Abstract
For most Western Australian plant species, no information is available on the effect of Phytophthora cinnamomi on seedling emergence, early survival, and early plant growth. Such information is required when selecting species for rehabilitating Eucalyptus marginata (jarrah) forest areas affected by Phytophthora dieback. This study evaluates the response of several native species to P. cinnamomi to identify those resistant to the pathogen at the early stages of plant development. Firstly, the effect of P. cinnamomi on seedling emergence and early survival was evaluated for 50 native species. Then, 24 species were selected and tested for their resistance to P. cinnamomi when more mature. They were infested at 5–7 months old and disease impact assessed after two months. Of 38 species that emerged in sufficient numbers for statistical analysis, P. cinnamomi did not affect either emergence or survival of 9 species, emergence or survival was decreased in 14 species, and both emergence and survival were significantly decreased in 15 species. Of the species tested at 5–7 months old, two were resistant, six were moderately resistant, ten were susceptible, three were highly susceptible, and three were tolerant hosts of P. cinnamomi. These results indicate that field resistance recorded from mature plants may be due to asymptomatic infection or resistance may not be present in seedlings. Therefore, data from mature plants may not be appropriate when selecting species for seed-based restoration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The soil-borne root pathogen Phytophthora cinnamomi has caused a significant reduction in biodiversity in many Australian natural ecosystems (Cahill et al. 2008). For example, in Western Australia, more than a million hectares of native flora have been extensively damaged (Department of Environment 2014) including the biodiverse ecosystems of the jarrah (Eucalyptus marginata) forest (Shearer and Tippett 1989) and the Swan Coastal Plain (Shearer and Dillon 1996). Even though the initial introduction of the pathogen to the jarrah forest occurred in the 1920s, species diversity in the infested areas has not recovered (Podger 1972; Shearer et al. 2007, 2009). The pathogen can persist in areas where susceptible woody plant species have been killed through its asymptomatic infections of annual and herbaceous perennial plants (Crone et al. 2013). Rehabilitation of infested areas through broadcasting seeds requires the use of species known to be resistant to the pathogen at the stage of seedling emergence and early seedling survival, as well as when plants are mature.
Many studies report the disease response of Australian species to P. cinnamomi (Shearer and Dillon 1995, 1996; Shearer et al. 2004; Kueh et al. 2012; Belhaj et al. 2018; Migliorini et al. 2019). These are mainly based on field observation of which species die as the disease front progresses and glasshouse studies of plants established in pots before inoculation. Species responses to P. cinnamomi have been compiled by McDougall (2005) and Groves et al. (2009). However, McDougall (2005), lists information on only approximately 8% of Western Australian species, and Groves et al. (2009) include only Western Australian species suitable for domestic or amenity horticulture in infested soil.
Thus, the disease response of most Western Australian species to P. cinnamomi is unknown, and the available records provide information on the species losses expected should the pathogen be introduced into a particular area. However, there is less information on the species’ response to the pathogen at seedling emergence and early seedling survival. The effect of the pathogen on seedling emergence and early survival of five mallee eucalypt taxa and several plant species native to kwongan plant communities has been reported (Simamora et al. 2017; Shaw 2019). Information on responses at the stages of emergence and early survival is essential as it cannot be assumed that species showing resistance as mature plants will also be resistant at these early stages. Plants may become more resistant to P. cinnamomi as they mature due to changes in physiology and biochemistry between the juvenile and mature growth phases, the formation of protective sheaths of mycorrhizae or the acquisition of protective endophytes, all of which will alter the disease response (Simamora et al. 2017). It is more difficult to hypothesise why a species might become less resistant as an adult. This study aimed to identify the plant species resistant to P. cinnamomi at the early stages of plant development. Firstly, the effect of P. cinnamomi was evaluated on seedling emergence and early survival of fifty species native to Western Australia. These included species with desirable properties for rehabilitation programs but for which there was no information on their disease response at seedling emergence and early seedling survival, and some species for which reported disease ratings were variable. Then 24 species, ranging from susceptible to resistant, were selected from the first experiment and were evaluated for their resistance to P. cinnamomi when more mature at five to seven months old.
Materials and methods
Study location and overview
Two experiments were conducted in an evaporatively cooled glasshouse environment at Murdoch University, Perth, Western Australia. The first experiment was conducted between February to April 2021 to evaluate the effect of P. cinnamomi on seedling emergence and early survival of fifty native plant species. The glasshouse temperature during the experiment was between 17.6 ± 0.26°C and 27.7 ± 0.45°C. The second experiment was conducted between July 2021 to May 2022 to evaluate the effect of P. cinnamomi on 5–7-month-old plants of twenty-four species selected from experiment one. The glasshouse temperature was between 17.7 ± 0.23°C and 26.7 ± 0.29°C during the experiment.
Isolation of Phytophthora cinnamomi
A soil sample was collected from a dying Xanthorrhoea gracilis tree in a Phytophthora-infested area of the Black Cockatoo Reserve, Mundaring, Western Australia, in November 2020. The topsoil layer was removed around the lower trunk, and around 150 g of soil and fine roots were collected into a zip-lock bag. The soil sample was transported to the laboratory and baited to isolate the pathogen according to the methods described by Simamora et al. (2018). Plates were incubated at 25°C, and P. cinnamomi growing out of baits was identified based on morphological characteristics. The isolate was freed of contaminants by transferring mycelial tips to NARH (Simamora et al. 2018) and then to half-strength potato dextrose agar to maintain a pure culture. Per ml of deionised water, NARP contains, 17 g of oxiod cornmeal agar, 1 ml nystatin (Nilsat, Wywth-Ayerst Australia Pty Ltd, Baulkham Hills, NSW), 100 mg ampicillin sodium (Fisons Pty Ltd, Sydney, NSW), 10 mg rifampcin (Rifadin, Hoechst Marion Roussel Australia Pty Ltd, Lane Cove, NSW), and 50 mg hymexazol (Tachigaren, Sankyo Company, Tokyo, Japan). The antibiotics were dissolved in sterile water and added to cooled agar (about 50°C) prior to pouring into plates. The identification of the P. cinnamomi isolate (MUCC845—GenBank accession number OR244346) was further confirmed by amplifying the ITS gene region using ITS IF and ITS4 primers at the Centre of Phytophthora Science and Management, Murdoch University, Western Australia.
Inoculum preparation
About 150 ml of millet seeds (Panicum miliaceum L.) and 120 ml of distilled water were added to 1000 ml conical flasks and sealed with a non-absorbent cotton plug. The flasks were covered with aluminium foil and autoclaved at 121°C for 20 min for three consecutive days to ensure the sterilisation of the millet seeds. The flasks were allowed to cool for 24 h and then inoculated with the P. cinnamomi isolate (MUCC845) grown in two V8 (vegetable juice) agar plates incubated at 25°C for 7 days. The flasks were shaken to distribute the agar plugs evenly, and then flasks were placed into ziplock bags and incubated at 25°C in the dark. The flasks were shaken weekly for even colonisation. Phytophthora cinnamomi colonisation was confirmed by plating a sub-sample of the millet inoculum on NARH medium and incubating it in the dark at 25°C (Simamora et al. 2018). The plant growth medium (river sand or potting mix) was inoculated with P. cinnamomi at 1% of the dry sand weight. Sterilised non-inoculated millet seeds were used for the controls.
Experiment 1 - Effect of P. cinnamomi on seedling emergence and early survival of fifty native species
Species selection
Seeds of 50 species from eight families, native to Western Australia, were sourced from Nindethana Seed Service (Albany, Western Australia) (Tables 1 and S1). The species were selected based on seed availability, plant height (to include a wide range of plant heights), distribution through the southwest of Western Australia, drought tolerance, provision of food and refuge to the wildlife and available records of P. cinnamomi susceptibility. Assessment of drought tolerance and value to wildlife through the provision of food and/or refuge was based on experimental evidence where available, species distributions and information from seed suppliers (Table S2). Hard seeds that required heat treatment for softening were soaked in hot water at 100°C for 2 min, and those that responded to smoke water were treated with smoke water (Grayson Australia, Bayswater, Victoria) diluted at 1:10 with deionised water for 24 h (Fryer 2006).
Experimental design
Washed river sand (Richgro, Canning Vale, Western Australia) steam pasteurised for two hours at 65°C was used as the growth medium. Propagation punnets (138 × 66 × 48 mm, Garden City Plastics, Forrestfield, Western Australia) with the base lined with cloth (Superwipes, Chux, Clorox Holdings Pty Limited, Australia) were filled with pasteurised river sand for controls and inoculated sand for treatments. Seeds of each species were sown at a depth of half the seed size for each species, approximately 30 mm apart. The number of seeds sown in each punnet varied based on the seed size of each species (Table 1). Multiple seeds (10–12) were sown in each indentation for the species that had tiny seeds. Five control and five treatment punnets were set up for each species to give 500 punnets. Ten punnets of randomly chosen species were placed on a tray (35 × 295 × 50 mm, Garden City Plastics, Forrestfield, Western Australia), each tray was positioned randomly within the glasshouse, and the position of the trays was randomised weekly. Separate trays were used for the control and infested punnets to avoid contamination. Punnets were hand-watered daily. The number of emerging seedlings was counted every third day. Plant species with tiny seeds had a cluster of seedlings per indentation. The number of seedlings in each indentation was also counted to determine the number of seedlings that had emerged per punnet. After three months, the final number of surviving seedlings was counted. Seedlings that died during the experiment before the final harvest were tested for P. cinnamomi in the roots by plating a root sample onto NARH.
Statistical analysis
One-way ANOVA was used to determine whether there was an impact of P. cinnamomi on seedling emergence and survival of seedlings during the first three months for each species. The seedling emergence or survival percentage was the response variable, and treatment was the predictor variable. The normality of residuals and homogeneity of variances were checked visually and statistically (Shapiro test and LeveneTest). For the species where normality and homogeneity of variances assumption were not met, the Kruskal–Wallis test and Welch ANOVA were used, respectively. Statistical analyses were performed in R version 4.1.2 (R Core Team 2021).
Experiment 2 – Screening selected plant species susceptibility from experiment 1 to P. cinnamomi
Species selection
Twenty-four plant species were selected from experiment 1 based on high seedling emergence and early survival in the presence of P. cinnamomi. Most had no information on their disease resistance as older plants. Five species in which P. cinnamomi affected both seedling emergence and early survival and were known to be susceptible as adult plants in the field were also included as controls (Table 1).
Experiment design
Native plant potting mix (Richgro, Canning Vale, Western Australia) steam pasteurised for two hours at 65°C was used as the growth medium. Three to five months old seedlings were taken from the seedlings germinating in propagation punnets and transferred to slimline pots (125 mm, 1.1 L, Garden City Plastics, Forrestfield, Western Australia) lined with Superwipes as described before. Two sterile plastic tubes (10 ml) were inserted on either side of the seedling. Seedlings were grown for two months and then inoculated with P. cinnamomi by removing the two plastic tubes and placing inoculum in each hole created by the tube. Sterilised non-inoculated millet seeds were used for the controls. At the time of inoculation, plant height was measured. There were 20 replicates for each species (10 for controls and 10 for inoculation), but deaths after transplantation reduced this number to 18 for four and 16 for seven species. The pots were placed randomly within the glasshouse, and the position of the pots was randomised weekly. The pots were hand-watered daily. Quarter-strength Dynamic Lifter liquid fertilizer (Yates Gardening Products) was used fortnightly.
Two months after inoculation, the height of the plants was measured, then plants were harvested, and the roots were gently washed with running water and blotted dried using paper towels. The root system of each plant was visually assessed and scored for root damage (1 = healthy roots, 2 = 1–25% damaged roots, 3 = 26–50% damaged roots, 4 = 51–75% damaged roots and 5 = 76–100% damaged roots) (Farooq et al. 2023). A surface sterilised root sample of each plant was plated in NARH to confirm the presence of P. cinnamomi. Roots were then dried at 37°C for one week, and the dry weight was recorded. Plant deaths observed during the experiment were recorded, and root samples from the dead plants were also plated onto NARH to reisolate P. cinnamomi.
Statistical analysis
The mean and standard deviation of shoot growth, root weight and root score for each species were calculated using the aggregate function. To determine whether P. cinnamomi inoculation affected the shoot growth, root weight and root score, the metafor package (Viechtbauer 2010) was used. The effect size estimates (mean difference) and the variance of each parameter separately for each species were calculated using the escalc function. Effect size estimates, and variance were fitted into a random model to calculate the random meta-analysis using rma function. To visualise the results, forest plots were created for each parameter using the forest function. Statistical analyses were performed in R version 4.1.2 (R Core Team 2021).
Results
Experiment 1 - Effect of P. cinnamomi on seedling emergence and early survival of fifty native plant species
Of the 50 plant species used, 38 emerged in sufficient numbers in both the control and inoculated treatments to perform the statistical analysis. The effect of P. cinnamomi on seedling emergence and early survival varied between species. For nine species, the presence of P. cinnamomi caused no significant (p > 0.05) changes to seedling emergence or survival (Table 2). Seedling emergence but not survival was significantly (p < 0.05) reduced in nine species, and seedling survival was significantly (p < 0.05) reduced in five species in the presence of P. cinnamomi (Table 2). In fifteen species, both seedling emergence and early survival were significantly (p < 0.05) reduced by P. cinnamomi (Table 2).
P. cinnamomi was not isolated from non-inoculated controls that died during the experiment, and it was isolated from approximately 60% of the dead seedlings from inoculated pots across all the species.
Experiment 2 – Screening selected plant species susceptibility from experiment 1 to P. cinnamomi
Of twenty-four species inoculated at 5–7 months old, no deaths were observed in P. cinnamomi inoculated plants of six species, and no P. cinnamomi was reisolated from the roots of these species (Table 3). In three species, no deaths were observed even though P. cinnamomi was reisolated from the roots (Table 3). One or more of the ten plants died in inoculated soil of thirteen species, and P. cinnamomi was reisolated from their roots (Table 3). Some deaths of plants of nine species occurred in the control non-inoculated pots, but no P. cinnamomi was reisolated from any of the dead plants. Plant deaths were observed in all five species previously recorded to be susceptible.
Shoot growth
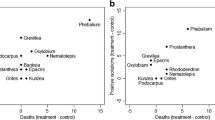
In 15 species, there was no effect of P. cinnamomi on shoot growth (Fig. 1). However, for eight species: Acacia dentifera, Banksia sessilis, Eucalyptus macrocarpa, Grevillea pterosperma, Hakea laurina, Kunzea pulchella, K. recurva and Regelia ciliata, shoot growth was significantly reduced by P. cinnamomi (Fig. 1). In contrast, significantly higher shoot growth was observed in Rhagodia preissii inoculated plants (Fig. 1).
Shoot growth of 24 species after inoculation with Phytophthora cinnamomi versus control Control and P. cinnamomi treatments are significantly (p < 0.05) different when the confidence interval for each species does not overlap with the line of no effect (Mean difference = 0). The size of the circle indicates the magnitude of the effect
Root weight and root score
The root weight of 18 species was not affected by the pathogen. P. cinnamomi significantly reduced the root weight of six species compared to the controls: A. dentifera, E. macrocarpa, G. pterosperma, K. pulchella, K. recurva and R. ciliata (Fig. 2).
Root weight of 24 species after inoculation with Phytophthora cinnamomi versus control Control and P. cinnamomi treatments are significantly (p < 0.05) different when the confidence interval for each species does not overlap with the line of no effect (Mean difference = 0). The size of the circle indicates the magnitude of the effect
Significantly different root damage scores compared to the controls were observed for B. sessilis, G. pterosperma, K. pulchella, K. recurva, Templetnia retusa and Xylomelum angustifolium (Fig. 3).
Root score of 24 species after inoculation with Phytophthora cinnamomi versus control Control and P. cinnamomi treatments are significantly different (p < 0.05) when the confidence interval for each species does not overlap with the line of no effect (Mean difference = 0). The size of the circle indicates the magnitude of the effect
Screening 24 species (at 5–7 months old) susceptibility to P. cinnamomi
Twenty-four plant species tested in the second experiment were allocated into five different categories based on the results of experiments 1 and 2 (Table 3). Species that had no mortality in inoculated plants in which P. cinnamomi was not isolated from the roots and did not affect any of the measured parameters for seedlings or 7–9 months old plants were categorised as resistant. Acacia acuminata and C. gilessii were the only two resistant species identified. (Table 3). Six species, including B. elderiana, C. phoeniceus, C. sanguineus, H. laurina, M. seriata and R. preissii were classified as moderately resistant to the pathogen (Table 3). No P. cinnamomi was isolated from the roots, there was less than 30% mortality after nine months and only up to two measured parameters were impacted.
When P. cinnamomi was isolated from the roots species were grouped as tolerant, susceptible, or highly susceptible. Acacia saligna, T. retusa, and C. hirsutus were categorised as tolerant hosts of P. cinnamomi. Even though P. cinnamomi was present in the roots, no plants died, and the pathogen significantly affected at most two measured parameters (Table 3). Ten species: A. dentifera, B. caleyi, B. lemanniana, B. sessilis, E. macrocarpa, H. petiolaris, H. trifurcata, L. lanceolata, R. ciliata, and X. angustifolium were classified as susceptible. These showed 11- < 80% mortality in inoculated plants, P. cinnamomi was isolated from the roots and up to three measured parameters were affected. Three species: G. pteroperma, K. pulchella, and K. recurva were categorised as highly susceptible to P. cinnamomi (Table 3). Mortality was higher than 80%, the pathogen was reisolated from the dead plants, and P. cinnamomi significantly affected all or most measured parameters.
Amongst the four species previously reported to be resistant, C. sanguineus and M. seriata showed moderate resistance in the current study, while A. saligna was infected asymptomatically, and H. petiolaris was susceptible. Of the eight species previously categorized as susceptible, six were susceptible or highly susceptible, while two appeared moderately resistant.
Discussion
Of the 24 plant species tested for resistance to P. cinnamomi, both as young seedlings and when inoculated 5–7-month-old plants, two species were resistant, six were moderately resistant, ten were susceptible, and three were highly susceptible. Three species were tolerant hosts of P. cinnamomi.
In the present study, data on the responses of 12 species to P. cinnamomi are reported for the first time. These included A. acuminata and C. gilesii, which were the most resistant to P. cinnamomi. Of the six moderately resistant species observed, C. sanguineus and M. seriata have also been recorded as resistant in the Stirling Range National Park, Western Australia (McDougall 2005; Groves et al. 2009). However, B. elderiana, which appeared moderately resistant in the current study, was recorded as susceptible in an experiment that used transplanted 7–9 months old plants in a Banksia woodland site and inoculated with P. cinnamomi (McCredie et al. 1985). It is possible that root damage during transplantation resulted in the plant’s susceptibility. Hakea laurina, which was observed to be susceptible to P. cinnamomi in the Stirling Range National Park, Western Australia (McDougall 2005) was moderately resistant in the current study. Callistemon phoeniceus and R. preissii, for which there is no previous information, were moderately resistant in the present study.
The susceptibility of B. caleyi, B. lemanniana, B. sessilis, H. trifurcata and X. angustifolium to P. cinnamomi is consistent with the previous records by McCredie et al. (1985) and Alcoa of Australia (2002). In the present study, many inoculated H. petiolaris plants died, and the pathogen was isolated from the roots of dead plants indicating that the species was susceptible to P. cinnamomi. This result contradicts McDougall (2005) and Groves et al. (2009) who recorded H. petiolaris as resistant to P. cinnamomi in the field. This is possibly due to plants being susceptible when young but showing improved resistance when they mature. Acacia dentifera, E. macrocarpa, L. lanceolata and R. ciliata were susceptible to P. cinnamomi in the present study, and no records are available regarding their responses to the pathogen.
Grevillea pterosperma, K. pulchella and K. recurva were highly susceptible to P. cinnamomi, with more than 85% deaths in the inoculated plants. Kunzea recurva has previously been recorded as susceptible to P. cinnamomi in the field (McDougall 2005), but this is the first record of the susceptibility of G. pterosperma and K. pulchella.
No deaths were observed in the inoculated plants of A. saligna, C. hirsutus, and T. retusa, and only one or two parameters tested were affected by the pathogen. However, P. cinnamomi was isolated from the roots of surviving plants indicating that these species were tolerant hosts to P. cinnamomi. Crone et al. (2013) revealed that some annual and herbaceous perennial native plant species in the jarrah forest can also survive in dieback areas as symptomless hosts. Similarly, the three species identified in the current study were resistant and able to survive in the P. cinnamomi inoculated soil without exhibiting disease symptoms even though the pathogen was present in the roots. Such species should be monitored further to determine if they are appropriate for rehabilitation work or if they may act as a reservoir of P. cinnamomi and increase soil inoculum levels. Further investigation of the species which would be valuable for rehabilitation work, but which have shown variable responses to the pathogen should be investigated to determine whether this variation is due to intra-species variation in susceptibility and whether seeds from some regions would be appropriate to use.
The species tested in the present study were mainly from Myrtaceae, Proteaceae and Fabaceae. Most species were resistant within the Myrtaceae and Fabaceae, whereas, within the Proteaceae, most species were susceptible to the pathogen, which is in accordance with previous reports (Kueh et al. 2012; Shearer et al. 2013). All three Calothamnus species from the Myrtaceae were resistant to the pathogen, but the Kunzea species, K. pulchella and K. recurva were highly susceptible. Within Fabaceae, A. acuminata and A. saligna were resistant, and A. dentifera was susceptible. The only resistant species in the Proteaceae were H. laurina and B. elderiana.
Most species susceptible to P. cinnamomi as young seedlings were also impacted when plants inoculated at 5–7 months. However, B. elderiana was susceptible as young seedlings, but the resistance increased when the plants were older. Shaw (2019) has revealed that the species susceptible at early stages may not be susceptible to the pathogen when they mature or vice versa. In addition, several other studies, including work both on eucalypts and crop species, also reported that the resistance of plants to Phytophthora or Pythium increased with plant age (Chun and Schneider 1998; Raftoyannis and Dick 2002; Simamora et al. 2017).
This is the first evaluation of species native to Western Australia for their response to P. cinnamomi at seedling emergence and early seedling growth. It has shown that some species showing field resistance may be susceptible at early stages and thus unsuitable for use in projects to rehabilitate P. cinnamomi infested forest sites. Screening of seedlings of species recorded as resistant from field observation or experiments with mature plants in the glasshouse is highly desirable before species selection for seed-based rehabilitation.
Data availability
Data are available on request.
References
Alcoa of Australia (2002) Indicators of Phytophthora cinnamomi used by Interpreters. Environment Research Bulletin Alcoa World Alumina, Booragoon, Western Australia
Belhaj R, McComb J, Burgess TI, Hardy GESJ (2018) Pathogenicity of 21 newly described Phytophthora species against seven Western Australian native plant species. Plant Pathol 67:1140–1149. https://doi.org/10.1111/ppa.12827
Cahill D, Rookes J, Wilson B, Gibson L, McDougall KL (2008) Phytophthora cinnamomi and Australia’s biodiversity: impacts, predictions and progress towards control. Aust J Bot 56:279–310. https://doi.org/10.1071/BT07159
Chun SC, Schneider RW (1998) Sites of infection by Pythium species in rice seedlings and effects of plant age and water depth on disease development. Phytopathology 88:1255–1261. https://doi.org/10.1094/PHYTO.1998.88.12.1255
Crone M, McComb JA, O’brien PA, Hardy GESJ (2013) Annual and herbaceous perennial native Australian plant species are symptomless hosts of Phytophthora cinnamomi in the Eucalyptus marginata (jarrah) forest of Western Australia. Plant Pathol 62:1057–1062. https://doi.org/10.1111/ppa.12016
Department of Environment (2014) Background: Threat abatement plan for disease in natural ecosystems caused by Phytophthora cinnamomi. Department of Environment, Commonwealth of Australia. https://www.dcceew.gov.au/sites/default/files/documents/background-threat-abatement-plan-disease-natural-ecosystems-caused-phytophthora-cinnamomi.pdf. Accessed 10 Jul 2022
Farooq QUA, McComb J, Hardy GSJ, Burgess TI (2023) Soil amendments and suppresssion of Phytophthora root rot in avocado (Persea indica). Australas Plant Pathol 52:1–11. https://doi.org/10.1101/2022.01.31.478582
Fryer F (2006) Seed germination records. In: Sweedman L, Merritt DJ (eds) Australian seeds: a guide to their collection, identification and biology. CSIRO Publishing, Collingwood, Victoria, pp 199–252
Groves E, Hollick P, Hardy GESJ, McComb J (2009) Western Australian native plants susceptible and resistant to Phytophthora cinnamomi. Centre for Phytophthora Science & Management (CPSM), Murdoch University, Western Australia. https://www.cpsm-phytophthora.org/downloads/natives_susceptible.pdf. Accessed 07 May 2020
Kueh KH, Mckay SF, Facelli E, Facelli JM, Velzeboer RMA, Able AJ, Scott ES (2012) Response of selected South Australian native plant species to Phytophthora cinnamomi. Plant Pathol 61:1165–1178. https://doi.org/10.1111/j.1365-3059.2012.02593.x
McCredie TA, Dixon KW, Sivasithamparam K (1985) Variability in the resistance of Banksia L.f. species to Phytophthora cinnamomi rands. Aust J Bot 33:629–637. https://doi.org/10.1071/BT9850629
McDougall KL (2005) The responses of native Australian plant species to Phytophthora cinnamomi. Appendix 4. In: O’Gara E, Howard K, Wilson B, Hardy GESJ (ed) Management of Phytophthora cinnamomi for biodiversity conservation in Australia: Part 2. National best practice guidelines. Centre for Phytophthora Science and Management, Murdoch University, Western Australia
Migliorini D, Khdiar MY, Padrón CR, Vivas M, Barber PA, Hardy GESJ, Burgess TI (2019) Extending the host range of Phytophthora multivora, a pathogen of woody plants in horticulture, nurseries, urban environments and natural ecosystems. Urban For Urban Green 46:126460. https://doi.org/10.1016/j.ufug.2019.126460
Podger FD (1972) Phytophthora cinnamomi, a cause of lethal disease in indigenous plant communities in Western Australia. Phytopathology 62:972. https://doi.org/10.1094/Phyto-62-972
R Core Team (2021) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing Vienna, Austria
Raftoyannis Y, Dick MW (2002) Effects of inoculum density, plant age and temperature on disease severity caused by pythiaceous fungi on several plants. Phytoparasitica 30:67–76. https://doi.org/10.1007/BF02983972
Shaw CJ (2019) Damping-off within natural and disturbed kwongan plant communtities. Dissertation, Murdoch University
Shearer BL, Crane CE, Barrett S, Cochrane A (2007) Phytophthora cinnamomi invasion, a major threatening process to conservation of flora diversity in the South-west Botanical Province of Western Australia. Aust J Bot 55:225–238. https://doi.org/10.1071/BT06019
Shearer BL, Crane CE, Cochrane A (2004) Quantification of the susceptibility of the native flora of the South-West Botanical Province, Western Australia, to Phytophthora cinnamomi. Aust J Bot 52:435–443. https://doi.org/10.1071/BT03131
Shearer BL, Crane CE, Cochrane JA, Dunne CP (2013) Variation in susceptibility of threatened flora to Phytophthora cinnamomi. Australas Plant Pathol 42:491–502. https://doi.org/10.1007/s13313-013-0215-1
Shearer BL, Crane CE, Fairman RG, Dunne CP (2009) Ecosystem dynamics altered by pathogen-mediated changes following invasion of Banksia woodland and Eucalyptus marginata forest biomes of south-western Australia by Phytophthora cinnamomi. Australas Plant Pathol 38:417–436. https://doi.org/10.1071/AP09018
Shearer BL, Dillon M (1995) Susceptibility of plant species in Eucalyptus marginata forest to infection by Phytophthora cinnamomi. Aust J Bot 43:113–134. https://doi.org/10.1071/BT9950113
Shearer BL, Dillon M (1996) Impact and disease centre characteristics of Phytophthora cinnamomi infestations of Banksia Woodlands on the Swan Coastal Plain, Western Australia. Aust J Bot 44:79–90. https://doi.org/10.1071/BT9960079
Shearer BL, Tippett JT (1989) Jarrah Dieback: The dynamics and management of Phytophthora cinnamomi in the jarrah (Eucalyptus marginata) forest of South-western Australia, Department of Conservation and Land Management Como, Western Australia
Simamora AV, Paap T, Howard K, Stukely MJC, Hardy GESJ, Burgess TI (2018) Phytophthora contamination in a nursery and its potential dispersal into the natural environment. Plant Dis 102:132–139. https://doi.org/10.1094/PDIS-05-17-0689-RE
Simamora AV, Stukely MJC, Barber PA, Hardy GESJ, Burgess TI (2017) Age-related susceptibility of Eucalyptus species to Phytophthora boodjera. Plant Pathol 66:501–512. https://doi.org/10.1111/ppa.12592
Viechtbauer W (2010) Conducting meta-analyses in R with the metafor package. J Stat Softw 36:1–48. https://doi.org/10.18637/jss.v036.i03
Western Australian Herbarium (1998-) Florabase—the Western Australian Flora. Department of Biodiversity, Conservation and Attractions. https://florabase.dpaw.wa.gov.au. Accessed 17 Oct 2022
Acknowledgements
We thank Murdoch University, Shire of Mundaring, and Holsworth Wildlife Research Endowment for providing financial support for this project.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Contributions
Giles Hardy, Treena Burgess and Shanika Harshani conceived and designed the research. Shanika Harshani performed the gashouse experiments and analysed the data. Shanika Harshani and Jen McComb wrote the first draft. All authors contributed critically to edit the draft.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare that are relevant to this article.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Harshani, H.S., McComb, J., Burgess, T.I. et al. Selecting plant species to rehabilitate Phytophthora cinnamomi infested forest. Australasian Plant Pathol. 52, 463–475 (2023). https://doi.org/10.1007/s13313-023-00934-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13313-023-00934-8