomy with osteotomy [32], acute pain following an oral surgical procedure [33] and hysterectomy-related pain [34].
References
Shan N, Perry ML, Weyna DR, Zaworotko MJ. Impact of pharmaceutical cocrystals: the effects on drug pharmacokinetics. Expert Opin Drug Metab Toxicol. 2014;10(9):1255–71. https://doi.org/10.1517/17425255.2014.942281.
Sekhon BS. Drug–drug co-crystals. Daru. 2012;20(1):45. https://doi.org/10.1186/2008-2231-20-45.
Duggirala NK, Perry ML, Almarsson O, Zaworotko MJ. Pharmaceutical cocrystals: along the path to improved medicines. Chem Commun (Camb). 2016;52(4):640–55. https://doi.org/10.1039/c5cc08216a.
Chou R, Gordon DB, de Leon-Casasola OA, Rosenberg JM, Bickler S, Brennan T, et al. Management of postoperative pain: a Clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17(2):131–57. https://doi.org/10.1016/j.jpain.2015.12.008.
Yaksh TL, Woller SA, Ramachandran R, Sorkin LS. The search for novel analgesics: targets and mechanisms. F1000Prime Rep. 2015;7:56. https://doi.org/10.12703/p7-56.
Moore RA, Derry S, Aldington D, Wiffen PJ. Single dose oral analgesics for acute postoperative pain in adults—an overview of Cochrane reviews. Cochrane Database Syst Rev. 2015;9:CD008659. https://doi.org/10.1002/14651858.cd008659.pub3.
Almansa C, Mercè R, Tesson N, Farran J, Tomàs J, Plata-Salamán CR. Co-Crystal of Tramadol hydrochloride–Celecoxib (CTC): a novel API–API co-crystal for the treatment of pain. Cryst Growth Des. 2017;17(4):1884–92. https://doi.org/10.1021/acs.cgd.6b01848.
Merlos M, Portillo-Salido E, Brenchat A. Administration of tramadol and celecoxib in a 1:1 molecular ratio produces synergistic antinociceptive effects in postoperative pain models: Preclinical rationale for clinical development of Co-Crystal of Tramadol–Celecoxib. IASP 2016; Yokohama: Abstract 2312 Poster Number PW.290.
López-Cedrún J, Videla S, Burgueño M, Juárez I, Aboul-Hosn S, Martín-Granizo R, et al. Co-Crystal of Tramadol–Celecoxib in patients with moderate to severe acute post-surgical oral pain: a dose-finding, randomised, double-blind, placebo- and active-controlled, multicentre, phase II trial. Drugs R D. 2018;18(2):137–48. https://doi.org/10.1007/s40268-018-0235-y.
Videla S, Lahjou M, Vaque A, Sust M, Escriche M, Soler L, et al. Pharmacokinetics of multiple doses of Co-Crystal of Tramadol–Celecoxib: findings from a 4-way randomized open-label phase I clinical trial. Br J Clin Pharmacol. 2018;84(1):64–78. https://doi.org/10.1111/bcp.13428.
Videla S, Lahjou M, Vaque A, Sust M, Encabo M, Soler L, et al. Single-dose pharmacokinetics of Co-Crystal of Tramadol–Celecoxib: results of a four-way randomized open-label phase I clinical trial in healthy subjects. Br J Clin Pharmacol. 2017;83(12):2718–28. https://doi.org/10.1111/bcp.13395.
Wang B, Wang J, Huang SQ, Su HH, Zhou SF. Genetic polymorphism of the human cytochrome P450 2C9 gene and its clinical significance. Curr Drug Metab. 2009;10(7):781–834.
Leppert W. CYP2D6 in the metabolism of opioids for mild to moderate pain. Pharmacology. 2011;87(5–6):274–85. https://doi.org/10.1159/000326085.
Gan SH, Ismail R, Wan Adnan WA, Zulmi W. Impact of CYP2D6 genetic polymorphism on tramadol pharmacokinetics and pharmacodynamics. Mol Diagn Ther. 2007;11(3):171–81.
Kubota T, Yamaura Y, Ohkawa N, Hara H, Chiba K. Frequencies of CYP2D6 mutant alleles in a normal Japanese population and metabolic activity of dextromethorphan O-demethylation in different CYP2D6 genotypes. Br J Clin Pharmacol. 2000;50(1):31–4.
Kim SH, Kim DH, Byeon JY, Kim YH, Kim DH, Lim HJ, et al. Effects of CYP2C9 genetic polymorphisms on the pharmacokinetics of celecoxib and its carboxylic acid metabolite. Arch Pharm Res. 2017;40(3):382–90. https://doi.org/10.1007/s12272-016-0861-2.
Liu R, Gong C, Tao L, Yang W, Zheng X, Ma P, et al. Influence of genetic polymorphisms on the pharmacokinetics of celecoxib and its two main metabolites in healthy Chinese subjects. Eur J Pharm Sci. 2015;79:13–9. https://doi.org/10.1016/j.ejps.2015.09.005.
European Medicines Agency. Summary of product characteristics for tramadol hydrochloride 50 mg capsules. 2017. http://www.medicines.org.uk/EMC/medicine/24186/SPC. Accessed 23 Oct 2017.
European Medicines Agency. Summary of product characteristics for Celebrex 100 mg capsule. 2016. http://www.medicines.org.uk/emc/medicine/14534. Accessed 25 Oct 2017.
Saarikoski T, Saari TI, Hagelberg NM, Backman JT, Neuvonen PJ, Scheinin M, et al. Effects of terbinafine and itraconazole on the pharmacokinetics of orally administered tramadol. Eur J Clin Pharmacol. 2015;71(3):321–7. https://doi.org/10.1007/s00228-014-1799-2.
Stamer UM, Musshoff F, Kobilay M, Madea B, Hoeft A, Stuber F. Concentrations of tramadol and O-desmethyltramadol enantiomers in different CYP2D6 genotypes. Clin Pharmacol Ther. 2007;82(1):41–7. https://doi.org/10.1038/sj.clpt.6100152.
de Moraes NV, Lauretti GR, Coelho EB, Godoy AL, Neves DV, Lanchote VL. Impact of fraction unbound, CYP3A, and CYP2D6 in vivo activities, and other potential covariates to the clearance of tramadol enantiomers in patients with neuropathic pain. Fundam Clin Pharmacol. 2016;30(2):153–61. https://doi.org/10.1111/fcp.12168.
Lassen D, Damkier P, Brosen K. The pharmacogenetics of tramadol. Clin Pharmacokinet. 2015;54(8):825–36. https://doi.org/10.1007/s40262-015-0268-0.
Miotto K, Cho AK, Khalil MA, Blanco K, Sasaki JD, Rawson R. Trends in tramadol: pharmacology, metabolism, and misuse. Anesth Anal. 2017;124(1):44–51. https://doi.org/10.1213/ane.0000000000001683.
Xu J, Zhang XC, Lv XQ, Xu YY, Wang GX, Jiang B, et al. Effect of the cytochrome P450 2D6*10 genotype on the pharmacokinetics of tramadol in post-operative patients. Pharmazie. 2014;69(2):138–41.
Li Q, Wang R, Guo Y, Wen S, Xu L, Wang S. Relationship of CYP2D6 genetic polymorphisms and the pharmacokinetics of tramadol in Chinese volunteers. J Clin Pharm Ther. 2010;35(2):239–47. https://doi.org/10.1111/j.1365-2710.2009.01102.x.
Yamaori S, Yamazaki H, Iwano S, Kiyotani K, Matsumura K, Saito T, et al. Ethnic differences between Japanese and Caucasians in the expression levels of mRNAs for CYP3A4, CYP3A5 and CYP3A7: lack of co-regulation of the expression of CYP3A in Japanese livers. Xenobiotica. 2005;35(1):69–83. https://doi.org/10.1080/00498250400021796.
Raffa RB. Tramadol in Japanese population: the relative contribution of M1 metabolite as assessed by CYP2D6*10 genotype. Pharmacol Pharm. 2012;3:337–41. https://doi.org/10.4236/pp.2012.33045.
Gong L, Thorn CF, Bertagnolli MM, Grosser T, Altman RB, Klein TE. Celecoxib pathways: pharmacokinetics and pharmacodynamics. Pharmacogenet Genom. 2012;22(4):310–8. https://doi.org/10.1097/FPC.0b013e32834f94cb.
Kirchheiner J, Stormer E, Meisel C, Steinbach N, Roots I, Brockmoller J. Influence of CYP2C9 genetic polymorphisms on pharmacokinetics of celecoxib and its metabolites. Pharmacogenetics. 2003;13(8):473–80.
Prieto-Perez R, Ochoa D, Cabaleiro T, Roman M, Sanchez-Rojas SD, Talegon M, et al. Evaluation of the relationship between polymorphisms in CYP2C8 and CYP2C9 and the pharmacokinetics of celecoxib. J Clin Pharmacol. 2013;53(12):1261–7. https://doi.org/10.1002/jcph.169.
ClinicalTrials.gov. Co-crystal E-58425 vs tramadol and celecoxib for moderate to severe acute pain after bunionectomy. Phase III clinical trial. 2017. https://clinicaltrials.gov/ct2/show/NCT03108482. Accessed 18 Dec 2017.
ClinicalTrials.gov. Efficacy and safety in a randomised acute pain study of MR308 (Tramadol/Celecoxib). (STARDOM1). 2017. https://clinicaltrials.gov/ct2/show/NCT02982161?term=MR308&rank=1. Accessed 12 Oct 2017.
ClinicalTrials.gov. Efficacy and safety in a randomised acute pain study of MR308: STARDOM2. (STARDOM2). 2017. https://clinicaltrials.gov/ct2/show/NCT03062644?term=MR308&rank=2. Accessed 12 Oct 2017.
Acknowledgements
We thank all study participants and staff. The study was funded by Mundipharma Research Ltd (Cambridge, UK) and was carried out by Richmond Pharmacology Ltd (London, UK). Bioanalytical analyses were conducted by Laboratorios del Doctor Esteve, S.A.U. (ESTEVE). Pharmacokinetic analyses were conducted by Mundipharma Research Ltd. Medical writing support was provided by Hannah Mace MPharmacol, CMPP at Aspire Scientific (Bollington, UK), and was funded by Mundipharma Research GmbH & Co. KG (Limburg, Germany). CTC is in development by Mundipharma Research and ESTEVE, as MR308 and E-58425.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by Mundipharma Research Ltd (Cambridge, UK).
Conflict of interest
UL is an employee of Richmond Pharmacology Ltd. MEn, MEs and GE are employees of Esteve. HD, GM, CB and KS are employees of Mundipharma Research Ltd. SM was an employee of Mundipharma Research GmbH & Co. KG at the time the study was conducted. Esteve and Mundipharma are in collaboration for the development and commercialisation of CTC.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (South Central-Berkshire B Research Ethics Committee, UK; IRAS:188440; REC reference: 15/SC/0549) and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Dooner, H., Mundin, G., Mersmann, S. et al. Pharmacokinetics of Tramadol and Celecoxib in Japanese and Caucasian Subjects Following Administration of Co-Crystal of Tramadol-Celecoxib (CTC): A Randomised, Open-Label Study. Eur J Drug Metab Pharmacokinet 44, 63–75 (2019). https://doi.org/10.1007/s13318-018-0491-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-018-0491-9