Abstract
Tasmannia lanceolata (native pepper) has been reported as susceptible to Phytophthora cinnamomi and the objective of this study was to identify variability in native pepper resistance to P. cinnamomi. Plant material was collected from native pepper populations across Tasmania (four regions) and selected commercially grown cultivars, and 47 clones were successfully propagated. Two disease screening experiments were conducted in “soil-free plant growth system” (SPS) units. Native pepper roots were inoculated with P. cinnamomi zoospores and maintained in controlled conditions. After one week, the pathogen was re-isolated to confirm infection success, and after two weeks root discolouration was visually assessed with images, which were then analysed with an automated machine learning system, using colour thresholds. The SPS was a successful approach to screen the early response of native pepper to P. cinnamomi. Based on pathogen re-isolation success and total root discolouration percentage, clones were categorised using a susceptibility rating method across multiple categories from highly resistant to highly susceptible. In the first experiment, all 47 propagated clones were challenged with one isolate of P. cinnamomi (Pc1), and pathogen re-isolation percentage and total root discolouration (brown and black) percentage differed significantly with clone (P < 0.001). In the second experiment, three representative clones of experiment 1 were challenged with two isolates of P. cinnamomi (Pc1 and Pc2) and clone response was similar. This study has highlighted that there is a range of responses to P. cinnamomi, from highly susceptible to highly resistant, in native pepper clones from different regions of Tasmania.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Native pepper (Tasmannia lanceolata (Poir.) A.C. Smith), also known as mountain pepper or Tasmanian pepper berry, is a species of the Winteraceae family which is endemic to Tasmania and parts of the SE Australian mainland (Clarke 2012; Cock 2013; Pengelly 2002; Read 1995). The berries of the plant contain large quantities of polygodial (Cock 2013; Jansen and De Groot 1991; Sultanbawa 2016) and it has traditionally been used as a food and medicine, and in recent decades as a boutique food product or plant extract (Cock 2013; Sultanbawa 2016). Very few pests and diseases (bacterial and fungal) have been recorded in T. lanceolata, possibly due to its antibacterial and antifungal properties. However, the species is susceptible to the soil borne oomycete Phytophthora cinnamomi, (Department of Primary Industries Parks Water and Environment of Tasmania 2018) which is a major threat to many Australian native plant species (Burgess et al. 2017a, 2021). Rotting of fine and fibrous roots, stem cankers, leaf chlorosis, leaf curl, wilting of the foliage, dieback of young shoots and necrosis are the common symptoms of P. cinnamomi infection (Graham et al. 2013; Hardham and Blackman 2018; Jung et al. 2013).
The current market need is for a continuous and a reliable supply of the berries, however production in native stands can vary considerably from year to year. Bushfood producers harvest native pepper from a range of locations to cover the low yielding fruiting seasons (Wilson 2015). Several small operators have established plantations to increase supply. The potential to utilise this species into the future relies on both conservation of native stands and successful cultivation of productive plant material.
The vigour of native pepper individuals can be identified by visual assessment prior to cultivation (Menary et al. 1999). Native pepper shows considerable variation in phenotypes depending on its distribution, and its range of extract composition is likewise varied. The selection of suitable populations of plants and timing of harvest are crucial aspects of commercialisation of essential oil crops for maintaining their extract quality (Menary et al. 1999). Successful cultivation relies on selecting plants from an appropriate provenance and finding sites with low summer temperatures and high rainfall (Wilson 2015). No native pepper cultivars or varieties are currently formally registered for commercial production (AgriFutures Australia 2017). However, some clones have been selected for small scale commercial production.
Studies of Phytophthora species have shown that the usual reactions after root or bark infection are a gradual water uptake reduction, the fast closure of stomata and, as a result, a decrease in photosynthesis (Oßwald et al. 2014). Sometimes plants can survive with P. cinnamomi infection for many years without showing disease symptoms (Jung et al. 2013) and invasive annual species can be asymptomatic hosts of P. cinnamomi (Crone et al. 2013a). Management of the pathogen in native vegetation is made more difficult due to asymptomatic, biotrophic growth in some annual and herbaceous perennials and the production of a range of survival structures (Crone et al. 2013b). Phytophthora disease management in commercial production of susceptible species can be improved by selecting resistant varieties (Sangchote et al. 2004).
Resistance to P. cinnamomi is often found among certain phenotypes within a species, for example Acacia pulchella R.Br. (family Mimosaceae), Eucalyptus ovata Labill. (Myrtaceae) (Islam et al. 2017; O’Gara et al. 2005) and Eucalyptus marginata (Stukely et al. 2007). In Tasmania, 136 native plant species have been recorded as hosts of P. cinnamomi and 31 species had highly susceptible elements in field populations (Podger et al. 1990). The families Proteaceae, Fabaceae, Xanthorrhoeacae, Dilleniaceae and Ericaceae tend to be highly susceptible to Phytophthora dieback (Cahill et al. 2008). One of the major conservation threats for biodiversity in Australia is the introduction and subsequent impact of Phytophthora cinnamomi within native vegetation (Burgess et al. 2017b).
In a recent study of native pepper in a small commercial plantation, 65% of cultivars grown showed symptoms of dieback and P. cinnamomi was detected at the site (K. Barry, unpublished). This high level of susceptibility was supported by controlled inoculation studies conducted in glasshouse soil grown plants (K Barry, unpublished). The cultivars in this plantation were from unknown original locations and therefore an assessment of variability due to origin could not be made. To increase knowledge of variability of resistance to P. cinnamomi in native pepper, screening material from a range of geographical locations would be useful. Screening plant material using artificial inoculation with a pathogen enables plant responses to be categorised. For example, Barker and Wardlaw (1995) developed categories for susceptibility of a range of Tasmanian native plant species to P. cinnamomi based on pathogen re-isolation success, root discolouration and other symptoms.
Understanding the factors that influence variation in susceptibility of native pepper cultivars to P. cinnamomi would support selection of commercial cultivars. In addition, rapid and reliable screening methods to determine susceptibility of young plants to P. cinnamomi is required to assist selection of cultivars. In this paper, we present studies which address several questions including, (1) Is a soil-free system suitable for inoculation and development of infection in native pepper by P. cinnamomi, (2) Is there evidence of variability between native pepper clones at early stages of infection, (3) Is there a relationship between root discolouration and pathogen re-isolation, which would aid rapid visual assessment of infection, (4) Do clonal properties (e.g. size, age) show a relationship with infection and root discolouration, (5) Do clones from different locations (regions) show similar results for root discolouration and/or pathogen re-isolation?
Materials & methods
Plant material
Fresh plant material (healthy stems with foliage) from approximately 70 individual native pepper plants was obtained from several regions of Tasmania and from three commercially grown cultivars, from June-October 2020. Plant material was obtained from either public land, private land with permission, or conservation areas with an approved permit (Wellington Park Management Trust collection permit). The sex of plant material selected from native stands was not always possible to determine at the time of sampling, therefore a mix of male and female cultivars was represented. While the production of native pepper relies on female plants (as production of pepper berries arises only from their flowers), disease resistant males could potentially be used as rootstocks and were therefore not excluded from the trial.
The fresh material from each individual was prepared as cuttings, as described by Barnes et al. (2000). In brief, the stem base of cuttings (shoot tips with 1–2 healthy leaves) were treated with Clonex RED rooting hormone and placed in a pasteurised seedling mixture. Cuttings were raised in mist beds at 18–26 °C to encourage root growth and under a photoperiod of 16 h with natural sunlight, supplemented with a mixture of fluorescent and incandescent light. Survival of plants was monitored over several months as the species is very slow growing. Plants were used in the experiments when they were between 5 and 9 months old, after the root system was well-established. Details of the clones that were used for screening experiments are provided in Table S1.
Phytophthora cinnamomi isolation and culture
Isolation of P. cinnamomi was achieved from soil samples with a lupin baiting technique (Eden et al. 2000; Horticulture Innovation Australia 2016), as lupins (Lupinus angustifolius) are highly susceptible to P. cinnamomi. In brief, lupin seeds were sterilised by soaking in 1% bleach solution for 1 min, then rinsed and soaked in sterile water for 40 min. Pre-germination of the sterile water-soaked lupin seeds was carried out in sterile vermiculite at room temperature. Baiting of soil used lupin seedlings which had roots that were no longer than 2 cm. Seedlings were planted in clear plastic cups (250 ml) which contained the fresh soil (approximately 3 cm depth) and enough sterile water to enable contact with the lupin radicles. All the soil samples were baited in triplicate cups, with four lupins per cup. A control sample without any soil was used in addition to the soil samples. Lupin symptoms were noted at two days intervals, and at the end of six days all the seedlings were weighed, and symptoms were noted for each lupin seedling.
A selective medium, NARPH (17 g Oxoid cornmeal agar, 1 mL nystatin, 100 mg ampicillin sodium, 10 mg rifampicin 100 mg PCNB and 50 mg hymexazol per L deionised water) was used for the isolation of P. cinnamomi from the lupin roots (Hüberli et al. 2000). Diseased roots with visible symptoms were plated on NARPH medium. These NARPH plates were incubated in the dark at 24 °C for three days. At the end of three days, plates were checked for pathogen growth and the well grown cultures were sub-cultured on NARPH medium to produce pure cultures.
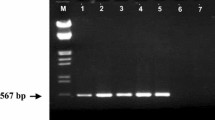
A fresh culture of P. cinnamomi was isolated from soil of a small commercial native pepper plantation in southern Tasmania and named as P. cinnamomi isolate 1 (Pc1). Another fresh culture of P. cinnamomi was isolated from soil of native forest in northern Tasmania (Mathinna Plains) and named as P. cinnamomi isolate 2 (Pc2). The purity, identity and mating type of P. cinnamomi cultures were confirmed by morphological features, growth on NARPH selective medium and PCR analysis with ITS sequencing and with P. cinnamomi species specific Ycin3F & Ycin4R primers (Schena et al. 2007). Both isolates were determined to be the A2 mating strain, with the use of reference (A1 & A2 mating strains) cultures. Genbank accession numbers were created for Pc1 (OR047869) and Pc2 (OR047870) for future reference.
Experimental design
Experiments were designed to explore the plant-pathogen interaction between P. cinnamomi and native pepper. The first experiment involved screening a large number (47) of propagated clones and examining infection within two weeks post-inoculation, while the second experiment was focussed only on three representative clones.
Both experiments were conducted in “soil-free plant growth system” (SPS) units (Gunning and Cahill 2009). Native pepper plants, raised in mist beds, were collected and rinsed with sterile water to remove all soil particles, then roots were surface sterilised in 70% alcohol for 10 s. Each SPS unit had four vertical trays and four native pepper plants could be positioned in each tray. For each clone, four plants were held in an “inoculated” SPS unit, and another four plants were held in a “control” unit. For each SPS unit, four lupin seedlings were held in one vertical tray. The base of each SPS unit was filled with 1 L of sterile water and 20 mL of nutrient solution (nutrient solution consists of 5.625 mL of KNO3, 5.625 mL of Ca (NO3)2.4H2O, 2.25 mL of MgSO4, 1.125 mL of KH2PO4, 2.25 mL of Micronutrients, 2.25 mL of Iron Chelate, 1.24 g of CaCl2 for 500 mL sterile water). Filter paper held vertically in each tray enabled moisture to wick upwards and keep plants moist. The SPS units were all housed within a single temperature-controlled growth cabinet with temperature maintained at 21 °C and 16/8 h light/dark photoperiod.
Experiment 1-inoculation of clones with isolate Pc1
Of the 70 clones collected, 47 exhibited strong enough growth with enough replicates to use in the first experiment, and they were used in two trials of 24 and 23 clones separately. Both trials were conducted identically and 2 weeks apart in time. Plants were established in the SPS units for 24 h prior to being removed briefly for direct inoculation with fresh zoospores of the Pc1 isolate. In order to inoculate the roots of native pepper clones, P. cinnamomi zoospores were produced by subculturing the mycelium from NARPH medium to V8 agar medium in accordance with the method described by Allardyce (2011) and Islam (2017). P. cinnamomi zoospores were counted with a haemocytometer and adjusted to the density of 1 × 105 zoospores/mL and applied to all roots of all plants in the inoculation units. The roots were inoculated with 3–5 sequential 20 µL drops 1 cm apart commencing from the tip of all plants. For the control (not inoculated) treatment, 20 µL drops of sterile water were applied to all roots of all plants similarly. After inoculation the plants were returned to the SPS units and growth cabinet.
For each plant, either control (C) or inoculated (I), the same sampling method was followed. For assessment of infection and re-isolation of the pathogen, at seven days post-inoculation(dpi) three root tips of each plant were excised, surface sterilised by 70% ethanol and then plated onto NARPH medium. After this root sampling, plants were returned to the SPS units. Plates were incubated and fungal growth monitored. Roots not excised as above, were monitored for visual symptoms of root disease over a 2-weeks period. The health of shoots was also monitored. Successful inoculation and infection were confirmed via observation of symptoms on simultaneously inoculated lupin roots and re-isolation of the pathogen on NARPH medium from native pepper roots.
The experiment was concluded at 14 dpi. Each plant was removed from the SPS unit and photographed under the same light conditions. In brief, colour thresholds were first determined by visual assessment. For infected roots, the first symptoms observed were light brown discolouration, which then turned to dark brown and black. In this study we combined colouration from light brown to black to report as total root discolouration. IMAGEJ (National Institute of Health, New York, NY, USA) software was used to determine colour thresholds and then root discolouration percentage of the roots of all plants was computed by an automated machine learning system.
After the experiment concluded, the plants were divided into different tissues (leaves, stem, and roots), placed in paper bags, and dried at 60 °C until a constant weight was achieved. Dry weight of each plant tissue was determined. As plants had only been infected for two weeks, we did not anticipate the inoculation would impact dry weight. The decision to determine dry weight after the trial was due to it requiring destructive sampling. Dry weight was considered the best objective measure of plant size given growth from cuttings is variable in structure.
The pathogen re-isolation results and root discolouration symptoms were used to categorise the clones into susceptible or resistant categories, following adaptation of a method used by Barker and Wardlaw (1995). The ratings used were: (1) Highly Resistant (HR)-Clones with 0% pathogen re-isolation success and less than 20% total root discolouration; (2) Moderately Resistant (MR)-Clones with 0% pathogen re-isolation success and more than 20% total root discolouration; (3) Slightly Resistant (SR)-Clones with variable pathogen re-isolation success (average which is > 0% and < 100%) and less than less than 20% total root discolouration; (4) Slightly Susceptible (SS)-Clones with variable pathogen re-isolation success (average which is > 0% and < 100%) and more than 20% total root discolouration; (5) Moderately Susceptible (MS)-Clones with 100% pathogen re-isolation success and less than 20% total root discolouration; (6) Highly Susceptible (HS)-Clones with 100% pathogen re-isolation success and more than 20% total root discolouration.
Experiment 2-inoculation of selected clones with isolates Pc1 and Pc2
Experiment 2 was conducted to confirm the responses of a selected number of clones to two different Pc isolates. Clone 17, 36 (East and North-East regions respectively, highly susceptible in experiment 1) and 45 (southern region, highly resistant in experiment 1) were used in the experiment. This was conducted in a similar way to experiment 1 in SPS units, with the use of lupin positive controls and inoculation with a zoospore solution. Four plants of each clone were challenged with each isolate (Pc1 and Pc2) of P. cinnamomi and four plants of each clone were used as controls. Re-isolation of the pathogen was attempted from inoculated and control roots harvested at seven dpi, but more root lengths were used for this experiment (1, 3, 5 and 7 cm above the root tip), then plated on NARPH medium. At 14 dpi plants were removed from the SPS unit, and root discolouration was visually assessed.
Statistical analysis of data
Statistical analysis was conducted using IBM SPSS software (IBM Corp, v27, Armonk, NY, USA). Two-way ANOVA was performed on each variable to test the effect of factors (i.e. clone, inoculation treatment and clone x inoculation treatment interaction) and P values less than 0.05 indicated statistical significance. Correlation between two variables was determined by calculating the Pearson Correlation Coefficient.
Results
Experiment 1
Pathogen re-isolation
In the first experiment with 47 clones and one isolate of P. cinnamomi, the pathogen was not isolated from any control plants. However, it was consistently re-isolated from all the inoculated lupin plants, which indicated that the zoospores were infective. From inoculated native pepper plants, the pathogen could not be re-isolated from roots of 17 clones, while it was re-isolated from all root samples of plants of 13 clones, and results were variable for the remaining 17 clones (Table 1). Pathogen re-isolation percentage differed significantly by clone (P < 0.001), inoculation treatment (P < 0.001), and the clone x treatment interaction (P < 0.001).
Symptoms development and root discolouration
Dark discolouration was found more often in the roots of inoculated plants compared to control plants (Fig. 1). Total root discolouration percentage (brown and black) differed significantly by clone (P < 0.001), treatment (P < 0.001), and the clone x treatment interaction (P < 0.001). Similarly, root discolouration percentage (black only) differed significantly by clone (P < 0.001), treatment (P < 0.001), and the clone x treatment interaction (P < 0.001).
There was a large range in percentage root discolouration of both inoculated and control native pepper plants. For inoculated plants the highest percentage of total root discolouration was 70% (clone 5, 69.8%) and the lowest was 8% (clone 41, 7.6%). About three-quarters of all clones (35 clones) had higher total root discolouration percentage in inoculated compared to control plants. In the other 12 clones, which included one commercially grown, five from the NE region and six from the Southern region, discolouration was lower in the inoculated roots than controls (Fig. 2a).
When examining black only discolouration, similar trends were found to total discolouration (Fig. 2b). For inoculated plants, the highest percentage of black only root discolouration was almost 40% (38.9%, clone 5) while the lowest was around 1% (1.2%, clone 41). As above, about three-quarters of all clones had higher black only root discolouration percentage in inoculated compared to control plants except in 11 clones, which included five from the NE region and six from the Southern region (Fig. 2b). There was no significant effect of region on total root discolouration percentage (Figure S1).
Difference of average root discolouration percentage in P. cinnamomi inoculated (I) and control (C) plants, per native pepper clone, categorised by source of clone origin (1-NW Tasmania, 2-Eastern Tasmania, 3-Commercial plantation, 4-NE Tasmania, 5-Southern Tasmania). a) total (brown and black) discolouration, b) black discolouration. NB. As data points are the difference between averages, no variability of replicates(n = 4) within clones is shown
In addition to discolouration, other symptoms were observed in some inoculated plants such as water soaking, wilting in shoots, termination of root growth and tissue necrosis. Death of the whole plant was visible in one inoculated plant of clone 5 and 24, and in two inoculated plants of clone 10.
Resistance categories
Based on the results of pathogen re-isolation success and total root discolouration percentage, clones from different regions were allocated across multiple resistance categories, and at least one clone from each region was allocated to the highly resistant category (Table 1).
Clone size
To determine if clone vigour (based on size at the time of the experiments) had any influence on experimental results, dry weight of plant tissues was determined after the experiment. As expected, dry weight of all tissues (leaves, stem, and roots) differed significantly with clone (P < 0.001), but not with inoculation treatment (P > 0.05). There was no significant effect of region on dry weight of each plant (Fig. 3).
Relationship between variables
Clonal material was 5–9 months old at the time of the experiment (Table S1), however, there was no significant effect of clone age on pathogen re-isolation percentage. Also, there was no significant effect of clone age on total root discolouration percentage (Fig. 4). There was no significant relationship between total root discolouration percentage and pathogen re-isolation percentage (Fig. 5).
In this study, there was a significant (P = 0.000) and positive correlation between total root discolouration percentage and total dry mass for both the control plants and inoculated plants. However, the strength of the correlation was greater for the inoculated plants (r = 0.622) than for the controls (r = 0.264).
Experiment 2
Pathogen re-isolation from plant roots in experiment 2 led to the same results as experiment 1, for both Pc1 and Pc2. That is 0% pathogen re-isolation success was detected for clone 45, while 100% pathogen re-isolation success was detected for clone 17 and 36. P. cinnamomi was detected in all root sections from the root tip to x 7 cm of its length, in clone 17 and 36. The pathogen was consistently isolated from inoculated lupin plants and never from the non-inoculated native pepper controls.
Root discolouration was mostly consistent from tip to the top of each inoculated root. Some root lengths were not discoloured along the whole length examined; however, the pathogen could still be re-isolated.
Discussion
Evidence of disease resistance
In this study of native pepper with early symptoms (two weeks post-inoculation), we detected considerable variation in pathogen re-isolation success and total root discolouration percentage among control and inoculated plants. Within clones propagated from plant material collected from different regions in Tasmania, clones were placed in several categories, from highly resistant to highly susceptible. Certain clones from all regions displayed some resistance to P. cinnamomi and we observed highly resistant clones in all regions.
Disease expression is dependent on the environmental conditions. Due to lack of visibility of disease symptoms in the field, confirmation of the level of resistance may be challenging. Allardyce et al. (2012) explained a method to assess plant resistance/susceptibility on P. cinnamomi infection using parameters such as plant fresh weight, root length, root lesion length, extent of pathogen colonisation and leaf chlorophyll content. The challenge is to accurately define at what point the resistance-susceptibility continuum the plants’ response to infection occurs (Shearer et al. 2007).
Podger and Brown (1989) defined a five-class classification scale of the relative response of fifty-four taxa of higher plants populations to the presence of P. cinnamomi using controlled and field trials. “Resistant” was species in which individuals showed no or only slight symptoms of infection while “Highly Susceptible” was species in which there appeared to be no resistant element in the populations, and which were eliminated in one to three years of first infection.
Barker and Wardlaw (1995) outlined a P. cinnamomi susceptibility rating for different plant species based on pathogen re-isolation success and root discolouration. “Resistant” was species in which individuals showed no symptoms and from which P. cinnamomi was not isolated. “Resistant host” included plants for which the pathogen was isolated but there were no symptoms. Susceptibility to P. cinnamomi has also been evaluated with disease progress curves; as conducted by Shearer et al. (2007) to assess susceptibility of species in the South-West botanical province of Western Australia. To analyse disease progress, a logistic model was used as it described numerous observed disease progress curves.
A previous study found that P. cinnamomi was isolated from 61% native pepper plants (out of plants tested) in a disturbed rainforest (Podger and Brown 1989). According to the results of experiment 1 of this study, 28 (60%) of 47 clones were susceptible. Therefore, the selection of clones we used appears to represent a natural population in terms of infection rate.
Use of soil-free system to study native pepper - P. cinnamomi pathosystem
The SPS units were initially developed for root disease studies of plants with simple root systems, such as cereals and grasses (Gunning and Cahill 2009; Islam et al. 2017). This system provides easy access to the root system to visualise root and pathogen interactions without involvement of soil or other adhering particulate matter. Therefore, the system is ideal for studies of pathogens that colonize the primary tissues of young roots (Gunning and Cahill 2009).
Native pepper is slow growing and survival rates from propagated material is low. However, when plants were handled carefully, they were able to survive in the SPS units for several weeks. The soil-free system enabled zoospores to be applied in a consistent way, directly to plant roots. It also allowed easy access to roots for sampling, without the need to wash away soil for pathogen re-isolation or assessment of root discolouration. P. cinnamomi could be re-isolated from roots within seven days of inoculation, which highlights that infection is relatively rapid. Additionally, significant differences in root discolouration were detected within 14 days, highlighting that the SPS system is a valid method for the early screening of infection in native pepper.
This system has also been used for young plants of woody species, such as pineapple (Lu et al. 2019) and therefore the system is likely to be useful for a range of woody plants. Aeroponics systems have been used successfully to study infection development of P. cinnamomi (Groves et al. 2015) on woody perennials such as E. marginata (Burgess et al. 1998; Jackson et al. 2000). An aeroponics system could also be explored in future trials on native pepper, but we suspect the species requires greater amount of free water to survive.
As above, there are several advantages of conducting the screening trials in the SPS system, however, the responses illustrated under the controlled conditions do not represent field conditions and would have to be tested with growth of the plants in field trials. Therefore, these early screening results would need to be validated to indicate the practical use of the results for clone selection. Also, in field conditions the inoculum concentration would likely be lower and may lead to less infection. However, understanding the innate level of resistance that a species can exhibit is required prior to evaluating other influences (Islam et al. 2017), and susceptibility levels to P. cinnamomi in a shade house environment following soil inoculation was similar to that recorded in disease centres in natural environments and therefore represents a realistic assessment of host response to the pathogen (Shearer et al. 2007).
Variation between native pepper clones
Based on the results shown in Table 1, there were some regional trends, suggesting NW clones were generally more susceptible, while the Southern clones were less susceptible, and East, NE and commercially sourced clones had a range of resistant and susceptible clones. Infection and mortality commenced across clones from different age classes (within the 5–9 months range). Age differences of clonal material from these different regions may have confounded results to some extent-that is, the oldest plants in the study were from the NW region and the youngest from the South region.
Increased resistance with plant age has been found for different Phytophthora species and agriculture host pathosystems (Lazarovits et al. 1981) but the trend does not seem to be as clear in native Australian plants infected with P. cinnamomi (Shearer et al. 2007). Eucalyptus seedlings in Western Australian nurseries are susceptible to P. cinnamomi at all life stages tested, however the mortality rates have been found to decline with the plant age (Simamora et al. 2017). Susceptibility of Banksia species was similar to P. cinnamomi in 2–3 weeks old and 10 months old seedlings (Tynan et al. 1998) and in 1–2 years old seedlings and 6 years old saplings of Eucalyptus marginata (Stukely and Crane 1994). Native pepper clones from the Southern region were the youngest when included in experiment 1 of this study, and most of these were categorised as highly resistant. The outcome of the interactions between the pathogen and individual plants may depend on both growth rates of the pathogen and the speed of the host response to infection.
According to a study of silver birch (Betula pendula) seedling roots, there was a smaller number of root segments in inoculated compared to control seedlings, suggesting root loss after Phytophthora inoculation (Hamberg et al. 2021). In a study of Fagus sylvatica seedlings infected with the root pathogen Phytophthora citricola, results showed a higher shoot and root dry weight in inoculated compared to control seedlings. This was speculated to be due to increased concentration of Phytophthora hyphae in the plant tissues (Portz et al. 2011). However, in this present study there was not a significant difference in dry weight of leaf, stem or root tissues compared to inoculated and control plants, except the clonal difference of dry weight.
Due to the potential for variation in pathogenicity among P. cinnamomi isolates, usage of more than one virulent isolate for testing host susceptibility is necessary (Dudzinski et al. 1993; Shearer et al. 2007). Weste (1975), for example, examined two Australian P. cinnamomi isolates, one each of the A1 and A2 mating strains and found variations in their pathogenicity to Nothofagus cunninghamii seedlings. It is worth noting, however, that for the present study it is only the A2 isolate that is found in Tasmania. Dudzinski et al. (1993) suggested that pathogenicity is a relatively stable characteristic and detection of variation in susceptibility to P. cinnamomi between selected E. marginata clones was affected by the pathogenicity of isolates. Their study confirmed that variation in pathogenicity within Australian populations of P. cinnamomi must be considered while selecting isolates for testing for resistance in trees and other woody hosts, however, they found no recognisable differences in pathogenicity of their isolates, against the host species tested. The three native pepper clones assessed in experiment 2 of this study responded similarly with two different Pc isolates, and therefore there was not any difficulty in categorising resistant and susceptible native pepper varieties.
Conclusion
The SPS design was a successful approach to screen the early responses of native pepper to infection by P. cinnamomi. There were differences in infection severity of individual clones. There was a range of responses to infection for clones from each region, and no region from which clones were derived was more or less likely to be associated with resistance versus susceptibility. This finding is important for both plant selection for production, and for conservation, as certain cloned plants from all regions displayed some resistance to P. cinnamomi. It is clear from our study that resistance of native pepper to P. cinnamomi is not necessarily related to one factor such as location of origin, age, or clone size. Methods used in this study could be used as a model approach to select native plants for commercial improvement and to produce plants which are most resistant to P. cinnamomi for commercial and conservation purposes.
References
AgriFutures A (2017) Mountain pepper https://www.agrifutures.com.au/farm-diversity/mountain-pepper/. Accessed 28 January 2020
Allardyce JA (2011) Defence mechanisms of a resistant monocot model to Phytophthora cinnamomi. PhD thesis, Deakin University, Geelong
Allardyce JA, Rookes JE, Cahill DM (2012) Defining Plant Resistance to Phytophthora cinnamomi: a standardized Approach to Assessment. 160(6):269–276. https://doi.org/10.1111/j.1439-0434.2012.01895.x
Barker P, Wardlaw T (1995) Susceptibility of selected tasmanian rare plants to Phytophthora cinnamomi. Aust J Bot 43(4):379–386
Barnes RW, Jordan GJ, Hill RS, McCoull CJ (2000) A common boundary between distinct northern and southern morphotypes in two unrelated tasmanian rainforest species. Aust J Bot 48(4):481. https://doi.org/10.1071/bt98044
Burgess T, McComb J, Hardy G, Colquhoun I (1998) Influence of low oxygen levels in aeroponics chambers on eucalypt roots infected with Phytophthora cinnamomi. Plant Dis 82(4):368–373
Burgess TI, Scott JK, McDougall KL, Stukely MJC, Crane C, Dunstan WA, Brigg F, Andjic V, White D, Rudman T, Arentz F, Ota N, Hardy GESJ (2017a) Current and projected global distribution of Phytophthora cinnamomi, one of the world’s worst plant pathogens. Glob Change Biol 23(4):1661–1674. https://doi.org/10.1111/gcb.13492
Burgess TI, White D, McDougall KM, Garnas J, Dunstan WA, Català S, Carnegie AJ, Worboys S, Cahill D, Vettraino A-M (2017b) Distribution and diversity of Phytophthora across Australia. Pac Conserv Biology 23(2):150–162
Burgess T, Edwards J, Drenth A, Massenbauer T, Cunnington J, Mostowfizadeh-Ghalamfarsa R, Dinh Q, Liew E, White D, Scott P (2021) Current status of Phytophthora in Australia. Persoonia-Molecular Phylogeny and Evolution of Fungi 47(1):151–177
Cahill DM, Rookes JE, Wilson BA, Gibson L, McDougall KL (2008) Phytophthora cinnamomi and Australia’s biodiversity: impacts, predictions and progress towards control. Aust J Bot 56(4):279–310
Clarke M (2012) Australian native food industry stocktake. RIRDC, Canberra
Cock I (2013) The phytochemistry and chemotherapeutic potential of Tasmannia lanceolata (tasmanian pepper): a review. Pharmacognosy Commun 3:13–25
Crone M, McComb JA, O’Brien PA, Hardy GE (2013a) Annual and herbaceous perennial native australian plant species are symptomless hosts of Phytophthora cinnamomi in the Eucalyptus marginata(jarrah) forest of western Australia. Plant Pathol 62(5):1057–1062. https://doi.org/10.1111/ppa.12016
Crone M, McComb JA, O’Brien PA, Hardy GE (2013b) Survival of Phytophthora cinnamomi as oospores, stromata, and thick-walled chlamydospores in roots of symptomatic and asymptomatic annual and herbaceous perennial plant species. Fungal Biol 117(2):112–123. https://doi.org/10.1016/j.funbio.2012.12.004
Department of Primary Industries Parks Water and Environment of Tasmania (2018) How to Identify Phytophthora cinnamomi Infection in Tasmania. https://dpipwe.tas.gov.au/biosecurity-tasmania/plant-biosecurity/pests-anddiseases/phytophthora/identification-of-phytophthora-cinnamomi. Accessed 13 March 2020
Dudzinski MJ, Old KM, Gibbs RJ (1993) Pathogenic variability in australian Isolates of Phytophthora cinnamomi Australian. J Bot 41:721–732. https://doi.org/10.1071/BT9930721
Eden MA, Hill RA, Galpoththage M (2000) An efficient baiting assay for quantification of Phytophthora cinnamomi in soil. Plant Pathol 49(4):515–522. https://doi.org/10.1046/j.1365-3059.2000.00478.x
Graham JH, Johnson EG, Gottwald TR, Irey MS (2013) Presymptomatic Fibrous Root decline in Citrus Trees caused by Huanglongbing and potential Interaction with Phytophthora spp. Plant Dis 97(9):1195–1199. https://doi.org/10.1094/PDIS-01-13-0024-RE
Groves E, Howard K, Hardy G, Burgess T (2015) Role of salicylic acid in phosphite-induced protection against Oomycetes; a Phytophthora cinnamomi-Lupinus augustifolius model system. Eur J Plant Pathol 141:559–569
Gunning T, Cahill D (2009) A soil-free plant growth system to facilitate analysis of Plant Pathogen interactions in roots. J Phytopathol 157:497–501. https://doi.org/10.1111/j.1439-0434.2008.01503.x
Hamberg L, Poimala A, Velmala S, Perttunen J, Muilu-Mäkelä R, Sievänen R (2021) Root discoloration and shoot symptoms in silver birch after Phytophthora infection in vitro. Plant Biol 23(1):162–171. https://doi.org/10.1111/plb.13198
Hardham AR, Blackman LM (2018) Phytophthora cinnamomi. Mol Plant Pathol 19(2):260–285. https://doi.org/10.1111/mpp.12568
Horticulture Innovation Australia (2016) Baiting for Phytophthora and Pythium in production nurseries. title. https://www.horticulture.com.au/growers/help-your-business-grow/research-reports-publications-fact-sheets-and-more/baiting-for-phytophthora-and-pythium-in-production-nurseries-a-2016-nursery-paper/
Hüberli D, Tommerup IC, Hardy GESJ (2000) False-negative isolations or absence of lesions may cause mis-diagnosis of diseased plants infected with Phytophthora cinnamomi. 29 3:164. https://doi.org/10.1071/ap00029
Islam MT (2017) Interaction of Phytophthora cinnamomi with model and native plant species. PhD thesis, Deakin University, Geelong
Islam MT, Rookes JE, Cahill DM (2017) Active defence by an australian native host, Lomandra longifolia, provides resistance against Phytophthora cinnamomi. Funct Plant Biol 44(4):386. https://doi.org/10.1071/fp16266
Jackson B, Colquhoun H (2000) Action of the fungicide phosphite on Eucalyptus marginata inoculated with Phytophthora cinnamomi. Plant Pathol 49(1):147–154. https://doi.org/10.1046/j.1365-3059.2000.00422.x
Jansen BJM, De Groot A (1991) The occurrence and biological activity of drimane sesquiterpenoids. Nat Prod Rep 8(3):309. https://doi.org/10.1039/np9910800309
Jung T, Colquhoun IJ, Hardy GESJ (2013) New insights into the survival strategy of the invasive soilborne pathogen Phytophthora cinnamomi in different natural ecosystems in western Australia. 43(4):266–288. https://doi.org/10.1111/efp.12025
Lazarovits G, Stossel R, Ward E (1981) Age-related changes in specificity and glyceollin production in the hypocotyl reaction of soybeans to Phytophthora megasperma var. Sojae. Phytopathology 71:94–97. https://doi.org/10.1094/Phyto-71-94
Lu X, Sun D, Rookes JE, Kong L, Zhang X, Cahill DM (2019) Nanoapplication of a resistance inducer to reduce Phytophthora Disease in Pineapple (Ananas comosus L). Front Plant Sci 10. https://doi.org/10.3389/fpls.2019.01238
Menary R, Garland SM, Dragar VA (1999) Tasmannia lanceolata: developing a new commercial flavour product: a report for the Rural Industries Research and Development Corporation. University of Tasmania
O’Gara E, Howard K, Wilson B, Hardy G (2005) Management of Phytophthora cinnamomi for Biodiversity Conservation in Australia. Murdoch University, Western Australia
Oßwald W, Fleischmann F, Rigling D, Coelho AC, Cravador A, Diez J, Dalio RJ, Horta Jung M, Pfanz H, Robin C, Sipos G, Solla A, Cech T, Chambery A, Diamandis S, Hansen E, Jung T, Orlikowski LB, Parke J, Prospero S, Werres S (2014) Strategies of attack and defence in woody plant-Phytophthora interactions. Forest Pathol 44(3):169–190. https://doi.org/10.1111/efp.12096
Pengelly A (2002) Indigenous and naturalised herbs: Tasmannia lanceolata: mountain pepper. Australian J Med Herbal 14 (2)
Podger F, Brown M (1989) Vegetation damage caused by Phytophthora cinnamomi on disturbed Sites in Temperate rain-forest in western Tasmania. Aust J Bot 37(6):443. https://doi.org/10.1071/bt9890443
Podger F, Palzer C, Wardlaw T (1990) A guide to the tasmanian distribution of Phytophthora cinnamomi and its effects on native vegetation. Tasforests 2(1):125–128
Portz RL, Fleischmann F, Koehl J, Fromm J, Ernst D, Pascholati SF, Osswald WF (2011) Histological, physiological and molecular investigations of Fagus sylvatica seedlings infected with Phytophthora citricola. Forest Pathol 41(3):202–211. https://doi.org/10.1111/j.1439-0329.2010.00667.x
Read C (1995) Aspects of leaf and extract production from Tasmannia lanceolata. PhD thesis, University of Tasmania, Hobart
Sangchote S, Poonpolgul S, Sdoodee R, Kanjanamaneesathian M, Baothong T, Lumyong P (2004) Phytophthora Diseases in Thailand. In: DRENTH A, GUEST DI (eds) Diversity and management of Phytophthora in Southeast Asia. Australian Centre for International Agricultural Research Canberra
Schena L, Duncan JM, Cooke DEL (2007) Development and application of a PCR-based ‘molecular tool box’ for the identification of Phytophthora species damaging forests and natural ecosystems. Plant Pathol 0(0):070921225609001. https://doi.org/10.1111/j.1365-3059.2007.01689.x
Shearer BL, Crane CE, Barrett S, Cochrane A (2007) Assessment of threatened flora susceptibility to Phytophthora cinnamomi by analysis of disease progress curves in shadehouse and natural environments. Australas Plant Path 36(6):609. https://doi.org/10.1071/ap07074
Simamora AV, Stukely MJC, Barber PA, Hardy GES, Burgess TI (2017) Age-related susceptibility of Eucalyptus species to Phytophthora boodjera. Plant Pathol 66(3):501–512. https://doi.org/10.1111/ppa.12592
Stukely M, Crane C (1994) Genetically based resistance of Eucalyptus marginata to Phytophthora cinnamomi. Phytopathology 84:650–656. https://doi.org/10.1094/Phyto-84-650
Stukely MJC, Crane CE, McComb JA, Bennett IJ (2007) Field survival and growth of clonal, micropropagated Eucalyptus marginata selected for resistance to Phytophthora cinnamomi. For Ecol Manag 238(1–3):330–334. https://doi.org/10.1016/j.foreco.2006.10.028
Sultanbawa Y (2016) Chap. 93 - Tasmanian Pepper Leaf (Tasmannia lanceolata) oils. In: Preedy VR (ed) Essential oils in Food Preservation, Flavor and Safety. Academic Press, San Diego, pp 819–823. doi:https://doi.org/10.1016/B978-0-12-416641-7.00093-6
Tynan KM, Scott ES, Sedgley M (1998) Evaluation of Banksia species for response to Phytophthora infection. Plant Pathol 47(4):446–455. https://doi.org/10.1046/j.1365-3059.1998.00248.x
Weste G (1975) Pathogenicity of Phytophthora cinnamomi towards Nothofagus cunninghamii. Aust J Bot 23(2):277–283
Wilson MD (2015) Aspects of developing Tasmannia lanceolata for commercial extract production. PhD thesis, University of Tasmania, Hobart
Acknowledgements
This research was funded by Diemen Pepper and a Research Training Postgraduate scholarship provided by the Australian government. We thank Dr Chris Read for supplying commercially grown native pepper cultivars and providing advice to locate natural provenances of native pepper. We thank Mr Philip Andrews for assistance to maintain glasshouse plants, Prof Andre Drenth and Ms Cecilia O’Dwyer (Queensland Alliance for Agriculture and Food Innovation) for providing P. cinnamomi A1 and A2 mating strains, Dr Tohidul Islam (Deakin University) for providing advice on P. cinnamomi zoospore production, Mr Tianyu Wang for helping with machine learning to process root images, and Dr Ian Hunt for providing advice on data presentation and statistical analysis.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
13313_2023_931_MOESM1_ESM.docx
Supplementary Material 1: Figure S1 Total root discolouration percentage in P. cinnamomi inoculated (I) and control (C) native pepper plants (4 replicates per each clone), categorised by source of clone origin (1-NW Tasmania, 2-Eastern Tasmania, 3-Commercial plantation, 4-NE Tasmania, 5-Southern Tasmania).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sinhalagoda, C., Wilson, M.D., Tran, S.N. et al. Screening native pepper, Tasmannia lanceolata (Poir.) A.C. Smith, for resistance against Phytophthora cinnamomi dieback. Australasian Plant Pathol. 52, 427–437 (2023). https://doi.org/10.1007/s13313-023-00931-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13313-023-00931-x