Abstract
Background and Objective
Amiodarone (AMD) is one of the most effective drugs for rhythm control of atrial fibrillation. The use of AMD is also associated with adverse effects in multiple tissues. Both the parent compound and its major metabolite desethylamiodarone (DEA) contribute to the drug’s therapeutic and toxic action. The present study aimed to build a whole-body physiologically based pharmacokinetic (PBPK) model for AMD and DEA in rats.
Methods
Pharmacokinetic data from multiple studies were collected. Some of the data were pooled together to develop the PBPK model; others were used to evaluate the model. Development of the model also involved in vitro to in vivo extrapolation based on in vitro metabolism data.
Results
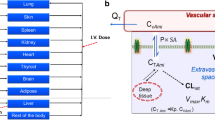
The final model consisted of 11 tissue compartments, including therapeutic target organs and those to which AMD and DEA may be harmful. Model simulations were in good agreement with the observed time courses of the drug–metabolite pair in tissues, under various dosing scenarios. The key pharmacokinetic properties of AMD, such as extensive tissue distribution, substantial storage in the fat tissue, and long half-lives in many tissues, were closely reflected.
Conclusion
The developed PBPK model can be regarded as the first step towards a PBPK–pharmacodynamic model that can used to mechanistically evaluate and explain the high adverse event rate and potentially to determine which factors are the primary drives for experiencing an adverse event.






Similar content being viewed by others
References
Kodama I, Kamiya K, Toyama J. Amiodarone: ionic and cellular mechanisms of action of the most promising class III agent. Am J Cardiol. 1999;84(9A):20R–8R.
Kodama I, Kamiya K, Toyama J. Cellular electropharmacology of amiodarone. Cardiovasc Res. 1997;35(1):13–29.
Gill J, Heel RC, Fitton A. Amiodarone. An overview of its pharmacological properties, and review of its therapeutic use in cardiac arrhythmias. Drugs. 1992;43(1):69–110.
Doval HC, et al. Randomised trial of low-dose amiodarone in severe congestive heart failure. Grupo de Estudio de la Sobrevida en la Insuficiencia Cardiaca en Argentina (GESICA). Lancet. 1994;344(8921):493–8.
Singh BN. Antiarrhythmic actions of amiodarone: a profile of a paradoxical agent. Am J Cardiol. 1996;78(4A):41–53.
Greene HL, et al. Toxic and therapeutic effects of amiodarone in the treatment of cardiac arrhythmias. J Am Coll Cardiol. 1983;2(6):1114–28.
Marchlinski FE, et al. Amiodarone pulmonary toxicity. Ann Intern Med. 1982;97(6):839–45.
Lewis JH, et al. Amiodarone hepatotoxicity: prevalence and clinicopathologic correlations among 104 patients. Hepatology. 1989;9(5):679–85.
Martino E, et al. The effects of amiodarone on the thyroid. Endocr Rev. 2001;22(2):240–54.
Charness ME, Morady F, Scheinman MM. Frequent neurologic toxicity associated with amiodarone therapy. Neurology. 1984;34(5):669–71.
Kounis NG, et al. Dose-dependent appearance and disappearance of amiodarone-induced skin pigmentation. Clin Cardiol. 1996;19(7):592–4.
Vorperian VR, et al. Adverse effects of low dose amiodarone: a meta-analysis. J Am Coll Cardiol. 1997;30(3):791–8.
Rotmensch HH, et al. Steady-state serum amiodarone concentrations: relationships with antiarrhythmic efficacy and toxicity. Ann Intern Med. 1984;101(4):462–9.
Sloskey GE. Amiodarone: a unique antiarrhythmic agent. Clin Pharm. 1983;2(4):330–40.
Araki R, et al. Population pharmacokinetic investigation for optimization of amiodarone therapy in Japanese patients. Ther Drug Monit. 2011;33(6):750–6.
Connolly SJ. Evidence-based analysis of amiodarone efficacy and safety. Circulation. 1999;100(19):2025–34.
Kosior DA, Krzykwa A, Postula M. Amiodarone administered orally or intravenously—the same or different drug? Pol Merkur Lekarski. 2013;34(202):183–7.
Libersa CC, et al. Dramatic inhibition of amiodarone metabolism induced by grapefruit juice. Br J Clin Pharmacol. 2000;49(4):373–8.
Yamreudeewong W, et al. Potentially significant drug interactions of class III antiarrhythmic drugs. Drug Saf. 2003;26(6):421–38.
Fenner KS, et al. Drug-drug interactions mediated through P-glycoprotein: clinical relevance and in vitro-in vivo correlation using digoxin as a probe drug. Clin Pharmacol Ther. 2009;85(2):173–81.
Gerlowski LE, Jain RK. Physiologically based pharmacokinetic modeling: principles and applications. J Pharm Sci. 1983;72(10):1103–27.
Boonpawa R, et al. A physiologically based kinetic (PBK) model describing plasma concentrations of quercetin and its metabolites in rats. Biochem Pharmacol. 2014;89(2):287–99.
Rostami-Hodjegan A. Physiologically based pharmacokinetics joined with in vitro-in vivo extrapolation of ADME: a marriage under the arch of systems pharmacology. Clin Pharmacol Ther. 2012;92(1):50–61.
Barrett JS, et al. Physiologically based pharmacokinetic (PBPK) modeling in children. Clin Pharmacol Ther. 2012;92(1):40–9.
Zhao Y, Hu ZY. Physiologically based pharmacokinetic modelling and in vivo [I]/K(i) accurately predict P-glycoprotein-mediated drug-drug interactions with dabigatran etexilate. Br J Pharmacol. 2014;171(4):1043–53.
Price PS, et al. Modeling interindividual variation in physiological factors used in PBPK models of humans. Crit Rev Toxicol. 2003;33(5):469–503.
Jiang XL, et al. Application of physiologically based pharmacokinetic modeling to predict acetaminophen metabolism and pharmacokinetics in children. CPT Pharmacometrics Syst Pharmacol. 2013;2:e80.
Machavaram KK, et al. A physiologically based pharmacokinetic modeling approach to predict disease-drug interactions: suppression of CYP3A by IL-6. Clin Pharmacol Ther. 2013;94(2):260–8.
Khalil F, Laer S. Physiologically based pharmacokinetic modeling: methodology, applications, and limitations with a focus on its role in pediatric drug development. J Biomed Biotechnol. 2011;2011:907461.
Bellen JC, Penglis S, Tsopelas C. Radiolabeling and biodistribution of amiodarone and desethylamiodarone. Nucl Med Biol. 1995;22(7):953–5.
Weir SJ, Ueda CT. Amiodarone pharmacokinetics. II. Disposition kinetics following subchronic administration in rats. Biopharm Drug Dispos. 1987;8(5):449–60.
Wyss PA, Moor MJ, Bickel MH. Single-dose kinetics of tissue distribution, excretion and metabolism of amiodarone in rats. J Pharmacol Exp Ther. 1990;254(2):502–7.
Nanas JN, Mason JW. Pharmacokinetics and regional electrophysiological effects of intracoronary amiodarone administration. Circulation. 1995;91(2):451–61.
Brien JF, et al. Disposition of amiodarone and its proximate metabolite, desethylamiodarone, in the dog for oral administration of single-dose and short-term drug regimens. Drug Metab Dispos. 1990;18(6):846–51.
Riva E, et al. Pharmacokinetics of amiodarone in rats. J Cardiovasc Pharmacol. 1982;4(2):270–5.
Plomp TA, et al. Pharmacokinetics and body distribution of amiodarone and desethylamiodarone in rats after intravenous administration. In Vivo. 1989;3(1):33–47.
Shayeganpour A, et al. The impact of experimental hyperlipidemia on the distribution and metabolism of amiodarone in rat. Int J Pharm. 2008;361(1–2):78–86.
Weir SJ, Ueda CT. Amiodarone pharmacokinetics. I. Acute dose-dependent disposition studies in rats. J Pharmacokinet Biopharm. 1986;14(6):601–13.
Elsherbiny ME, Brocks DR. The effect of CYP1A induction on amiodarone disposition in the rat. J Pharm Sci. 2010;99(1):539–48.
Shayeganpour A, El-Kadi AO, Brocks DR. Determination of the enzyme(s) involved in the metabolism of amiodarone in liver and intestine of rat: the contribution of cytochrome P450 3A isoforms. Drug Metab Dispos. 2006;34(1):43–50.
Campos Moreno E, et al. Population modelling to describe pharmacokinetics of amiodarone in rats: relevance of plasma protein and tissue depot binding. Eur J Pharm Sci. 2007;30(2):190–7.
Shayeganpour A, Hamdy DA, Brocks DR. Pharmacokinetics of desethylamiodarone in the rat after its administration as the preformed metabolite, and after administration of amiodarone. Biopharm Drug Dispos. 2008;29(3):159–66.
Brown RP, et al. Physiological parameter values for physiologically based pharmacokinetic models. Toxicol Ind Health. 1997;13(4):407–84.
Kagan L, et al. Physiologically based pharmacokinetic model of amphotericin B disposition in rats following administration of deoxycholate formulation (Fungizone(R)): pooled analysis of published data. AAPS J. 2011;13(2):255–64.
Meno-Tetang GM, et al. Physiologically based pharmacokinetic modeling of FTY720 (2-amino-2[2-(-4-octylphenyl)ethyl]propane-1,3-diol hydrochloride) in rats after oral and intravenous doses. Drug Metab Dispos. 2006;34(9):1480–7.
Hu ZY, Lu J, Zhao Y. A physiologically based pharmacokinetic model of alvespimycin in mice and extrapolation to rats and humans. Br J Pharmacol. 2014;171(11):2778–89.
Elsherbiny ME, El-Kadi AO, Brocks DR. The metabolism of amiodarone by various CYP isoenzymes of human and rat, and the inhibitory influence of ketoconazole. J Pharm Pharm Sci. 2008;11(1):147–59.
Deng P, et al. Identification of amiodarone metabolites in human bile by ultraperformance liquid chromatography/quadrupole time-of-flight mass spectrometry. Drug Metab Dispos. 2011;39(6):1058–69.
Davies B, Morris T. Physiological parameters in laboratory animals and humans. Pharm Res. 1993;10(7):1093–5.
Latini R, Tognoni G, Kates RE. Clinical pharmacokinetics of amiodarone. Clin Pharmacokinet. 1984;9(2):136–56.
McLure JA, Miners JO, Birkett DJ. Nonspecific binding of drugs to human liver microsomes. Br J Clin Pharmacol. 2000;49(5):453–61.
Houston JB. Utility of in vitro drug metabolism data in predicting in vivo metabolic clearance. Biochem Pharmacol. 1994;47(9):1469–79.
Mager DE, et al. Physiologically based pharmacokinetic model for composite nanodevices: effect of charge and size on in vivo disposition. Pharm Res. 2012;29(9):2534–42.
Pertinez H, Chenel M, Aarons L. A physiologically based pharmacokinetic model for strontium exposure in rat. Pharm Res. 2013;30(6):1536–52.
Ohno Y, et al. General framework for the prediction of oral drug interactions caused by CYP3A4 induction from in vivo information. Clin Pharmacokinet. 2008;47(10):669–80.
Nadanaciva S, et al. A high content screening assay for identifying lysosomotropic compounds. Toxicol In Vitro. 2011;25(3):715–23.
Morissette G, et al. Intracellular sequestration of amiodarone: role of vacuolar ATPase and macroautophagic transition of the resulting vacuolar cytopathology. Br J Pharmacol. 2009;157(8):1531–40.
Trachsel D, et al. Pharmacokinetics and pharmacodynamic effects of amiodarone in plasma of ponies after single intravenous administration. Toxicol Appl Pharmacol. 2004;195(1):113–25.
Chen Y, Mao J, Hop CE. Physiologically based pharmacokinetic modeling to predict drug-drug interactions involving inhibitory metabolite: a case study of amiodarone. Drug Metab Dispos. 2015;43(2):182–9.
Yamashita F, et al. Modeling of rifampicin-induced CYP3A4 activation dynamics for the prediction of clinical drug-drug interactions from in vitro data. PLoS One. 2013;8(9):e70330.
Hirsch J, Han PW. Cellularity of rat adipose tissue: effects of growth, starvation, and obesity. J Lipid Res. 1969;10(1):77–82.
Ohyama K, et al. A significant role of human cytochrome P450 2C8 in amiodarone N-deethylation: an approach to predict the contribution with relative activity factor. Drug Metab Dispos. 2000;28(11):1303–10.
Zahno A, et al. The role of CYP3A4 in amiodarone-associated toxicity on HepG2 cells. Biochem Pharmacol. 2011;81(3):432–41.
Ohyama K, et al. Inhibitory effects of amiodarone and its N-deethylated metabolite on human cytochrome P450 activities: prediction of in vivo drug interactions. Br J Clin Pharmacol. 2000;49(3):244–53.
Mori K, et al. Cocktail-substrate assay system for mechanism-based inhibition of CYP2C9, CYP2D6, and CYP3A using human liver microsomes at an early stage of drug development. Xenobiotica. 2009;39(6):415–22.
Reasor MJ, Kacew S. An evaluation of possible mechanisms underlying amiodarone-induced pulmonary toxicity. Proc Soc Exp Biol Med. 1996;212(4):297–304.
Varbiro G, et al. Concentration dependent mitochondrial effect of amiodarone. Biochem Pharmacol. 2003;65(7):1115–28.
Spaniol M, et al. Toxicity of amiodarone and amiodarone analogues on isolated rat liver mitochondria. J Hepatol. 2001;35(5):628–36.
Hohol K, et al. Neuropathy due to amiodarone: schwann cells are the target. Neurology. 2012;78(Meeting Abstracts 1):P06.138.
Beddows SA, et al. Cytotoxic effects of amiodarone and desethylamiodarone on human thyrocytes. Biochem Pharmacol. 1989;38(24):4397–403.
Wiper A, Roberts DH, Schmitt M. Amiodarone-induced skin pigmentation: Q-switched laser therapy, an effective treatment option. Heart. 2007;93(1):15.
Acknowledgments
The authors thank David Colon-Smith in Department of Computer Science at Duke University for correcting spelling, grammar, usage and punctuation errors of the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
No sources of funding were used to conduct this study.
Conflict of interest
The authors declare no conflict of interest.
Appendix
Appendix
1.1 I. Model parameter abbreviations
Abbreviations | Parameter |
|---|---|
QC | Cardiac output |
Q Tissue | Blood flow to tissue |
V VB | Volume of venous blood |
V AB | Volume of arterial blood |
V Tissue | Volume of tissue |
V VTissueC | Fraction of vascular space in tissue |
PS Tissue | Permeability-surface area product between vascular space and extravascular space in tissue |
F u,Tissue | Fraction of unbound drug in tissue |
F u,Bld | Fraction of unbound drug in blood |
F u,plasma | Fraction of unbound drug in plasma |
F u,mic | Fraction of unbound drug in liver microsomes |
K a,Tissue | First-order association rate constant for drug binding to ‘deep tissue’ of tissue |
K d,Tissue | First-order dissociation rate constant for drug binding to ‘deep tissue’ of tissue |
K BP | Blood to plasma concentration ratio |
V max,LivMet,AMD | Maximum metabolic rate of AMD to DEA in liver |
K M,LivMet,AMD | AMD concentration at which half of V max,LivMet,AMD is achieved |
CL OthMet,AMD | Clearance of unbound AMD mediated by conversion to other metabolites rather than DEA |
CL Met,DEA | Clearance of unbound DEA mediated by metabolism |
A VTissue | Drug amount in vascular space of tissue |
A Tissue | Drug amount in tissue extravascular space except in ‘deep tissue’ |
A TissueDeep | Drug amount in ‘deep tissue’ of tissue extravascular space |
A AB | Drug amount in arterial blood |
A VB | Drug amount in venous blood |
C VTissue | Drug concentration in vascular space of tissue |
C Tissue | Drug concentration in tissue extravascular space except in ‘deep tissue’ |
C VMix | Drug concentration in mixed venous blood |
C V | Drug concentration in venous blood |
C plasma | Drug concentration in venous plasma |
C A | Drug concentration in arterial blood |
DoseRate | Dosing rate |
1.2 II. Equations
Since model equations describing drug disposition in different tissue compartments are very similar, only the equations for liver, arterial blood pool and venous blood pool were presented.
1.2.1 A. Liver
1. For AMD
Liver vascular space:
Liver extravascular space:
Equation 3 describes the changing rate of AMD amount in vascular space of liver; Eq. 5 describes the changing rate of AMD amount in extravascular space of liver except in ‘deep tissue’; Eq. 7 describes the changing rate of AMD amount in ‘deep tissue’ of liver; Eq. 8 describes the AMD concentration in extravascular space of liver.
2. For DEA
Liver vascular space:
Liver extravascular space:
1.2.2 B. Venous blood
1. For AMD
Equation 16 describes the changing rate of AMD amount in venous blood.
2. For DEA
1.2.3 C. Arterial blood
1. For AMD
Equation 23 describes the changing rate of AMD amount in arterial blood.
2. For AMD
Rights and permissions
About this article
Cite this article
Lu, JT., Cai, Y., Chen, F. et al. A Physiologically Based Pharmacokinetic Model of Amiodarone and its Metabolite Desethylamiodarone in Rats: Pooled Analysis of Published Data. Eur J Drug Metab Pharmacokinet 41, 689–703 (2016). https://doi.org/10.1007/s13318-015-0295-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-015-0295-0