Abstract
Background and Objectives
Acute kidney injury (AKI) is frequently observed after heart transplantation and is associated with morbidity and mortality. However, many confounding factors also contribute to the development of AKI in heart transplants. We hypothesized that supratherapeutic whole-blood tacrolimus trough concentrations are associated with AKI.
Methods
In a retrospective observational cohort from April 2005 to December 2012, all adult heart transplantation patients were included. AKI was assessed in the first 2 weeks after transplantation as classified by the Kidney Disease Improving Global Outcomes Network (KDIGO). Whole-blood tacrolimus trough concentrations were determined from day 1 to day 14 and at 1, 3, 6 and 12 months post-transplantation. The therapeutic range was 9 to 15 ng/ml in the first 2 months and tapered to 5–8 ng/ml thereafter. The relationship between supratherapeutic tacrolimus trough concentrations and AKI was evaluated. The impact of various potentially confounding factors on tacrolimus concentrations and AKI was considered.
Results
We included 110 patients. AKI occurred in 57% of patients in the first week. Recovery from AKI was seen in 24%. The occurrence of chronic kidney disease (CKD) was 19% at 1 year. Whole-blood tacrolimus trough concentrations were often supratherapeutic and, despite correction for confounding factors, independently associated with AKI (OR 1.66; 95% CI 1.20–2.31).
Conclusions
Supratherapeutic whole-blood tacrolimus trough concentrations are independently associated with the development of AKI in adult heart transplantation patients. More stringent dosing of tacrolimus early after transplantation may be critical in preserving the kidney function.
Similar content being viewed by others
AKI often occurs in the early phase after heart transplantation, is caused by variable factors and is associated with morbidity and mortality |
Supratherapeutic whole-blood tacrolimus trough concentrations often occur early after heart transplantation |
A supratherapeutic whole-blood tacrolimus trough concentration is a cofactor of AKI in the first week after heart transplantation |
1 Introduction
Over 104,000 heart transplantations have been performed worldwide since 1967 [1]. The immunosuppressive regimen has improved considerably since then [1,2,3]. The introduction of tacrolimus, a very effective immunosuppressive drug, has substantially contributed to increased survival and decreased rejection rates [1,2,3,4]. Despite its success, tacrolimus often has serious side effects, such as nephrotoxicity [3]. Tacrolimus-induced nephrotoxicity frequently evolves into chronic kidney disease (CKD) [5]. The occurrence of CKD in heart-transplanted patients is reported to be 26% after 1 year, 52% after 5 years and in 68% by 10 years. Of these patients, 81% has been treated with the immunosuppressant tacrolimus as the preferred calcineurin inhibitor [6]. It has been acknowledged that CKD after heart transplantation contributes considerably to increasing mortality rates over time [7,8,9]. Logically, prevention of acute kidney injury (AKI) might prevent subsequent CKD in heart transplants [10].
The etiology of AKI in the perioperative phase is often multifactorial. Various factors collectively contribute to the development of AKI, e.g., a high baseline creatinine, a long surgery time, the use of cardiopulmonary bypass, shock, inflammation, the administration of blood products, and nephrotoxic drugs [10,11,12,13,14]. At present, the evidence on tacrolimus trough concentrations being related to AKI after heart transplantation is circumstantial and largely derived from other solid organ transplantations. Therefore, the association between AKI after heart transplantation and tacrolimus is still not fully elucidated.
Our research hypothesis was that supratherapeutic whole-blood tacrolimus trough concentrations are an independent factor in the development of AKI in adult heart transplant recipients. In addition, we analyzed whether AKI after heart transplantation is associated with subsequent development to CKD.
2 Patients and Methods
2.1 Inclusion and Exclusion Criteria
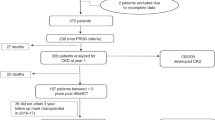
Data of all heart transplantation patients at the University Medical Center Utrecht between April 2005 and December 2012 were retrospectively examined. No multi-organ transplantations were performed. Patients who died within 24 h were excluded as well as patients with preoperative glomerular filtration rate (GFR) < 40 ml/min defined by the Modification of Diet in Renal Disease formula (MDRD) [15]. In these patients, tacrolimus was postponed for several days and basiliximab was used as immunosuppression. Patients who have died on the first day could not be analyzed for kidney injury, because of low exposure to tacrolimus and the delayed increase in plasma creatinine.
2.2 Immunosuppressive Regimen and Dosing
The protocol of the transplantation center demanded that tacrolimus was started at an oral dose of 2 mg twice daily. Further, dosing was based on tacrolimus whole-blood trough concentrations at 6 a.m. (12 h post-dose). A whole-blood tacrolimus trough concentration between 9 and 15 ng/ml was considered therapeutic in the first 2 months and thereafter tapered towards 5–8 ng/ml providing no rejection was encountered [16]. Blood samples for tacrolimus concentration were immediately drawn before the administration and, therefore, represent trough levels. Steady state was not needed for dose adjustments. Corrections on basis of trough concentrations, kidney and liver function, gut motility and interactions with other drugs were left to the discretion of the transplantation cardiologist. Accompanying immunosuppression comprised corticosteroids [prednisolone 50 mg intravenously directly postoperative followed by 25 mg twice daily and tapered off to 20 mg twice daily orally after 6 days] and mycophenolate mofetil [1000 mg orally twice daily]. Basiliximab was not administered in combination with tacrolimus.
2.3 Tacrolimus Assay
The measurements from days 1 to 14, and at 1, 3, 6 and 12 months after transplantation were used for analysis using a micro-particle enzyme immunoassay in accordance with the required quality standards. The lower limit of quantification was 2 ng/ml and intraday imprecision was ± 15% (Abbott IMx™ assay II, Abbott laboratories, Malvern, USA) [17].
2.4 Definition of Kidney Injury
Acute kidney injury (AKI) was classified according to the Kidney Disease Improving Global Outcomes Network (KDIGO) criteria, which distinguishes three classes (See also online resource Table S.1) [18]. Urine data were unavailable; therefore, AKI classification was solely based on serum creatinine concentration: AKI stage 1; Increase in serum creatinine ≥ 26 μmol/L or 150–200% from baseline, AKI stage 2; Increase in serum creatinine > 200% and ≤ 300% from baseline, AKI stage 3; Increase in serum creatinine > 300% or ≥ 354 μmol/L with an acute increase of minimally 44 μmol/L or initiation of renal replacement therapy. Baseline creatinine was the last creatinine prior to surgery. Indications for renal replacement therapy were stage 3 combined with one of the following characteristics: hyperkalemia, severe hypervolemia, uncorrectable metabolic acidosis and serious uremia.
Recovery of AKI was defined as a reduction in peak AKI stage within 3 days, which is consistent for 48 h between day 1 to day 14 [19]. Persistence of AKI was determined at 1 month by comparing the creatinine at 1 month to the baseline creatinine among patients with AKI between day 1 and day 14. Chronic kidney disease (CKD) was determined as the estimated GFR using the “CKD Epidemiology Collaboration equation” [20]. CKD was defined as having a stage 3, 4 or 5 and was assessed after 3 and 6 months and 1 year.
2.5 Collection and Definitions of other Covariates Considered in the Analyses
Drugs interacting with tacrolimus were also documented. Co-medication increasing tacrolimus blood concentrations encompassed macrolides, azoles, calcium antagonists, haloperidol and amiodarone (Additional information is given in the online resource Table S.1, showing the definitions of the covariates). Corticosteroids are known to decrease tacrolimus blood concentrations [21]. Liver dysfunction increases tacrolimus blood concentrations. Liver injury was defined as bilirubin > 34 μmol/L or alanine aminotransferase (ALT) > 90 U/L for men and > 70 U/L for women [22].
Systemic inflammatory response syndrome (SIRS) was determined according to the definition of the American College of Chest Physicians (ACCP) and the Society of Critical Care Medicine Consensus Conference (SCCM) [23]. The only modification to the SIRS criteria was that the heart frequency, providing one SIRS point, was established as 100/min instead of 90/min, because in many heart transplant patients the standard pacemaker configuration is set at 100/min. Shock was specified as mean arterial pressure < 60 mmHg or use of at least one of the following vasopressors/inotropes: norepinephrine, epinephrine, phenylephrine, vasopressin, dopamine, dobutamine or milrinone. We also collected information on potentially nephrotoxic drugs other than tacrolimus that were used in this cohort of heart transplantation patients, particularly (val)acyclovir or (val)ganciclovir (CMV prophylaxis), tobramycin, gentamicin, trimethoprim/sulfamethoxazole (Pneumocystis jiroveci prophylaxis), penicillins, furosemide, vancomycin and amphotericin B. The covariates SIRS, shock and nephrotoxic drugs were not available between days 7 and 14.
2.6 Statistics
Statistical analyses are outlined in the online resource Table S.2. Variables are presented as mean (with standard deviation [SD]), median (with interquartile range [IQR]) or number (proportion) where appropriate. A Generalized Estimating Equations (GEE) procedure was used to test whether AKI was significantly associated with prior supratherapeutic whole-blood tacrolimus trough concentrations, after correction for possible confounders like shock and baseline characteristics (preoperative ventricular assist device (VAD), ischemic cardiomyopathy (ICM), diabetes mellitus (DM), surgery time > 400 min and extracorporeal membrane oxygenation postoperative (ECMO)). GEE analysis was chosen, because it accounts for the correlation among the repeated observations for a given patient [24]. The outcome variable “AKI” was “0” when not meeting the KDIGO criteria and “1” when meeting one of the KDIGO classes (1, 2, or 3).
Linear mixed models were used to study the relationships between different variables (liver injury, other administered drugs and time) and supratherapeutic whole-blood tacrolimus trough concentration. Effects were considered significant when p values were lower than or equal to 0.005 (instead of 0.05 as usual, to compensate for multiple comparisons). Kaplan–Meier analyses were used to compare two groups of patients: group 1 included patients without AKI between day 1 and day 14; group 2 included patients having AKI between day 1 and day 14. Statistical analyses were carried out using SPSS version 15.0 for Windows and SAS version 9.2 for Windows (SAS Institute Inc., USA).
3 Results
3.1 Demographics
Between April 2005 and December 2012, 114 patients underwent heart transplantation in our hospital. In 4 patients, tacrolimus was postponed and basiliximab was part of the initial immunosuppressive strategy. No patient died within 24 h. We analyzed 110 patients of whom five died within the first 30 days and additionally three within 1 year. Causes of death were right ventricular failure (2), pulmonary bleeding (1), primary graft failure (2), acute rejection (1), and infection (2). Two patients were transferred to other hospitals and three were lost to follow-up. Table 1 shows the patient characteristics. Within 1 year, three patients stopped tacrolimus due to severe side effects as neuro- and nephrotoxicity and switched to sirolimus (N = 1) or cyclosporine (N = 2). (The frequencies of AKI and CKD are also shown in the online resource Figure S.1).
3.2 Renal Function
Figure 1 shows the distribution of serum creatinine over time. The median baseline creatinine was 102 µmol/L (IQR 84-126 µmol/L). The highest median creatinine of 117 µmol/L was observed at day 3 and slowly decreased to 91 µmol/L at 1 year. The frequency of patients presenting at least one episode of AKI between day 1 and day 6 was 57% (63 out of 110). AKI was most prevalent on day 3 (44%). There was a decrease in frequency of AKI to 17% at day 14, with an increase at 1 month to 25%. The most serious AKI stage 3 [based on “KDIGO criteria”] was most often observed on day 4 (6%). Renal replacement therapy was needed in 6% of patients within the first 14 days (7 out of 110). Recovery from AKI was observed in 15 out of 64 patients (24%). Five patients among the 110 (4.5%) had a recurrent AKI. Persistent kidney injury at one month was not associated with the occurrence of CKD (p = 0.7). The frequency of patients with CKD was 19% at one year. CKD or death was not significantly related to the occurrence of AKI (p = 0.14).
3.3 Variables Influencing AKI
GEE analyses showed that AKI was associated with prior supratherapeutic whole-blood tacrolimus trough concentrations both in the first week (OR 1.66; 95% CI 1.20–2.31) and in the first 2 weeks (OR 2.10; 95% CI 1.35–3.25) after transplantation, even after correction for the variables ICM, VAD, DM, age, contrast administration, surgery time > 400 min and ECMO post-operation. Among the patients who presented a supratherapeutic level, the median duration was 2 days (minimum 1 day, maximum 5 days). Nephrotoxic drugs as described in the method section were frequently co-administered during the first week after transplantation (95%, 104 out of 110) and most often on day one and two (72 and 75%, respectively). At day 6, 33% of the patients used nephrotoxic drugs. Shock was observed in 96% of patients (105 out of 110) during the first 6 days. The occurrence of shock was most often seen on day one (84%) and decreased to 50% on day 6. The frequency of SIRS was highest on day 2 (86%) with a decrease to 31% on day 6 (Table 2).
3.4 Tacrolimus Blood Concentrations
We analyzed a total of 473 tacrolimus whole-blood trough concentrations within the first week after transplantation. In 34% of patients (37 out of 110), a supratherapeutic concentration was measured at least once. Whole-blood tacrolimus trough concentrations ranged from 1.5 to 35 ng/mL with the highest median at day 4, 5, 6 and 7 of 10 ng/mL. At month 3, the median tacrolimus concentration was 9 ng/mL, indicating a supratherapeutic concentration. Median whole-blood tacrolimus trough concentrations showed to be below therapeutic range during the first 3 days, at day 10 and 12, at month 6 and at 1 year (see Fig. 2). At day 4, 5 and 6 the highest frequencies of supratherapeutic whole-blood tacrolimus trough concentrations were measured (16% on all days). At day, 14 3% had a supratherapeutic level and at 1 month this frequency increased to 8%. (See Table S.3 in the online resource, demonstrating the variables influencing whole-blood tacrolimus concentrations). Liver injury was often observed within the first week after transplantation (56%, 61 out of 110). The highest occurrence of liver injury was observed on day 2 post-transplantation (28%) with a decrease to 16% on day 6. Supratherapeutic whole-blood tacrolimus trough concentrations were not significantly associated with liver injury (p = 0.03) (See Table S.3 in the online resource, demonstrating the variables influencing whole-blood tacrolimus concentrations). Almost all patients used drugs increasing tacrolimus concentrations within the first week after transplantation (95%, 104 out of 110). Drugs increasing tacrolimus concentrations were most frequently used on day 1 (82%). From day 2 on drugs decreasing tacrolimus concentrations were applied. The prevalence of the administration of these drugs was highest on day 2 (71%). One patient was treated with high dose methylprednisolone for 6 days because of suspected rejection. Drugs, which could affect tacrolimus concentrations, increasing as well as decreasing, were not significantly associated with whole-blood tacrolimus trough concentrations (p values 0.86 and 0.07, respectively, See also in the online resource Table S.3).
Tukey boxplot of whole-blood tacrolimus trough concentrations between day 1 and year 1. The therapeutic range of 9–15 ng/ml and 5–8 ng/ml is indicated as gray bars in the figure. Box: 25th, median and 75th percentiles, whiskers: maximum value excluding outliers, dots: outliers more or less than 3/2 upper or lower quartile
4 Discussion
We found a high incidence rate of AKI after heart transplantation. AKI was independently associated with supratherapeutic tacrolimus concentrations, which were most often observed in the first week.
The high incidence rate of AKI (57%) in our cohort of heart transplant patients was in accordance with Tjahjono et al., in which AKI was seen in 59% of the patients within the first week [12]. These high incidence rates showed to be higher than in a cohort of lung transplants and may be based on the high frequency of shock and on the high median baseline creatinine, reflecting a decreased renal function pre-operatively [12, 25]. The high baseline creatinine is in accordance with previous observations of heart transplants [6]. A diminished renal function may further deteriorate in case of shock and inflammation, the use of a pulmonary bypass circuit, increased administration of blood products as well as with the administration of nephrotoxic drugs, such as tacrolimus [12]. It was suggested that a supratherapeutic tacrolimus concentration at day 3 is a risk factor for the development of AKI. This was observed in a pediatric heart transplantation cohort, though it did not reach significance [11]. We showed that a prior supratherapeutic whole-blood tacrolimus trough concentration was an independent predictor of AKI in an adult heart transplantation cohort.
The occurrence of AKI is a predictor of CKD. In a group of 300 heart transplantation patients, AKI was related to CKD [10]. The risk of progression to CKD is related to the stages of AKI. Even mild AKI, stage 1, increases the risk of CKD [26]. We could not relate AKI to CKD. This lack of association may have been caused by a too small sample size or too short follow-up period. Nonetheless, we observed a low recovery rate of AKI in our cohort [27, 28]. Recovery of AKI in heart transplants is important. AKI persistent at 1 month after transplantation has been shown to significantly decrease survival rates compared to patients with complete renal recovery [1, 10, 13, 28]. Moreover, earlier reports showed a steady increase in the percentage and severity of CKD after heart transplantation in the first 10 years with a median time to progression to CKD stage 4 of 3 years [1, 9]. Hence, the importance of AKI in the development of CKD shows to be pivotal and tacrolimus may have an important part in it.
Tacrolimus is an ongoing assault to the kidneys, because it is administered continuously. Nephrotoxicity induced by tacrolimus is assumed to be caused by acute vascular and tubular damage, and chronic irreversible tubule-interstitial fibrosis [29,30,31,32,33,34,35,36,37]. Renal biopsies show a gradual increase of arteriolar hyalinosis, glomerulosclerosis as well as interstitial fibrosis in patients treated with calcineurin inhibitors [38]. Paradoxically, anatomical abnormalities may go unnoticed and tacrolimus nephrotoxicity might be present without clinical loss of renal function. Nankivell et al. observed a mean GFR of 60 ml/min in the presence of grade I nephropathy and of 50 ml/min in the presence of chronic nephropathy of grade II or higher [38].
To avoid early tacrolimus nephrotoxicity, decreased doses and delayed introduction have been suggested. To preclude early rejection when tacrolimus is postponed, an interleukin-2 receptor inhibitor or mTOR inhibitor could be administered [39,40,41,42,43,44]. Unfortunately, mTOR inhibitors are poorly tolerated, with almost one-third discontinuing the drug within 1 year after transplantation [41, 44,45,46]. Rejection rates may be higher in tacrolimus free regimens; therefore, tacrolimus is still the preferred immunosuppressive drug after heart transplantation [41].
Tacrolimus was carefully dosed in our cohort, reflected by the low starting dose and the (sub-)therapeutic median whole-blood concentrations. Dosages were substantially lower than in a cohort of lung transplantation patients [25]. Yet, tapering of corticosteroids, increasing tacrolimus blood concentrations, was started in a later stage as in the lung transplantation cohort [25]. Nevertheless, we found a high frequency of supratherapeutic concentrations during the first 2 weeks. This cohort of heart transplants showed a higher frequency of liver injury and shock compared to the cohort of lung transplantation patients, reflecting the higher frequency of clinical instability [25]. Furthermore, we observed a high prevalence of drugs influencing the blood tacrolimus concentrations. Although none of the beforementioned variables individually influenced the development of subsequent supratherapeutic tacrolimus concentrations, all combined might still contribute to supratherapeutic tacrolimus concentrations. Tacrolimus dosing is complex and extremely difficult in the clinically unstable phase after heart transplantation.
To prevent from supratherapeutic whole-blood trough concentrations, an even more tentative dosing scheme could be used. Targeting at the narrow therapeutic range may have contributed to the supratherapeutic levels. Starting with a low dose and using only an upper level may prevent peaks in the concentrations. This qualitative dosing scheme may prevent erratic tacrolimus concentrations and thus supratherapeutic tacrolimus concentrations.
4.1 Limitations of this Study
There are some notable limitations to this study due to its retrospective character. Several variables influencing pharmacokinetics of tacrolimus, for instance, the effect of variations in HDL, alpha-1-acid glycoprotein, acidosis, changes in fluid balance, gut motility and variations in concentrations or activity of CYP 3A4/5 and P-glycoprotein, could not be studied.
In our study, only 18 patients were still followed at 1-year post-transplantation and developed a CKD. A larger sample size would increase the reliability of our results, e.g., the potential influence of tacrolimus on the occurrence of CKD is likely to remain undetected in our study (Kaplan–Meier analysis). Similarly, it is plausible that our study had inadequate power to detect the effect of other drugs and liver injury on the whole-blood tacrolimus trough concentration (mixed model analysis).
Trough concentrations were assumed, though with one sample per 24-h period the assumption may be insufficient. Especially, clinically unstable patients may lack steady state and large changes in half-life times may occur. This may hamper the interpretation of the blood concentrations.
We could not analyze all the known factors related to AKI after heart transplantation, e.g., the amount of blood products administered, inotrope and ventilation duration. Moreover, ultrasound, biopsy, and urine analyses have not been performed. Therefore, not all causes of kidney injury have been investigated in this study and they may have had an effect on kidney function.
Another limitation is the estimation of AKI itself. At present, the KDIGO criteria are considered to be the best option. However, plasma creatinine provides only an indication of renal function. Plasma creatinine in heart transplantation patients shortly after surgery may overestimate renal function [47]. Moreover, CKD-EPI tends to overestimate GFR [9]. Uniformity in defining kidney injury as well as recovery from AKI may be an important improvement for the comparison of renal outcome in heart transplant recipients.
5 Conclusions
This study shows that AKI after heart transplantation is correlated to supratherapeutic whole-blood tacrolimus trough concentrations. Low recovery rates of AKI might even be a reflection of ongoing tacrolimus toxicity. Therefore, the prevention of tacrolimus nephrotoxicity early after transplantation may be crucial in preserving kidney function. The appropriate tailoring of tacrolimus dosing early after transplantation could be a key factor in improving transplantation outcomes.
References
Stehlik J, Edwards LB, Kucheryavaya AY, Benden C, Christie JD, Dipchand AI, et al. The Registry of the International Society for Heart and Lung Transplantation: 29th official adult heart transplant report–2012. J Heart Lung Transpl. 2012;31:1052–64.
Guethoff S, Meiser BM, Groetzner J, Eifert S, Grinninger C, Ueberfuhr P, et al. Ten-year results of a randomized trial comparing tacrolimus versus cyclosporine a in combination with mycophenolate mofetil after heart transplantation. Transplantation. 2013;95:629–34.
Penninga L, Møller CH, Gustafsson F, Steinbrüchel DA, Gluud C. Tacrolimus versus cyclosporine as primary immunosuppression after heart transplantation: systematic review with meta-analyses and trial sequential analyses of randomised trials. Eur J Clin Pharmacol. 2010;66:1177–87.
Zijlstra LE, Constantinescu AA, Manintveld O, Birim O, Hesselink DA, van Thiel R, et al. Improved long-term survival in Dutch heart transplant patients despite increasing donor age: the Rotterdam experience. Transpl Int. 2015;28:962–71.
Nankivell BJ, P’Ng CH, O’Connell PJ, Chapman JR. Calcineurin inhibitor nephrotoxicity through the lens of longitudinal histology: comparison of cyclosporine and tacrolimus eras. Transplantation. 2016;100:1723–31.
Lund LH, Edwards LB, Kucheryavaya AY, Dipchand AI, Benden C, Christie JD, et al. The Registry of the International Society for Heart and Lung Transplantation: thirtieth official adult heart transplant report-2013; focus theme: age. J Heart Lung Transpl. 2013;32:951–64.
Healy AH, Stehlik J, Edwards LB, McKellar SH, Drakos SG, Selzman CH. Predictors of 30-day post-transplant mortality in patients bridged to transplantation with continuous-flow left ventricular assist devices—an analysis of the International Society for Heart and Lung Transplantation Transplant Registry. J Heart Lung Transpl. 2016;35:34–9.
Lund LH, Edwards LB, Kucheryavaya AY, Benden C, Dipchand AI, Goldfarb S, et al. The Registry of the International Society for Heart and Lung Transplantation: thirty-second Official Adult Heart Transplantation Report-2015; focus theme: early graft failure. J Heart Lung Transpl. 2015;34:1244–54.
Söderlund C, Löfdahl E, Nilsson J, Reitan Ö, Higgins T, Rådegran G. Chronic kidney disease after heart transplantation: a single-centre retrospective study at Skåne University Hospital in Lund 1988–2010. Transpl Int. 2016;29:529–39.
De Santo LS, Romano G, Amarelli C, Maiello C, Baldascino F, Bancone C, et al. Implications of acute kidney injury after heart transplantation: what a surgeon should know. Eur J Cardiothorac Surg. 2011;40:1355–61 (Oxford University Press; discussion1361).
MacDonald C, Norris C, Alton GY, Urschel S, Joffe AR, Morgan CJ, et al. Acute kidney injury after heart transplant in young children: risk factors and outcomes. Pediatr Nephrol. 2016;31:671–8 (Springer Berlin Heidelberg).
Tjahjono R, Connellan M, Granger E. Predictors of acute kidney injury in cardiac transplantation. Transpl Proc. 2016;48:167–72.
Gude E, Andreassen AK, Arora S, Gullestad L, Grov I, Hartmann A, et al. Acute renal failure early after heart transplantation: risk factors and clinical consequences. Clin Transpl. 2010;24:E207–13 (Blackwell Publishing Ltd).
Delgado JF, Crespo-Leiro MG, Gómez-Sánchez MA, Paniagua MJ, González-Vílchez F, Vázquez de Prada JA, et al. Risk factors associated with moderate-to-severe renal dysfunction among heart transplant patients: results from the CAPRI study. Clin Transpl. 2010;24:E194–200.
Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–70.
Aidong W, Zhenjie C, Tong L, Lei Z, Yin W, Shanqi Z, et al. Therapeutic drug monitoring of tacrolimus in early stage after heart transplantation. TPS. 2004;36:2388–9.
Salm P, Rutherford DM, Taylor PJ, Black MJ, Pillans PI. Evaluation of microparticle enzyme immunoassay against HPLC-mass spectrometry for the determination of whole-blood tacrolimus in heart- and lung-transplant recipients. Clin Biochem. 2000;33:557–62.
Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120(4):c179–84 (Karger Publishers).
Chawla LS, Bellomo R, Bihorac A, Goldstein SL, Siew ED, Bagshaw SM, et al. Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat Rev Nephrol. 2017;13:241–57 (Nature Publishing Group).
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12 (NIH Public Access).
Christians U, Jacobsen W, Benet LZ, Lampen A. Mechanisms of clinically relevant drug interactions associated with tacrolimus. Clin Pharmacokinet. 2002;41:813–51 (Springer International Publishing).
Kramer L, Jordan B, Druml W, Bauer P, Metnitz PGH. Incidence and prognosis of early hepatic dysfunction in critically ill patients—a prospective multicenter study. Crit Care Med. 2007;35:1099-e7.
Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock. Crit Care Med. 2012;2013:580–637.
Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–30.
Sikma MA, Hunault CC, van de Graaf EA, Verhaar MC, Kesecioglu J, de Lange DW, et al. High tacrolimus blood concentrations early after lung transplantation and the risk of kidney injury. Eur J Clin Pharmacol. 2017;73:573–80 (Springer Berlin Heidelberg).
Xu J-R, Zhu J-M, Jiang J, Ding X-Q, Fang Y, Shen B, et al. Risk factors for long-term mortality and progressive chronic kidney disease associated with acute kidney injury after cardiac surgery. Medicine (Baltimore). 2015;94:e2025.
Garzotto F, Piccinni P, Cruz D, Gramaticopolo S, Dal Santo M, Aneloni G, et al. RIFLE-based data collection/management system applied to a prospective cohort multicenter italian study on the epidemiology of acute kidney injury in the intensive care unit. Blood Purif. 2011;31:159–71.
Lee JM, Lee S-A, Cho H-J, Yang H-M, Lee H-Y, Hwang HY, et al. Impact of perioperative renal dysfunction in heart transplantation: combined heart and kidney transplantation could help to reduce postoperative mortality. Ann Transpl. 2013;18:533–49.
Hortelano S, Castilla M, Torres AM, Tejedor A, Boscá L. Potentiation by nitric oxide of cyclosporin A and FK506-induced apoptosis in renal proximal tubule cells. J Am Soc Nephrol. 2000;11:2315–23.
Nankivell BJ, Borrows RJ, Fung CLS, Oconnell PJ, Chapman JR, Allen RDM. Delta analysis of posttransplantation tubulointerstitial damage. Transplantation. 2004;78:434–41.
Esteva-Font C, Ars E, Guillen-Gomez E, Campistol JM, Sanz L, Jimenez W, et al. Ciclosporin-induced hypertension is associated with increased sodium transporter of the loop of Henle (NKCC2). Nephrol Dial Transpl. 2007;22:2810–6.
Catarsi P. Angiotensin-converting enzyme (ACE) haplotypes and cyclosporine A (CsA) response: a model of the complex relationship between ACE quantitative trait locus and pathological phenotypes. Hum Mol Genet. 2005;14:2357–67.
Randhawa PS, Shapiro R, Jordan ML, Starzl TE, Demetris AJ. The histopathological changes associated with allograft rejection and drug toxicity in renal transplant recipients maintained on FK506. Am J Surg Pathol. 1993;17:60–8.
Bai JPF, Lesko LJ, Burckart GJ. Understanding the genetic basis for adverse drug effects: the calcineurin inhibitors. Pharmacotherapy. 2010;30:195–209.
Naesens M, Kuypers DRJ, Sarwal M. Calcineurin inhibitor nephrotoxicity. Clin J Am Soc Nephrol. 2009;4:481–508.
Ozdemir BH, Ozdemir FN, Demirhan B, Haberal M. TGF-beta1 expression in renal allograft rejection and cyclosporine A toxicity. Transplantation. 2005;80:1681–5.
Myers BD, Ross J, Newton L, Luetscher J, Perlroth M. Cyclosporine-associated chronic nephropathy. N Engl J Med. 1984;311:699–705.
Nankivell BJ, Borrows RJ, Fung CLS, O’Connell PJ, Allen RDM, Chapman JR. The natural history of chronic allograft nephropathy. N Engl J Med. 2003;349:2326–33.
Sánchez-Lázaro IJ, Almenar Bonet L, Martínez-Dolz L, Buendía Fuentes F, Navarro-Manchón J, Agüero Ramon-Llin J, et al. Repeated daclizumab administration to delay the introduction of calcineurin inhibitors in heart transplant patients with postoperative renal dysfunction. Rev Esp Cardiol. 2011;64:237–9.
González-Vílchez F, de Prada JAV, Exposito V, García-Camarero T, Fernández-Friera L, Llano M, et al. Avoidance of calcineurin inhibitors with use of proliferation signal inhibitors in de novo heart transplantation with renal failure. J Heart Lung Transpl. 2008;27:1135–41.
Kaczmarek I, Zaruba M-M, Beiras-Fernandez A, Reimann R, Nickel T, Grinninger C, et al. Tacrolimus with mycophenolate mofetil or sirolimus compared with calcineurin inhibitor-free immunosuppression (sirolimus/mycophenolate mofetil) after heart transplantation: 5-year results. HEALUN. 2013;32:277–84 (Elsevier).
Arora S, Gude E, Sigurdardottir V, Mortensen SA, Eiskjær H, Riise G, et al. Improvement in renal function after everolimus introduction and calcineurin inhibitor reduction in maintenance thoracic transplant recipients: The significance of baseline glomerular filtration rate. HEALUN. 2012;31:259–65 (Elsevier Inc).
Gullestad L, Mortensen S-A, Eiskjær H, Riise GC, Mared L, Bjørtuft Ø, et al. Two-Year Outcomes in Thoracic Transplant Recipients After Conversion to Everolimus With Reduced Calcineurin Inhibitor Within a Multicenter, Open-Label. Randomized Trial. Transplantation. 2010;90:1581–9.
Kaplinsky E, González-Costello J, Manito N, Roca J, Barbosa MJ, Nebot M, et al. Renal function improvement after conversion to proliferation signal inhibitors during long-term follow-up in heart transplant recipients. Transpl Proc. 2012;44:2564–6.
Thibodeau JT, Mishkin JD, Patel PC, Kaiser PA, Ayers CR, Mammen PPA, et al. Tolerability of sirolimus: a decade of experience at a single cardiac transplant center. Clin Transpl. 2013;27:945–52.
Manito N, Delgado JF, Crespo-Leiro MG, Arizón JM, Segovia J, González-Vílchez F, et al. Twelve-month efficacy and safety of the conversion to everolimus in maintenance heart transplant recipients. World J Transpl. 2015;5:310–9.
Bragadottir G, Redfors B, Ricksten S-E. Assessing glomerular filtration rate (GFR) in critically ill patients with acute kidney injury—true GFR versus urinary creatinine clearance and estimating equations. Crit Care. 2013;17:R108.
Acknowledgements
All authors made substantial intellectual contributions to conception, design and acquisition of data and data analysis and writing or interpretation of the data. All authors contributed to drafting the article or revising it critically for important intellectual content and gave final approval of the version to be published and approved of the order in which their names will be listed in the manuscript. We thank E.M. van Maarseveen, PharmD PhD, by supporting us in the data collection”.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics
This study was conducted in compliance with the Declaration of Helsinki, Good Clinical Practice guidelines and in accordance with local and national regulatory requirements and laws. The accredited ethics committee of the University Medical Center Utrecht has approved of the use of patient data (IRB UMC Utrecht protocol number 12-071).
Disclosure of interest
Claudine C. Hunault, Marianne C. Verhaar, Johannes H. Kirkels, Jozef Kesecioglu and Dylan W. de Lange declare that they have no conflicts of interest.
Funding
No source of funding was used to conduct this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Sikma, M.A., Hunault, C.C., Kirkels, J.H. et al. Association of Whole Blood Tacrolimus Concentrations with Kidney Injury in Heart Transplantation Patients. Eur J Drug Metab Pharmacokinet 43, 311–320 (2018). https://doi.org/10.1007/s13318-017-0453-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-017-0453-7