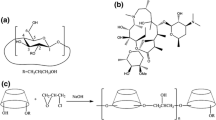
Abstract
Cyclodextrin (CD) inclusions are generally used to increase the solubility of poorly soluble drugs. In this study, magnolol (MAG) was used as a model drug for exploring the effects of CD on the degradation of pharmaceutical drugs by intestinal microflora. MAG/β-cyclodextrin (β-CD) and MAG/hydroxypropyl-β-CD (HP-β-CD) inclusion complexes were successfully prepared by the saturated aqueous solution and freeze-drying methods, respectively. Structural characterisation along with analyses of solubility, residual water content and drug content of the inclusion complexes was performed. The intestinal microflora of male rats was used to study MAG degradation in vitro. At three concentrations, the degradation of both the inclusion complexes was slower than that of the MAG monomer, MAG and CD mixtures and the MAG-poloxamer 188 micelle. There were no statistically significant differences in the degradation of the MAG/β-CD and MAG/HP-β-CD inclusion complexes. A simulation first-order equation of the degradation parameters revealed that the degradation of the inclusion complexes was slower and pronounced, judging by slope. The experimental findings were verified by molecular docking for predicting the stable molecular structure of the inclusion complexes. In conclusion, the inclusion complexes partially protected MAG from degradation by the intestinal bacteria.







Similar content being viewed by others
Abbreviations
- CD:
-
Cyclodextrin (Fw=~1135, purity ≧98%)
- MAG:
-
Magnolol
- MAG/β-CD:
-
MAG/β-cyclodextrin
- MAG/HP-β-CD:
-
MAG/hydroxypropyl-β-cyclodextrin (DS = 6.1, Fw = 1489, purity ≥ 91%)
- RSD:
-
Relative standard deviation
References
Salustio PJ, Pontes P, Conduto C, et al. Advanced technologies for oral controlled release: cyclodextrins for oral controlled release. AAPS PharmSciTech. 2011;12(4):1276–92.
Lv H-X, Zhang Z-H, Jiang H, Waddad A, Zhou J-P. Preparation, physicochemical characteristics and bioavailability studies of an atorvastatin hydroxypropyl-beta-cyclodextrin complex. Pharmazie. 2012;67(1):46–53.
Gidwani B, Vyas A. Synthesis, characterization and application of epichlorohydrin-β-cyclodextrin polymer. Colloids Surf B: Biointerfaces. 2014;114:130–7.
Raoov M, Mohamad S, bin Abas M-R, Surikumaran H. New macroporous β-cyclodextrin functionalized ionic liquid polymer as an adsorbent for solid phase extraction with phenols. Talanta. 2014;130:155–63.
Gould S, Scott R-C. 2-Hydroxypropyl-β-cyclodextrin (HP-β-CD): a toxicology review. Food Chem Toxicol. 2005;43(10):1451–9.
Zhang Z-H, Liu L, Xu D-S. Extraction process of Radix Salviae Miltiorrhizae with the aid of cyclodextrion. Chin Tradit Pat Med. 2005;27(3):264–6.
Deroubaix X, Stockis A, Allemon A-M, Lebacq E, Acerbi D, Ventura P. Oral bioavailability of CHF1194, an inclusion complex of piroxicam and β-cyclodextrin, in healthy subjects under single dose and steady-state conditions. Eur J Clin Pharmacol. 1995;47(6):531–6.
Zhang X-W, Zhang T-P, Lan Y-L, Wu B-J, Shi Z-H. Nanosuspensions containing oridonin/HP-β-cyclodextrin inclusion complexes for oral bioavailability enhancement via improved dissolution and permeability. AAPS PharmSciTech. 2016;17(2):400–8.
Chen Y-H, Huang P-H, Lin F-Y, Chen W-C, Chen Y-L, Yin W-H, et al. Magnolol: a multifunctional compound isolated from the Chinese medicinal plant Magnolia officinalis. Eur J Integr Med. 2011;3(4):e317–e24.
Behbehani J, Shreaz S, Irshad M, Karched M. The natural compound magnolol affects growth, biofilm formation, and ultrastructure of oral Candida isolates. Microb Pathog. 2017;113:209–17.
Shen P, Zhang Z, He Y, Gu C, Zhu K, Li S, et al. Magnolol treatment attenuates dextran sulphate sodium-induced murine experimental colitis by regulating inflammation and mucosal damage. Life Sci. 2018;196:69–76.
Gao X, Patel M-G, Bakshi P, Sharma D, Banga AK. Enhancement in the transdermal and localized delivery of honokiol through breast tissue. AAPS PharmSciTech. 2018;19(8):3501–11.
Kim D-J, Kim Y-S. Magnolol protects against trimethyltin-induced neuronal damage and glial activation in vitro and in vivo. Neurotoxicology. 2016;53:173–85.
Chen X, Hao K, Yu X, Huang A, Zhu B, Wang G-X, et al. Magnolol protects Ctenopharyngodon idella kidney cells from apoptosis induced by grass carp reovirus. Fish Shellfish Immun. 2018;74:426–35.
Tang L, Qiu S-B, Wu L, Lv L-F, Lv H-X, Shan W-G. Preparation and in vitro dissolution of magnolol solid dispersion. Chin J Chin Mater Med. 2016;41(3):433–7.
Radu C-D, Parteni O, Ochiuz L. Applications of cyclodextrins in medical textiles—review. J Control Release. 2016;224:146–57.
Qiu N, Shen B, Li X, Zhang X, Sang Z, Yang T, et al. Inclusion complex of magnolol with hydroxypropyl-β-cyclodextrin: characterization, solubility, stability and cell viability. J Incl Phenom Macrocycl Chem. 2016;85(3–4):289–301.
Brandtzaeg P. The gut as communicator between environment and host: immunological consequences. Eur J Pharmacol. 2011;668:S16–32.
Sankar S-A, Lagier J-C, Pontarotti P, Raoult D, Fournier P-E. The human gut microbiome, a taxonomic conundrum. Syst Appl Microbiol. 2015;38(4):276–86.
Hyland N-P, Cryan J-F. Microbe-host interactions: influence of the gut microbiota on the enteric nervous system. Dev Biol. 2016;417(2):182–7.
Jiang D, Zhang L, Cao Y, Yao W. Application of gut microbiota in research of Chinese medicines. China Journal of Chinese Materia Medica. 2016;41(17):3218–25.
Umesaki Y, Setoyama H. Structure of the intestinal flora responsible for development of the gut immune systemin a rodent model. Microbes Infect. 2000;2(11):1343–51.
Nishimura M, Ohkawara T, Kagami-Katsuyama H, Sekiguchi S, Taira T, Tsukada M, et al. Alteration of intestinal flora by the intake of enzymatic degradation products of adlay (Coix lachryma-jobi L. var. ma-yuen Stapf) with improvement of skin condition. J Funct Foods. 2014;7:487–94.
Yu H-N, Zhu J, Pan W-S, Shen S-R, Shan W-G, Das UN. Effects of fish oil with a high content of n-3 polyunsaturated fatty acids on mouse gut microbiota. Arch Med Res. 2014;45(3):195–202.
Men W, Chen Y, Li YJ, Yang Q, Weng X-G, Gong Z-P, et al. Research progress of biotransformation on effective ingredients of Chinese medicine via intestinal bacteria. Chin J Exp Tradit Med Formulae. 2015;21(2):229–34.
Yu H, Guo Z, Shen S, Shan W. Effects of taurine on gut microbiota and metabolism in mice. Amino Acids. 2016;48(7):1601–17.
Yu H, Li R, Huang H, Yao R, Shen S. Short-chain fatty acids enhance the lipid accumulation of 3T3-L1 cells by modulating the expression of enzymes of fatty acid metabolism. Lipids. 2018;53(1):77–84.
Luo Y, Yang W, Sun H-Y, Sun J, Dong L, Chen S-Y, et al. Metabolism of gallic acid and protocatechuic acid in rat intestinal flora. Journal of Shenyang Pharmaceutical University. 2017;34(1):43–7.
Lin W-Z, Li K-P, Zeng Y-B, Zhou X-R. Analysis of the metabolites of isorhamnetin-3-O-β-D-rutinoside in rat intestinal flora in vitro by UPLC-ESI-Q-TOF-MS-MS. Chin J Exp Tradit Med Formulae. 2012;18(20):140–3.
Pei L-K, Guo B-L. Advances in studies on absorption and metabolism of flavonoids. J Chin Pharm. 2006;41(8):568–72.
Barman S, Barman B-K, Roy M-N. Preparation, characterization and binding behaviors of host-guest inclusion complexes of metoclopramide hydrochloride with α- and β-cyclodextrin molecules. J Mol Struct. 2018;1155:503–12.
Deng Y-H, Pang Y-H, Guo Y-F, Ren Y-F, Wang F, Liao X-L, et al. Host-guest inclusion systems of daidzein with 2-hydroxypropyl-β-cyclodextrin (HP-β-CD) and sulfobutyl ether-β-cyclodextrin (SBE-β-CD): preparation, binding behaviors and water solubility. J Mol Struct. 2016;1118:307–15.
Kundu M, Saha S, Roy M-N. Evidences for complexations of β-cyclodextrin with some amino acids by 1H NMR, surface tension, volumetric investigations and XRD. J Mol Liq. 2017;240:570–7.
Tejada G, Lamas M-C, Sortino M, Alvarez VA, Leonardi D. Composite microparticles based on natural mucoadhesive polymers with promising structural properties to protect and improve the antifungal activity of miconazole nitrate. AAPS PharmSciTech. 2018;19(8):3712–22.
Liao Y, Zhang X, Li C, Huang Y, Lei M, Yan M, et al. Inclusion complexes of HP-β-cyclodextrin with agomelatine: preparation, characterization, mechanism study and in vivo evaluation. Carbohydr Polym. 2016;147:415–25.
Yin M-Z, Yang Z-C, Bao Y, Gan J, Cai J-H. Preparation and characterization of norepinephrine cantharidi-poloxamer polymer nano-micelle. J Hubei Polytech Univ. 2015;31(6):43–6.
Bao Y, Zhen F, Lian Z, Zhang J-P, Tao Y, Qian M-S, et al. In vitro metabolic transformation of syringin by rat intestinal flora. China Tradit Herbal Drugs. 2015;46(9):1333–7.
Cao D, Fan Z, Zhu J-P, Yang B, Zhou L, Jin J. Effect of rat intestinal bacteria on metabolism of pedunculoside in vitro. China Pharm. 2016;19(4):621–4.
Tang L, Fu L-L, Shen L-T, Sun B-X, Zhu Z-F, Yu X-L, et al. Degradation of total saponins of Panax notoginsengby intestinal flora of rats in vitro. China Tradit Herbal Drugs. 2018;49(2):396–9.
Hattori M, Sakamoto T, Endo Y, Kakiuchi N, Kobashi K, Mizuno T, et al. Metabolism of magnolol from Magnoliae cortex. I. Application of liquid chromatography-mass spectrometry to the analysis of metabolites of magnolol in rats. Chem Pharm Bull. 1984;32:5010–7.
Jian W-J, Zeng Y, Xiong H-J, Pang J. Molecular simulation of the complex of konjac glucomannan–borate in water. Carbohydr Polym. 2011;85(2):452–6.
Yadav P, Bandyopadhyay A, Chakraborty A, Sarkar K. Enhancement of anticancer activity and drug delivery of chitosan-curcumin nanoparticle via molecular docking and simulation analysis. Carbohydr Polym. 2018;182:188–98.
Su D-H, Zhou M, Tang X-Z, Cui C-Y, Liu S, Wei W-X. Solubility determination and separation of magnolol and honoki. J Guangxi Univ. 2012;37(6):1131–5.
Yun F, Xu X, Di L, Kang A, Shan J, Zhao X, et al. Preparation of different osthole cyclodextrin inclusion complex and comparison on their bioavailability. Chinese Pharmaceutical Journal. 2014;47(8):341–8.
Zhu Z. Preparation, physicochemical properties and bioavailability of magnolol phospholipid complexes. Northwest University; 2013.
Zhang W, Huang Q, Xiang Z, Cheng W. Biotransformation of polydatin by rat intestinal bacteria. J Chin Pharm. 2012;47(8):631–4.
Li N, Song Y, Zhang WZ, Wang W, Chen J-S, Wong A-W, et al. Evaluation of the in vitro and in vivo genotoxicity of magnolia bark extract. Regul Toxicol Pharmacol. 2007;49(3):154–9.
Dai Y-H, Wang M, Zhu Y-N, Wang L-L, Ju J-M, Zhang Z-H. Effect of D-cellobiose on oral bioavailability of gentiopicroside. Chin J Chin Mater Med. 2016;41(10):1855–9.
Korte C, Quodbach J. 3D-printed network structures as controlled-release drug delivery systems: dose adjustment, API release analysis and prediction. AAPS PharmSciTech. 2018;19(8):3333–42.
Antenucci R-N, Palmer J-K. Enzymatic degradation of α- and β-cyclodextrins by bacteroides of the human colon. J Agric Food Chem. 1984;32(6):1316–21.
Fetzner A, Böhm S, Schreder S, Schubert R. Degradation of raw or film-incorporated β-cyclodextrin by enzymes and colonic bacteria. Eur J Pharm Biopharm. 2004;58(1):91–7.
Li X, Song F-R, Liu Z-Q, editors. Studies on the metabolites of diester-type aconitum alkaloids intestinal bacteria using direct analysis in real time (DART) mass spectrometry. The 11th National Doctoral Academic Annual Meeting (Biomedical Topics); 2013; Beijing.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The Laboratory Animal Welfare Ethics Committee of Zhejiang University of Technology approved the animal experiments.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
We studied the effects of β-cyclodextrin and hydroxypropyl-β-cyclodextrin inclusion on the degradation of MAG by intestinal bacteria. The degradation of both the inclusions was slower than that of the MAG monomer, MAG and CD mixtures and micelle which demonstrated that the inclusion had a protective effect on the degradation of intestinal flora.
Rights and permissions
About this article
Cite this article
Tang, L., Zhu, Z., Xie, M. et al. Effects of β-Cyclodextrin and Hydroxypropyl-β-Cyclodextrin Inclusions on the Degradation of Magnolol by Intestinal Bacteria. AAPS PharmSciTech 20, 244 (2019). https://doi.org/10.1208/s12249-019-1397-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-019-1397-9