Abstract
Background and objectives
β-Lapachone (βLAP) is a promising, poorly soluble, antitumoral drug. βLAP combination with cyclodextrins (CDs) improves its solubility and dissolution but there is not enough information about the impact of cyclodextrins on βLAP intestinal permeability. The objectives of this work were to characterize βLAP intestinal permeability and to elucidate cyclodextrins effect on the dissolution properties and on the intestinal permeability. The final goal was to evaluate CDs influence on the oral absorption of βLAP.
Methods
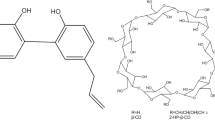
Binary systems (physical mixtures and inclusion complexes) including βLAP and CDs (β-cyclodextrin: βCD, random-methyl-β-cyclodextrin: RMβCD and sulfobutylether-β-cyclodextrin: SBEβCD) have been prepared and analysed by differential scanning calorimetry. βLAP (and its combinations with CDs) absorption rate coefficients and effective permeability values have been determined in vitro in MDCK or MDCK-Mdr1 monolayers and in situ in rat by a closed loop perfusion technique.
Results
DSC results confirmed the formation of the inclusion complexes. βLAP–CDs inclusion complexes improve drug solubility and dissolution rate in comparison with physical mixtures. βLAP presented a high permeability value which can provide complete oral absorption. Its oral absorption is limited by its low solubility and dissolution rate. Cyclodextrin (both as physical mixtures and inclusion complexes) showed a positive effect on the intestinal permeability of βLAP. Complexation with CDs does not reduce βLAP intestinal permeability in spite of the potential negative effect of the reduction in free fraction of the drug.
Conclusions
The use of RMβCD or SBEβCD inclusion complexes could benefit βLAP oral absorption by enhancing its solubility, dissolution rate and permeability.





Similar content being viewed by others
References
Cunha-Filho MS, Dacunha-Marinho B, Torres-Labandeira JJ, Martinez-Pacheco R, Landin M. Characterization of beta-lapachone and methylated beta-cyclodextrin solid-state systems. AAPS PharmSciTech. 2007;8(3):E60.
Ough M, Lewis A, Bey EA, Gao J, Ritchie JM, Bornmann W, et al. Efficacy of beta-lapachone in pancreatic cancer treatment: exploiting the novel, therapeutic target NQO1. Cancer Biol Ther. 2005;4(1):95–102.
Gomez-Castellanos JRPJ, Heinrich M. Red Lapacho (Tabebuia impetiginosa)—a global ethnopharmacological commodity? J Ethnopharmacol. 2009;121(1):1–13.
Reinicke KE, Bey EA, Bentle MS, Pink JJ, Ingalls ST, Hoppel CL, et al. Development of beta-lapachone prodrugs for therapy against human cancer cells with elevated NAD(P)H:quinone oxidoreductase 1 levels. Clin Cancer Res. 2005;11(8):3055–64.
Wuerzberger SM, Pink JJ, Planchon SM, Byers KL, Bornmann WG, Boothman DA. Induction of apoptosis in MCF-7:WS8 breast cancer cells by beta-lapachone. Cancer Res. 1998;58(9):1876–85.
Jang SB, Kim D, Kim SY, Park C, Jeong JH, Kuh HJ, et al. Impact of micellar vehicles on in situ intestinal absorption properties of beta-lapachone in rats. Korean J Physiol Pharmacol. 2013;17(1):9–13.
Nasongkla N, Wiedmann AF, Bruening A, Beman M, Ray D, Bornmann WG, et al. Enhancement of solubility and bioavailability of beta-lapachone using cyclodextrin inclusion complexes. Pharm Res. 2003;20(10):1626–33.
Tewari A, Raman JD, Chang P, Rao S, Divine G, Menon M. Long-term survival probability in men with clinically localized prostate cancer treated either conservatively or with definitive treatment (radiotherapy or radical prostatectomy). Urology. 2006;68(6):1268–74.
Cunha-Filho MS, Martinez-Pacheco R, Landin M. Dissolution rate enhancement of the novel antitumoral beta-lapachone by solvent change precipitation of microparticles. Eur J Pharm Biopharm. 2008;69(3):871–7.
Wang F, Blanco E, Ai H, Boothman DA, Gao J. Modulating beta-lapachone release from polymer millirods through cyclodextrin complexation. J Pharm Sci. 2006;95(10):2309–19.
Szejtli J. Introduction and general overview of cyclodextrin chemistry. Chem Rev. 1998;98(5):1743–54.
Gamsiz ED, Miller L, Thombre AG, Ahmed I, Carrier RL. Modeling the influence of cyclodextrins on oral absorption of low solubility drugs: II. Experimental validation. Biotechnol Bioeng. 2009;105(2):421–30.
Irie T, Uekama K. Pharmaceutical applications of cyclodextrins. III. Toxicological issues and safety evaluation. J Pharm Sci. 1997;86(2):147–62.
Li P, Zhao L, Yalkowsky SH. Combined effect of cosolvent and cyclodextrin on solubilization of nonpolar drugs. J Pharm Sci. 1999;88(11):1107–11.
Loftsson T, Brewster ME. Pharmaceutical applications of cyclodextrins. 1. Drug solubilization and stabilization. J Pharm Sci. 1996;85(10):1017–25.
Rajewski RA, Stella VJ. Pharmaceutical applications of cyclodextrins. 2. In vivo drug delivery. J Pharm Sci. 1996;85(11):1142–69.
Rao VM, Stella VJ. When can cyclodextrins be considered for solubilization purposes? J Pharm Sci. 2003;92(5):927–32.
Stella VJ, Rajewski RA. Cyclodextrins: their future in drug formulation and delivery. Pharm Res. 1997;14(5):556–67.
Carrier RL, Miller LA, Ahmed I. The utility of cyclodextrins for enhancing oral bioavailability. JCR. 2007;123(2):78–99.
Khan KA, Rhodes CT. Effect of compaction pressure on the dissolution efficiency of some direct compression systems. Pharm Acta Helv. 1972;47(10):594–607.
Loftsson T, Brewster ME. Pharmaceutical applications of cyclodextrins: basic science and product development. J Pharm Pharmacol. 2010;62(11):1607–21.
Loftsson T, Brewster ME. Pharmaceutical applications of cyclodextrins: effects on drug permeation through biological membranes. J Pharm Pharmacol. 2011;63(9):1119–35.
Loftsson T, Magnusdottir A, Masson M, Sigurjonsdottir JF. Self-association and cyclodextrin solubilization of drugs. J Pharm Sci. 2002;91(11):2307–16.
Loftsson T, Duchene D. Cyclodextrins and their pharmaceutical applications. Int J Pharm. 2007;329(1–2):1–11.
Dahan A, Miller JM, Hoffman A, Amidon GE, Amidon GL. The solubility–permeability interplay in using cyclodextrins as pharmaceutical solubilizers: mechanistic modeling and application to progesterone. J Pharm Sci. 2010;99(6):2739–49.
Lozoya-Agullo I, Zur M, Wolk O, Beig A, Gonzalez-Alvarez I, Gonzalez-Alvarez M, et al. In-situ intestinal rat perfusions for human Fabs prediction and BCS permeability class determination: investigation of the single-pass vs. the Doluisio experimental approaches. Int J Pharm. 2015;480(1–2):1–7.
Merino V, Freixas J, del Val Bermejo M, Garrigues TM, Moreno J, Pla-Delfina JM. Biophysical models as an approach to study passive absorption in drug development: 6-fluoroquinolones. J Pharm Sci. 1995;84(6):777–82.
Sanchez-Castano G, Ruiz-Garcia A, Banon N, Bermejo M, Merino V, Freixas J, et al. Intrinsic absolute bioavailability prediction in rats based on in situ absorption rate constants and/or in vitro partition coefficients: 6-fluoroquinolones. J Pharm Sci. 2000;89(11):1395–403.
Cunha-Filho MS, Martinez-Pacheco R, Landin M. Fast dissolving beta-lapachone particles and tablets: an approach using surface adsorption technique. Drug Dev Ind Pharm. 2012;38(7):866–71.
Costa P, Sousa Lobo JM. Modeling and comparison of dissolution profiles. Eur J Pharm Sci. 2001;13(2):123–33.
Doluisio JT, Billups NF, Dittert LW, Sugita ET, Swintosky JV. Drug absorption. I. An in situ rat gut technique yielding realistic absorption rates. J Pharm Sci. 1969;58(10):1196–200.
Gonzalez-Alvarez I, Fernandez-Teruel C, Casabo-Alos VG, Garrigues TM, Polli JE, Ruiz-Garcia A, et al. In situ kinetic modelling of intestinal efflux in rats: functional characterization of segmental differences and correlation with in vitro results. Biopharm Drug Dispos. 2007;28(5):229–39.
Fernandez-Teruel C, Gonzalez-Alvarez I, Casabo VG, Ruiz-Garcia A, Bermejo M. Kinetic modelling of the intestinal transport of sarafloxacin. Studies in situ in rat and in vitro in Caco-2 cells. J Drug Target. 2005;13(3):199–212.
Tsume Y, Mudie DM, Langguth P, Amidon GE, Amidon GL. The biopharmaceutics classification system: subclasses for in vivo predictive dissolution (IPD) methodology and IVIVC. Eur J Pharm Sci. 2014;57:152–63.
Irvine JD, Takahashi L, Lockhart K, Cheong J, Tolan JW, Selick HE, et al. MDCK (Madin–Darby canine kidney) cells: a tool for membrane permeability screening. J Pharm Sci. 1999;88(1):28–33.
Troutman MD, Thakker DR. Efflux ratio cannot assess P-glycoprotein-mediated attenuation of absorptive transport: asymmetric effect of P-glycoprotein on absorptive and secretory transport across Caco-2 cell monolayers. Pharm Res. 2003;20(8):1200–9.
Troutman MD, Thakker DR. Rhodamine 123 requires carrier-mediated influx for its activity as a P-glycoprotein substrate in Caco-2 cells. Pharm Res. 2003;20(8):1192–9.
Smith D, Artursson P, Avdeef A, Di L, Ecker GF, Faller B, et al. Passive lipoidal diffusion and carrier-mediated cell uptake are both important mechanisms of membrane permeation in drug disposition. Mol Pharm. 2014;11(6):1727–38.
Rodriguez-Ibanez M, Nalda-Molina R, Montalar-Montero M, Bermejo MV, Merino V, Garrigues TM. Transintestinal secretion of ciprofloxacin, grepafloxacin and sparfloxacin: in vitro and in situ inhibition studies. Eur J Pharm Biopharm. 2003;55(2):241–6.
Ming X, Knight BM, Thakker DR. Vectorial transport of fexofenadine across Caco-2 cells: involvement of apical uptake and basolateral efflux transporters. Mol Pharm. 2011;8(5):1677–86.
Ballatori N, Christian WV, Wheeler SG, Hammond CL. The heteromeric organic solute transporter, OSTalpha-OSTbeta/SLC51: a transporter for steroid-derived molecules. Mol Aspects Med. 2013;34(2–3):683–92.
Bermejo MV G-AI. How and where are drugs absorbed? In: Gad SC, editor. Preclinical development handbook: ADME and biopharmaceutical properties. New York: Wiley; 2008.
Kim I, Kim H, Ro J, Jo K, Karki S, Khadka P, et al. Preclinical pharmacokinetic evaluation of beta-lapachone: characteristics of oral bioavailability and first-pass metabolism in rats. Biomol Ther (Seoul). 2015;23(3):296–300.
Camenisch G, Folkers G, van de Waterbeemd H. Shapes of membrane permeability–lipophilicity curves: extension of theoretical models with an aqueous pore pathway. Eur J Pharm Sci. 1998;6(4):325–9.
Martin-Villodre A, Pla-Delfina JM, Moreno J, Perez-Buendia D, Miralles J, Collado EF, et al. Studies on the reliability of a bihyperbolic functional absorption model. I. Ring-substituted anilines. J Pharmacokinet Biopharm. 1986;14(6):615–33.
Salamat-Miller NJT. Current strategies used to enhance the paracellular transport of therapeutic polypeptides across the intestinal epithelium. Int J Pharm. 2005;294(1–2):201–16.
Fenyvesi F, Kiss T, Fenyvesi E, Szente L, Veszelka S, Deli MA, et al. Randomly methylated beta-cyclodextrin derivatives enhance taxol permeability through human intestinal epithelial Caco-2 cell monolayer. J Pharm Sci. 2011;100(11):4734–44.
Nakanishi K, Nadai T, Masada M, Miyajima K. Effect of cyclodextrins on biological membrane. II. Mechanism of enhancement on the intestinal absorption of non-absorbable drug by cyclodextrins. Chem Pharm Bull. 1992;40(5):1252–6.
Uekama K. Design and evaluation of cyclodextrin-based drug formulation. Chem Pharm Bull (Tokyo). 2004;52(8):900–15.
Lambert D, O’Neill CA, Padfield PJ. Depletion of Caco-2 cell cholesterol disrupts barrier function by altering the detergent solubility and distribution of specific tight-junction proteins. Biochem J. 2005;387(Pt 2):553–60.
Beig A, Miller JM, Dahan A. Accounting for the solubility–permeability interplay in oral formulation development for poor water solubility drugs: the effect of PEG-400 on carbamazepine absorption. Eur J Pharm Biopharm. 2012;81(2):386–91.
Dahan A, Miller JM. The solubility–permeability interplay and its implications in formulation design and development for poorly soluble drugs. AAPS J. 2012;14(2):244–51.
Miller JM, Dahan A. Predicting the solubility–permeability interplay when using cyclodextrins in solubility-enabling formulations: model validation. Int J Pharm. 2012;430(1–2):388–91.
Acknowledgments
This work was partially supported by the following projects: Red-Biofarma. Red para el desarrollo de metodologías biofarmacéuticas racionales que incrementen la competencia y el impacto social de las Industrias Farmacéuticas Locales (DCI ALA/19.09.01/10/21526/245-297/ALFA 111(2010)29), Foodomics evaluation of dietary polyphenols against colon cancer using in vitro and in vivo models (AGL2011-29857-C03-03) and “Diseño de formulaciones de fármacos de baja hidrosolubilidad mediante herramientas de Inteligencia Artificial” (SAF 2012-39878-C02-01) from Spanish Ministry of Science and Innovation. VM-S received a grant from Ministry of Education and Science of Spain and Miguel Hernandez University (FPU AP2010-2372). ME-L thanks the Galician Government (Xunta de Galicia) for her financial support by IN.CI.TE. 2009-2013_Isabel Barreto Program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
VM-S, JG-N, ME-L, IG-A, MG-A, VG-C, MB and ML have no conflicts of interest to declare.
Additional information
V. Mangas-Sanjuan and J. Gutiérrez-Nieto made equal contributions and are considered first co-authors.
V.-G. Casabó: deceased, July 7, 2013.
Rights and permissions
About this article
Cite this article
Mangas-Sanjuan, V., Gutiérrez-Nieto, J., Echezarreta-López, M. et al. Intestinal Permeability of β-Lapachone and Its Cyclodextrin Complexes and Physical Mixtures. Eur J Drug Metab Pharmacokinet 41, 795–806 (2016). https://doi.org/10.1007/s13318-015-0310-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-015-0310-5