Abstract
Colletotrichum species are one of the most common causes of postharvest fruit rot in mango in Australia, particularly in the tropical region of north Queensland, and can result in significant losses if not managed. The research aims were to identify sources of anthracnose tolerance and to determine if host material other than fruit could improve or fast track the screening process and result in improved breeding efficiency. Access to the Australian National Mango Genebank (ANMG) collection enabled fruit screening of more than 100 Mangifera indica cultivars or Mangifera species for tolerance to anthracnose by artificial inoculation with Colletotrichum asianum over a period of 14 years. Mean lesion diameters were compared with those on a known susceptible M. indica cultivar Kensington Pride (KP) and a tolerant M. laurina cultivar Lombok. Inoculation of leaf discs and entire leaves was evaluated in the laboratory and the field as alternative assays for tolerance to anthracnose and was assessed by presence/absence of disease. Screening of fruit has shown that anthracnose tolerance within the mango germplasm is highly variable and needs to be assessed over multiple years. None of the alternative laboratory bioassays provided consistent or reliable data. The in-field artificial inoculation of immature leaf flush was successful but was not deemed suitable for adoption due to practical restraints. While resistance to anthracnose in fruit has not yet been identified, some cultivars and Mangifera spp. showed promise for inclusion as parents in future breeding programs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anthracnose (Colletotrichum spp.) is recognised as one of the most prevalent mango diseases worldwide, infecting leaves, inflorescences, and fruit. Symptoms include leaf and inflorescence blight, spotting and abortion of immature fruitlets, and postharvest decay of mature fruits (Fig. 1). The disease is a major cause of fruit losses in the postharvest market chain (Arauz 2000; Diedhiou et al. 2014; Ploetz 2017). Losses between 20 and 60% are reported due to rots, particularly in areas with prolonged wet periods (Chang et al. 2012; Esguerra et al. 2006; Kamle and Kumar 2016). During fruit ripening the disease causes irregular brown to black lesions covering large areas of the fruit surface. As the disease develops, lesions become soft, sunken, with a pink to orange-coloured conidial mass. Fruit that exhibits any of the above symptoms are rendered unsaleable.
The fungal taxa Colletotrichum gloeosporioides (Penz.) Penz. & Sacc., C. gloeosporioides (Penz.) var. minor Simmonds and C. acutatum Simmonds were originally considered the main causal organisms of anthracnose on mango in Australia (Ploetz 2003). However, taxonomic revision has since identified 22 species and one subspecies within the C. gloeosporioides complex (Weir et al. 2012). Of the 22 species, C. asianum Prihast., L. Cai & K.D. Hyde, C. fructicola Prihast., L. Cai & K.D. Hyde, C. siamense Prihast., L. Cai & K.D. Hyde, and C. simmondsii R.G. Shivas & Y.P. Tan have been reported on fruits, whilst C. fioriniae R.G. Shivas & Y.P. Tan and C. karsti You L. Yang, Zuo Y. Liu, K.D. Hyde & L. Cai were recorded on mango stems in Australia (Damm et al. 2012a; Damm et al. 2012b). Coates et al. (2019) also found that C. alienum B.S. Weir & P.R. Johnst., C. kahawae subsp. cigarro B.S. Weir & P.R. Johnst. and C. theobromicola Delacr. isolated from avocado could also produce anthracnose symptoms on mango cv. R2E2.
Post-harvest fruit anthracnose infection is initiated in the field, before harvest, from conidia produced on dead and dying plant material within the tree. For infection to occur, conidia require free moisture to germinate, resulting in the formation of an infection peg that develops from an appressoria and penetrates the host tissue (Ploetz 1994). In early fruit development, disease symptoms may develop quickly and cause the infected fruit to shed. As fruit develop it is common for the fungus to remain in a latent phase for months due to the natural occurrence of an antifungal resorcinol compound present in the peel of the developing fruit (Cojocaru et al. 1986; Droby et al. 1987; Kobiler et al. 1998). When fruit reach maturity and begin to ripen, resorcinol concentration declines below fungi toxic levels and the fungus recommences the infection process. Antifungal compounds concentrations vary between cultivars (Pierre 2015; Zainuri et al. 2001) and are reduced with higher fruit nitrogen concentrations (Bally 2006). The tolerance of some mango cultivars to C. gloeosporioides has been attributed to the concentrations of antifungal in the peel and latex of mango remaining high as the fruit ripen (Karunanayake et al. 2011; Knödler et al. 2007).
In Australia, commercially grown mango cultivars have very low or no natural tolerance to anthracnose. As such, the disease is managed with infield and postharvest fungicides, and orchard hygiene practices such as pruning dead wood from the tree. In the field up to 25 fungicide applications (protectants and systemics) are applied throughout a growing season (Akem 2006), targeting the critical growth stages of leaf flush, flowering, and early fruit set (Jeffries et al. 1990). Postharvest fungicide treatment of fruit is required to reduce the diseases impact on the shelf life of fruit under challenging environmental and/or storage conditions (Bally et al. 2013). Fungicide control methods are expensive, not well regarded by consumers, have a negative impact on the environment and may lead to the development of pathogen resistance (Kumar et al. 2007).
True genetic resistance to anthracnose in mango has not been reported. However, varying levels of partial resistance or tolerance to the disease have been published since the 1950’s (Akem et al. 2007; Dinh et al. 2003; Do 2019; Felipe et al. 2022; Gong et al. 2013; Grice and Bally 2007; Gupta et al. 2015; Nishijima 1994; Pernezny and Ploetz 2000; Sanchez-Arizpe et al. 2021; Sudheeran et al. 2021; Vitale et al. 2020). Some of these are outlined in Table 1. Tolerance to the disease is expressed as a significant slowing or a delay in the onset of lesion development in the later stages of fruit ripening (Sudheeran et al. 2021). These observations were mostly of fruits naturally infected with the pathogen in the field, based on grower observations (Jeffries et al. 1981) or limited repetition of laboratory screenings. Reports of resistance levels in fruits are inconsistent and highly variable for any given cultivar, between seasons, and between countries.
A range of inoculation methods to determine levels of tolerance to Colletotrichum species have been assessed on other hosts and host organs. Bigirimana and Höfte (2001) assessed various inoculation methods (seed, seedling, and detached leaf) on bean (Phaseolus vulgaris L.) using the pathogen C. lindemuthianum (Sacc. & Magnus) Briosi & Carvara. The authors found that detached leaves sprayed with a spore suspension and incubated for one week was the most suitable technique. A similar technique using detached leaves of blueberry (Ehlenfeldt et al. 2006) and the pathogen C. acutatum J.H. Simmonds also gave good results. Denoyes-Rothan and Guérin (1996) screened strawberries (Fragaria ananassa Duch.) for resistance to C. acutatum using six different techniques. The authors concluded that the technique of dipping whole plants in a suspension of conidia and incubating for 28 days gave the best results.
A review of techniques used for other pathogens was also investigated by the authors. In the screening of sour cherry (Prunus cerasus L.) germplasm against Blumeriella jaapii (Rehm) Arx (Wharton et al. 2003), inoculation methods included spraying a spore suspension on actively growing glasshouse plants or detached branches from field grown trees. Alternatively, a droplet of the spore suspension was placed on detached leaves or leaf discs. Both methods produced comparable results, and the detached leaf/leaf disc method was chosen as an increased number of seedlings could be screened at any one time. In 2011, Santos et al. used detached leaves inoculated with a 200 µl aliquot of spore suspension followed by an incubation period of five to seven days to screen > 200 cacao (Theobroma cacao L.) genotypes for resistance to the organism Phytophthora palmivora E.J. Butler that causes pod rot, a serious disease of cacao.
Some of the above listed bioassays were assessed for their suitability and reliability as alternative methods to artificial fruit inoculations. These techniques had not been previously evaluated, making it difficult to assess and compare leaf and postharvest fruit tolerances to anthracnose. Although this research has not previously been conducted on mango, similar studies have occurred on other Colletotrichum species in blueberry to determine if there is a correlation between leaf and fruit infection (Ehlenfeldt et al. 2006). In this paper we present our development of an early screening leaf assay for anthracnose tolerance in mango before trees start fruiting in comparison to the standard fruit-based assay.
The Australian National Mango Genebank (ANMG) holds over 360 cultivars of mangoes and related Mangifera species from 12 distinct geographic origins (Bally 2009; Dillon et al. 2013). The genebank serves as a resevoir of genetic diversity and a source of novel alleles for the Australian National Mango Breeding Program (Bally and Dillon 2018). This is the only known paper that reports on the screening of more than 100 M. indica cultivars and Mangifera species from around the world for tolerance to anthracnose using the artificial inoculation method on fruit. This paper reports on their susceptibility or tolerance to the disease, following earlier reports (Akem et al. 2007; Grice and Bally 2007).
A detailed characterization of genetics-based resistance to Colletotrichum spp. in mango germplasm is imperative and will benefit breeders, growers, and consumers through the development of new varieties with anthracnose tolerance.
Materials and methods
Collection sites
Mango fruits (Mangifera indica L. and other Mangifera species) were sourced from three of the Department of Agriculture and Fisheries (DAF) ANMG sites located at the Ayr, Southedge and Walkamin Research Facilities.
Isolate details
Two isolates of C. asianum, both previously used in laboratory inoculation studies (Hassan et al. 2007), were attained from the Queensland Plant Pathology Herbarium (BRIP) and pathogenicity tested on cv. Kensington Pride (KP; M. indica) fruits. C. asianum was chosen based on its frequent recovery from mango fruit in north Queensland and its prior use by Hassan et al. (2007). The most aggressive isolate of the two viz., BRIP 28734 was used from 2007 to 2018 when it lost virulence. In the 2018 season, the pathogenicity of additional isolates obtained from unsprayed backyard trees in the Mareeba area were tested. One of these, identified using molecular methods as C. asianum (BRIP 66620), produced consistent, typical anthracnose symptoms and has been used from 2018 to date.
Isolate storage and inoculum production
Isolates of C. asianum were preserved long term at -80 oC by placing a concentrated conidial suspension in sterile 2ml tubes containing 10% glycerol solution or using the Protect™ (Protect System, Scientific and Technical Consultants Ltd, UK) microorganism preservation system. Isolates were revived from storage by taking a sterile wire loopful or extracting a single bead of spores that were streaked or rolled respectively onto oatmeal agar (OMA) in Petri dishes that were incubated at 25–26 oC for 48 h before exposure to near ultra-violet light (12 h light/12 h dark) for 2–4 days to induce spore production.
Fruit collection, preparation, and screening
Screening experiments were conducted from the 2008/2009 harvest season through to 2020/2021. No experiments were conducted in 2015/2016 due to unseasonal weather conditions, leading to poor pollination and fruit set. Between one and three fruit screening experiments, with up to 17 cultivars and two controls (susceptible: KP and tolerant: cv. Lombok (Mangifera laurina Blume)) in any one individual experiment, were conducted each harvest season, depending on the availability of fruit.
Eight to eleven fruits of each cultivar with minimal blemishes were picked randomly from each tree at the hard green stage and immediately transported back to the laboratory where they were de-sapped and washed in a solution of Mango Wash® (Shamrock Chemicals, NT, Australia) to remove sap from the fruit skin and eliminate symptoms of sap burn. Fruits were then air dried prior to being placed in a netted bag and dipped for 5 min in a hot water bath (52 oC) to reduce latent infection of anthracnose. Fruits were removed from the hot water bath and spread out on towels to dry and cool prior to being labelled with the cultivar name and replicate number. The dry, cool fruits were then placed on rubber matting inside a plastic container to minimise movement and raised above the 1000–1500 ml of water placed in the bottom of each plastic container to create high humidity during the incubation period. Two 10 mm diameter circles were ink marked on the surface of each fruit to indicate the sites of inoculum or sterile distilled water in the case of the non-inoculated controls.
Artificial inoculation of fruit and assessment methods
A spore suspension was prepared from 5 to 6-day old cultures as previously described by flooding the surface of sporulating cultures with sterile distilled water and dislodging the spores using a sterile glass spreader. A funnel lined with 3–4 layers of muslin was placed in a conical flask and the spore suspension passed through to remove unwanted mycelial fragments of the fungus. The inoculum was adjusted to a concentration of 1–3 × 106 conidia per ml with an Improved Neubauer haemocytometer (Boeco, Germany). For each cultivar, one 25 µl droplet of sterile distilled water was placed in the centre of each of the two marked spots on the single control fruit, while for all other fruit one droplet of 25 µl of conidial suspension (Fig. 2) was placed in the centre of each of the two marked spots. All fruits were then incubated in plastic containers for 48 h. Following the incubation period one fruit of each cultivar was packed into a mango box to form an experimental replicate before being placed in a ripening room set to approximately 24 oC for the duration of the experiment. Each cultivar had between seven and ten inoculated fruit and one non-inoculated control fruit.
Fruits were assessed every second day, post inoculation, for their stage of ripeness, lesion development and disease severity. The stage of fruit ripeness was determined by hand firmness and categorised into five stages (Holmes et al. 2010): 1 = hard (no ‘give’ in the fruit); 2 = rubbery (slight ‘give’ in the fruit); 3 = sprung (flesh deforms by 2–3 mm with extreme thumb pressure); 4 = firm soft (whole fruit deforms with moderate hand pressure); 5 = soft (whole fruit deforms with slight hand pressure). Even though all stages of ripeness were assessed, only stage 4 (eating ripe) data is presented here, as this is the stage that fruit are normally consumed, representing the end of the supply chain.
Disease development was assessed at each of the two marked inoculum sites on the fruit by measuring lesion diameter (mm) with digital callipers and calculating the mean lesion diameter per fruit. This process was repeated for all fruit in each experiment.
Fruits were also rated for disease severity using a 0 to 4 scale to differentiate between hypersensitive skin reactions and typical anthracnose lesions. The following rating scale was developed: 0 = no skin discolouration; 1 = skin speckling with no lesion expansion; 2 = blackening of inoculation point, with no lesion expansion; 3 = dark lesion expanding beyond the inoculation point, and 4 = dark lesion, sunken/sporing (Fig. 3).
Statistical analysis
Individual fruit screening experiments conducted in each year were analysed using linear mixed models. The random model comprised a term for the replicate effect and cultivar was fitted as the fixed model. All significance testing was performed at the 0.05 level and Fisher’s 95% protected least significant difference was used to make pairwise comparisons. All statistical analyses were conducted in GenStat for Windows (12th to 21st editions) (VSN_International 2020).
Alternative bioassays
Inoculation studies included leaf assays using leaf discs, whole immature detached leaves and branches consisting of immature leaf flush and were conducted in one season using the same isolate of C. asianum as above. A range of laboratory methods and field assays were assessed to ascertain if alternative methods could be a suitable substitute to using fruit to determine tolerance or susceptibility to anthracnose.
Leaf discs
In the initial experiment, inoculation via a conidial suspension was evaluated by inoculating 25 mm leaf discs cut from fully expanded immature leaves with pink or green pigment (leaf positions two to five) of KP with three different concentrations of a 10 µl of conidial suspension (concentrations 105, 106 and 107) deposited on the upper leaf surface. Leaf discs were assessed for presence or absence of disease development. The results also served to determine the most effective concentration of inoculum on the susceptible cultivar.
As an alternative to the 10 µl droplet technique described above, two other methods were assessed on KP and Lombok, with and without wounding the leaf material using a sterile needle: (1) 3.5 mm plugs of actively growing C. asianum isolate was placed on the upper leaf surface, with the mycelium in contact with the leaf; and (2) whole leaf discs were immersed in a spore suspension (106) for 30 s, then allowed to dry. All the in vitro techniques were conducted in glass Petri dishes containing a 5 mm layer of glass beads to support the leaf discs with the addition of benzimidazole (Sigma-Aldrich Pty Ltd) at 50 mg/L to reduce the rate of senescence (Mishra and Misra 1973) and assessed for the presence or absence of disease development.
Detached immature leaves
Two separate laboratory experiments were conducted using detached stems of fully expanded immature vegetative growth, cut immediately above the previous growth units, of KP, Keitt, Lombok, and Mangifera rubropetala Kosterm. Stems were collected from the field, kept cool and taken directly to the laboratory. A stock conidial suspension (106) was prepared from 5 to10 day old cultures of C. asianum and subsequently diluted 1:10 and 1:100 to provide suspension concentrations of 104 and 105, respectively.
In the first experiment, four stems of each cultivar and species (KP, Lombok, and M. rubropetala) were re-cut just above the original cut site before being placed in beakers containing portions of Oasis® floral foam brick and sterile distilled water. Individual stems were sprayed to the point of run-off with the conidial suspension using a Preval® atomiser (Bridgeview, Illinois, USA), whilst one stem of each was sprayed with sterile distilled water as the untreated control. Stems were incubated by covering with a plastic bag (Fig. 4) for 48 h, after which the leaves were assessed for disease development.
In the second experiment, four stems of each cultivar and species (KP, Lombok and Keitt), were cut and transported to the laboratory where all plant material was submerged in cold water for one h to reduce water loss and wilting of the sensitive foliage. As with the first experiment, stems were recut before being inserted into florist Oasis®, but instead of sterile distilled water, a solution of benzimidazole (Sigma-Aldrich Pty Ltd) at 50 mg/L was used to slow the rate of senescence. The beakers were also placed on a tray filled with water to increase the humidity in an air-conditioned environment. Treatments and incubation times used were the same as in the first experiment. Once the plastic bags were removed, the leaf material was sprayed intermittently with sterile distilled water to keep the leaves fresh. Leaves were assessed every two days for presence or absence of anthracnose.
Infield inoculation
Two infield inoculation experiments were conducted at the Southedge Research Facility. The first experiment used the documented tolerant and susceptible cultivars (Lombok and KP, respectively). Three conidial suspensions (104, 105 and 106) were applied to the point of run-off to a single branch of immature leaf material each using a Preval® atomiser. After inoculation, plastic bags were misted with sterile distilled water then placed over the inoculated stem and sealed for 48 h to maintain high humidity (Fig. 5). The inoculated stems were removed from the tree after nine days and assessed for presence or absence of disease symptoms.
The second experiment was conducted using the same methodology but with an increased number of cultivars: KP (2 trees), Lombok, Hybrid 17, Gudang, Lippens and Neelum.
Results
Artificial inoculation of fruit and assessment methods
Between 2008 and 2022, a total of 99 mango genebank cultivars of M. indica and 13 other Mangifera species have been assessed for their tolerance to postharvest anthracnose by artificial inoculation with C. asianum. Their origin, the number of screening experiments in which they have been assessed and the overall mean and maximum mean lesion diameter (mm) based on individual screening experiments at ripeness stage 4 are presented in Tables 2 and 3. Disease severity data were also recorded on all fruit and results matched those of the mean lesion diameters but are not reported or further discussed in this paper.
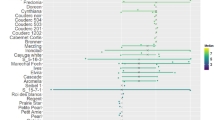
Thirty four taxa were screened against C. asianum in two or more screening experiments (Table 2) and compared with KP (Fig. 7), the standard susceptible cultivar (mean lesion diameter 8.85 mm (se = 1.328)), and Lombok (Fig. 8), the standard tolerant cultivar (mean lesion diameter 2.67 mm (se = 0.531)), where possible.
Mean anthracnose lesion diameters at eating ripe (stage 4) for Kensington Pride (KP), Lombok and cultivars screened for anthracnose tolerance between 2008–2022. Each data point represents the mean lesion diameter for a cultivar obtained from the linear mixed model analyses of each fruit screening experiment. A single cultivar could appear multiple times if it was assessed in several screening experiments in one season, as seen by KP and Lombok. Error bars for KP and Lombok represent +/- one standard error. For clarity, error bars are not shown for the other cultivars. The solid line represents the overall trend for the mean lesion diameter for KP, while the dashed line represents the overall trend for Lombok
Mean lesion diameters varied from 2.78 for M. rubropetala to 16.02 mm for Bangampalli. M. rubropetala was the only species where the mean lesion diameter was similar to the tolerant standard Lombok, whereas three M. indica cultivars (Van Dyke, Calypso and Bangampalli) measured mean lesion diameters greater than the susceptible standard KP. The maximum lesion diameter (Table 2) represents the largest mean lesion diameter obtained from a screening experiment and not on an individual fruit. In some taxa the variability between screening experiments was large as seen by the difference between the mean and maximum lesion diameters. For example, KP had a mean lesion diameter of 8.85 mm and a maximum mean lesion diameter of 19.83 mm.
Table 3 lists the 67 cultivars and 9 Mangifera species assessed on a single occasion only. In this instance, the mean lesion diameter ranged from 0.82 (Kimba) to 20.25 mm (Heidi), again indicating a high level of variability. Kyal was one of the more susceptible cultivars with a mean lesion diameter of 17.73 mm (Fig. 9), more than double that of the susceptible cultivar KP. The two cultivars (Akbar and Sensation) listed at the bottom of Table 3 measured mean lesion diameters of 18.08 and 5.86 mm, respectively. The results reported for Akbar are based on ripeness stage 3 only, due to both inoculation sites developing rapidly. The same result was observed with Sensation and only one fruit was assessed at ripeness stage 4. Both cultivars should be reassessed to confirm these results.
The mean lesion diameter for each cultivar from each fruit screening experiment over time (Fig. 6), indicates that susceptibility to anthracnose is variable. Where a cultivar was screened multiple times in a year, the mean is presented for each individual fruit screening experiment. The error bars on KP and Lombok represent +/- one standard error, and, for clarity, error bars are not shown for the other cultivars. The solid line represents the overall trend for the mean lesion diameter for KP, while the dashed line is the overall trend for Lombok. In the 2020/2021 season, Lombok was not screened as early flowering meant that all fruit of this species had abscised by the time other cultivars were mature and a comparative assessment was not possible. The overall mean lesion diameter for KP was consistently higher than Lombok, although in 2011/2012 they were not significantly different.
Alternative bioassays
Leaf discs
Observations from the preliminary experiment inoculating pink or green fully expanded immature leaves of KP with a 10 µl droplet of a 105, 106 or 107 conidial suspension showed some inconsistencies in disease development between the three concentrations, particularly at the low rate (105). A similar result was observed when the droplet (106) technique or mycelial plug method was applied to KP and Lombok, as disease symptoms progressed more on leaf discs that contained larger portions of midrib material. Neither of these methods were considered suitable as an alternative to the fruit inoculation method due to these inconsistencies (data not shown).
Dipping leaf discs in an inoculum suspension (106) is also not suitable as both KP and Lombok developed disease rapidly, as the cut edge of the disc (wounded tissue) allowed for rapid development of anthracnose, resulting in complete collapse of all tissue (data not shown).
In the treatments where tissue was wounded prior to the application of inoculum, disease development or infection was exacerbated (data not shown) and was again not considered a suitable alternative to fruit inoculation. It is speculated that the addition of wounding to the inoculation technique would enhance disease development and provide unrealistic and biased results.
Detached immature leaves
In the first experiment, the detached immature leaves started to wither and dry out three days post inoculation, before disease symptoms were observed. With the addition of benzimidazole solution, the detached immature leaves started to wither and dry out four days post inoculation, also before disease symptoms were observed. As there was no disease development, no disease measurements were made. Based on these results, it was evident that immature mango foliage was too fragile when removed from the tree and could not be kept alive for the length of time required for disease development to occur (data not shown).
Infield inoculation
Lesions developed on the susceptible cultivar KP at the inoculation rates of 104 and 105, but the foliage inoculated with the high rate (106) was broken from the tree when the plastic bag was removed and prior to disease development. In contrast, no lesions were observed on the Lombok inoculated foliage at any of the inoculation rates (Fig. 10). No results were obtained from the second experiment due to the unforeseen application of systemic fungicide applied to the trial area.
Discussion
This study was conducted over a period of 14 years and showed that, while the disease tolerance for two controls (KP and Lombok) remained relatively consistent (susceptible and tolerant, respectively) from year to year, there was some variation between seasons (Fig. 6). This seasonal variation was observed in all species and cultivars tested. Complete tolerance to mango anthracnose has not been identified and similar examples are also noted in the literature with other commodities and pathogens, including the cocoa pod rot pathogen (P. palmivora) (Santos et al. 2011), C acutatum in blueberry (Ehlenfeldt et al. 2006) and Botrytis cinerea in tomato (ten Have et al. 2007). One example was the evaluation of tomato accessions for resistance to B. cinerea where a fourfold variability in the susceptible control cultivar was encountered between experiments conducted over time (ten Have et al. 2007). In mango, variability in disease intensity under natural conditions was also observed by Gupta et al. (2015). The intensity of anthracnose in mango varied from 1.67% in cv. Thanking amadi to 48.29% cv. Hazur Pasand over two seasons. Dinh et al. (2003), also assessed anthracnose severity and incidence under natural field conditions in mango and observed variability between cultivars and across seasons. Therefore, to attain a reliable indication of anthracnose disease tolerance in any given mango cultivar, screening over multiple seasons is necessary. The cause of seasonal variability in anthracnose disease tolerance was not explored in our study, however, factors such as varied environmental conditions as observed by Gupta et al. (2015) and Dinh et al. (2003), changes in pathogen strains (Denoyes-Rothan and Guérin, 1996) or pathogenicity, variability in host susceptibility/tolerance or modification of screening methods may impact screening assessment results. Felipe et al. (2022) and Akem et al. (2006) identified M. indica cv. Tommy Atkins with tolerance to anthracnose if inoculated with wounding or under natural field conditions respectively. However, the same level of tolerance was not observed in our studies, and this could be attributed to a different species of the pathogen, or pathogenicity thereof, and environmental conditions. Seasonal differences were also observed in the M. indica cv. Fyhn. It performed exceptionally well in two consecutive years, then succumbed to natural infection from inoculum from mummified fruit hanging above developing fruit in the third year (data not shown). This illustrates the difficulty in assigning M. indica or M. laurina cultivars to a specific classification (tolerant or susceptible).
Similarly, the diversity in fruit reactions to anthracnose as seen in the M. indica cultivars is also reflected in the M. laurina cultivars. We have screened 13 cultivars of M. laurina, all showing varied levels of tolerance to anthracnose (Tables 2 and 3).
Poor repeatability or consistency of results is a feature of many reports of anthracnose resistance screening in mango fruit, particularly between natural and artificial inoculation techniques (Akem et al. 2007). Many of the cultivars that perform well under natural field conditions develop severe anthracnose symptoms when artificially inoculated. This inconsistency between natural and artificial inoculation of fruit was attributed to variable environmental conditions in the naturally infected fruit. In artificially inoculated fruit, the controlled environment and standard inoculation protocol (Akem et al. 2007; Grice and Bally, 2007) are more conducive to symptom development and disease expression is more consistent. Therefore, if tolerance is consistently observed using the artifical inoculation process, then it should also be upheld under natural conditions in the field as the latter is not always consistent due primarily to environmetal and other factors as previously stated.
The screening conducted to date has indicated that the artificial inoculation method of fruit described in this paper is able to distinguish cultivars that are consistently susceptible to anthracnose based on the mean lesion diameter (Tables 2 and 3). However, there was not always a clear distinction between other accessions, particularly those with variable mean lesion diameters across seasons. Unfortunately, there are time disadvantages of using fruit for screening, in that trees can take a minimum of four to five years to bear fruit, if at all, and screening opportunities only occur once a year. This is especially of concern when the screening is being used to evaluate germlines in a breeding programme. Seasonal changes in weather conditions can also hamper flowering, which can lead to poor pollination and low subsequent fruit set as observed in 2015/2016 where insufficient fruit were available in many cultivars for screening. The maturity of available fruit or unforeseen chemical applications prior to harvest can also have a significant effect on disease development and can impact the outcome of the artificial inoculation process. In addition, disease screening experiments are time consuming, meaning only a limited number of cultivars can be screened in any one season.
As previously stated, relying solely on the availability of fruit for anthracnose screening is an impediment to advancing the search for disease tolerance. For this reason, several field and laboratory experiments were conducted to develop alternative screening methods and evaluate them against the standard artificial fruit inoculation technique we used.
None of the leaf disc techniques, placing a droplet of conidial suspension, mycelial plug or immersion of leaf discs in a conidial suspension, provided consistent results and were deemed not suitable as an alternative to fruit screening. Anthracnose also spread rapidly on leaf discs with higher proportions of midrib present, particularly on those where the conidial suspension or mycelial plug method was applied. The dip method resulted in complete tissue collapse as the cut edge of the leaf disc was quickly colonised by the fungus. It is documented that Colletotrichum invades injured or wounded tissue (Bergstrom and Nicholson 1999; Timmer et al. 2000; Veloso et al. 2021) so, this result was not considered unusual. Dinh et al. (2003) also used wounding prior to the artificial inoculation of fruit and compared this to fruit with intact peel using M.indica cv. Nam Dok Mai. Lesion development was observed to be significantly higher in treatments where wounding was applied prior to inoculation.
The use of detached leaves as a method of inoculation has been successful for crops including tomato, bean and cherry where the material could be sustained for six, seven and 14 days respectively (ten Have et al. 2006; Wharton et al. 2003; Bigirimana and Höfte 2001). This is contrary to the results of our experiments using detached leaves or branches as the plant material declined rapidly and before disease development to occurred.
Infield spray inoculations of leaves were also attempted, with and without the addition of carborundum to help abrade the leaf tissue. Using this inoculation method, typical anthracnose symptoms developed on only known susceptible cultivars and this, therefore, represented a potential early infield assay. However, the technique was not progressed because: (1) insect damage and other fungal diseases can also affect immature leaves, potentially confounding results; (2) leaf flush is often not synchronous limiting the number of M. indica cultivars and M. laurina cultivars that can be screened at any one time; and (3) the method is an unsafe practice (applying inoculum from a ladder) as well as impractical. Similar experiments were conducted on blueberries to determine if there was any correlation between foliar and fruit rot infection caused by C. acutatum (Ehlenfeldt et al. 2006). They determined that the leaf assay could be used to screen seedlings for foliar resistance, however, there was no correlation between the foliar and fruit rot susceptibility to the pathogen.
Accessions with low mean lesion diameters identified in this study (Tables 2 and 3), may not be considered suitable for commercial production due to other non-disease related factors for example size, flavour, colour or aroma. However, they could be used as parents in future breeding programs and aid our understanding of the genetic basis of natural anthracnose tolerance in mango. We see merit in the adoption of molecular markers developed by Felipe et al. (2022) to enhance and improve the breeding process and eliminate inferior progeny at an earlier stage of development. Our studies have shown that the artificial inoculation method is currently the most reliable assay to determine susceptibility or tolerance to mango anthracnose in fruit under standard growing conditions and would therefore recommend the use of molecular markers in parallel with fruit screening.
References
Akem C, Grice K, Bally I, Barron Z, Mac Manus G, Boccalatte P Preliminary evaluation of mango varieties for postharvest disease resistance. In: Dunmall T (ed) Delivering Mango Research. The Amistar Sixth Australian Mango Conference Proceedings, Gold Coast, 2007. Australian Mango Industry Association, pp 3–4
Akem CN (2006) Mango anthracnose disease: Present status and future research priorities. Plant Pathology Journal 5(3):266-273
Arauz LF (2000) Mango anthracnose: economic impact and current options for integrated management. Plant Dis 84(6):600–611
Bally ISE (2006) The effect of preharvest nutrition and crop load on fruit quality and postharvest disease in mango (Mangifera indica L.). Doctor of Philosophy, University of Queensland, Brisbane, Australia
Bally ISE (2009) Australian National Mango Genebank. In: Redona E (ed) Proceedings of the joint 14th Australasian Plant Breeding Conference and the 11th Congress of the Society for the Advancement of Breeding Research in Asia and Oceania (SABRAO), Cairns, Australia, 10–14 August 2009. Society for Advancement of Breeding Research in Asia and Oceania, Bangkok, Thailand, p 5
Bally ISE, Akem CN, Dillon NL, Grice K, Lakhesar D, Stockdale K (2013) Screening and breeding for genetic resistance to anthracnose in mango. Acta Hort 992:239–244
Bally ISE, Dillon NL (2018) Mango (Mangifera indica L) breeding. In: Al-Khayri JM, Jain SM, Johnson DV (eds) Advances in plant breeding strategies: fruits. Advances in Plant Breeding Strategies, vol 3. Springer International Publishing, Cham, Switzerland, pp 811–896. doi:https://doi.org/10.1007/978-3-319-91944-7_20
Bergstrom GC, Nicholson RL (1999) The Biology of Corn Anthracnose: knowledge to exploit for Improved Management. Plant Dis 83(7):596–608. doi:https://doi.org/10.1094/pdis.1999.83.7.596
Bigirimana J, Höfte M (2001) Bean Anthracnose: Inoculation Methods and Influence of Plant Stage on Resistance of Phaseolus vulgaris Cultivars. J Phytopathol 149(7–8):403–408. doi:https://doi.org/10.1111/j.1439-0434.2001.tb03870.x
Chang C-H, Lin T-S, Yang W-J (2012) Invading time of Colletotrichum gloeosporioides affects fruit drop and infection rate in ‘Irwin’ mango (Mangifera indica L.). Horticulture, Environment, and Biotechnology 53:452–459. doi:https://doi.org/10.1007/s13580-012-1021-3
Coates L, Cooke A, Mitchell R (2019) Pathogenicity and host range of newly described Colletotrichum species on avocado fruit. Internal report for Queensland Department of Agriculture and Fisheries
Cojocaru M, Droby S, Glotter E, Goldman A, Gottlieb HE, Jacoby B, Prusky D (1986) 5-(12-Heptadecenyl)-resorcinol, the major component of the antifungal activity in the peel of mango fruit. Phytochemistry 25(5):1093–1095
Damm U, Cannon PF, Woudenberg JHC, Crous PW (2012a) The Colletotrichum acutatum species complex. Stud Mycol 73:37–113. doi:https://doi.org/10.3114/sim0010
Damm U, Cannon PF, Woudenberg JHC, Johnston PR, Weir BS, Tan YP, Shivas RG, Crous PW (2012b) The Colletotrichum boninense species complex. Stud Mycol 73:1–36. doi:https://doi.org/10.3114/sim0002
Denoyes-Rothan B, Guérin G (1996) Comparison of six inoculation techniques with Colletotrichum acutatum on cold stored strawberry plants and screening for resistance to this fungus in french strawberry collections. Eur J Plant Pathol 102(7):615–621. doi:https://doi.org/10.1007/BF01877242
Diedhiou PM, Diallo Y, Faye R, Mbengue AA, Sene A (2014) Efficacy of different fungicides against Mango Anthracnose in Senegalese Soudanian Agroclimate. Am J Plant Sci 05(15):2224–2229. doi:https://doi.org/10.4236/ajps.2014.515236
Dillon NL, Bally ISE, Wright CL, Hucks L, Innes DJ, Dietzgen RG (2013) Genetic diversity of the Australian National Mango Genebank. Sc Hort 150:213–226. doi:https://doi.org/10.1016/j.scienta.2012.11.003
Dinh SQ, Chongwungse J, Pongam P, Sangchote S (2003) Fruit infection by Colletotrichum gloeosporioides and anthracnose resistance of some mango cultivars in Thailand. Australas Plant Pathol 32(4):533–538
Do TK (2019) Mango anthracnose in Australia associated with varietal resistance, phenolic compounds and novel antifungal products. Masters, University of Queensland, Brisbane, Australia
Droby S, Prusky D, Jacoby B, Goldman A (1987) Induction of antifungal resorcinols in flesh of unripe mango fruits and its relation to latent infection by Alternaria alternata. Physiol Mol Plant Pathol 30(2):285–292
Ehlenfeldt MK, Polashock JJ, Stretch AW, Kramer M (2006) Leaf Disk infection by Colletotrichum acutatum and its relation to Fruit Rot in Diverse Blueberry Germplasm. Hortsci 41(1):270–271. doi:https://doi.org/10.21273/hortsci.41.1.270
Esguerra EB, Chavez SM, Traya RV, Philippines U (2006) Los Banos, College, Laguna (Philippines). Crop Science Cluster Modified and rapid heat treatment for the control of postharvest diseases of mango (Mangifera indica Linn. cv. carabao) fruits. 89
Felipe JEL, Lachica JAP, Dela Cueva FM, Laurel NR, Alcasid CE, Sison MLJ, Valencia LDC, Ocampo ETM (2022) Validation and molecular analysis of β-1,3-GLU2 SNP marker associated with resistance to Colletotrichum gloeosporioides in mango (Mangifera indica L.). Physiol Mol Plant Pathol 118:101804. doi:https://doi.org/10.1016/j.pmpp.2022.101804
Gong DQ, Zhu SJ, Gu H, Zhang LB, Hong KQ, Xie JH (2013) Disease resistance of ‘Zill’ and ‘Keitt’ mango fruit to anthracnose in relation to defence enzyme activities and the content of anti-fungal substances. J Hortic Sci Biotechnol 88(3):243–250. doi:https://doi.org/10.1080/14620316.2013.11512962
Grice K, Bally ISE Preliminary screening for anthracnose resistance in mango. Paper presented at the The Amistar 6th Australian mango conference, Surfers Paradise, Queensland, 22–25 May 2007
Gupta S, Singh K, Singh A (2015) Resistance to anthracnose disease in commercial cultivars and advanced hybrids of mango. Plant Pathol J (Faisalabad) 14(4):255–258
Hassan MK, Dann EK, Irving DE, Coates LM (2007) Concentrations of constitutive alk(en)ylresorcinols in peel of commercial mango varieties and resistance to postharvest anthracnose. Physiol Mol Plant Pathol 71:158–165
Holmes R, Hofman P, Barker L (2010) Mango quality assessment manual. A guide to assessing the post-harvest quality of australian mangoes, 1st edn. The state of Queensland, Department of Employment, Economic Development and Innovation, Brisbane
Jeffries P, Dodd JC, Jeger MJ, Plumbley RA (1990) The biology and control of Colletotrichum species on tropical fruit crops. Plant Pathol 39(3):343–366
Kamle M, Kumar P (2016) Colletotrichum gloeosporioides: Pathogen of Anthracnose Disease in Mango (Mangifera indica L.). In: Kumar P, Gupta VK, Tiwari AK, Kamle M (eds) Current Trends in Plant Disease Diagnostics and Management Practices. Springer International Publishing, Cham, pp 207–219. doi:https://doi.org/10.1007/978-3-319-27312-9_9
Karunanayake LC, Adikaram N, Kumarihamy BMM, Bandara BMR, Abayasekara C (2011) Role of Antifungal Gallotannins, Resorcinols and Chitinases in the Constitutive Defence of Immature Mango (Mangifera indica L.) against Colletotrichum gloeosporioides. J Phytopathol 159(10):657–664. doi:https://doi.org/10.1111/j.1439-0434.2011.01818.x
Knödler M, Berardini N, Kammerer DR, Carle R, Schieber A (2007) Characterization of major and minor alk(en)ylresorcinols from mango (Mangifera indica L.) peels by high-performance liquid chromatography/atmospheric pressure chemical ionization mass spectrometry. Rapid Commun Mass Spectrom 21(6):945–951. doi:https://doi.org/10.1002/rcm.2919
Kobiler I, Reved R, Artez L, Prusky D (1998) Antifungal compounds regulating quiescent diseases in mango. In: Johnson GI, Highley E, Joyce DC (eds) Disease Resistance in Fruit, Chiang Mai, Thailand, 18 – 12 May 1997. ACIAR Proceedings, vol 80. ACIAR, pp 109–114
Kumar AS, Reddy NE, Reddy KH, Devi MC (2007) Evaluation of fungicidal resistance among Colletotrichum gloeosporioides isolates causing mango anthracnose in Agri Export Zone of Andhra Pradesh, India. Plant Pathol Bull 16(3):157–160
Mishra D, Misra B (1973) Retardation of induced senescence of leaves from crop plants by benzimidazole and cytokinins. Exp Gerontol 8(4):235–239. doi:https://doi.org/10.1016/0531-5565(73)90032-6
Nishijima WT (1994) Mango diseases and their control. In: Conference on Mango, Manooa, Hawaii, March 9–11 1993. University of Hawaii, pp 20–24
Pernezny K, Ploetz RC (2000) Some Common Diseases of Mango in Florida. Tropical Research and Education Center. University of Florida, Homestead, USA
Pierre H (2015) Mangiferin as a biomarker for Mango Anthracnose Resistance. M.Sc. Florida International University, Florida, USA
Ploetz R (2017) The mango disease trilogy. Acta Hort 1183:221–228. doi:https://doi.org/10.17660/ActaHortic.2017.1183.31
Ploetz RC (1994) Distribution and prevalence of Fusarium subglutinans in mango trees affected by malformation. Can J Bot 72(1):7–9
Ploetz RC (2003) Diseases of Mango. In: Ploetz RC (ed) Diseases of Tropical Fruit crops. CABI Publishing, Cambridge, pp 327–364
Sanchez-Arizpe A, Galindo-Cepeda ME, Arispe-Vazquez J, Genis-Velazquez R, Vazquez-Badillo M, Antonio-Bautista A (2021) Natural resistance of two mango “Mangifera indica” L. commercial cultivars to anthracnose caused by “Colletotrichum gloeosporioides” Penz. Penz and Sacc 15. vol 8. Southern Cross Journalsdoi:https://doi.org/10.3316/informit.181088180094764
Sudheeran PK, Sela N, Carmeli-Weissberg M, Ovadia R, Panda S, Feygenberg O, Maurer D, Oren-Shamir M, Aharoni A, Alkan N (2021) Induced defense response in red mango fruit against Colletotrichum gloeosporioides. HortRes 8. doi:https://doi.org/10.1038/s41438-020-00452-4
ten Have A, van Berloo R, Lindhout P (2007) Partial stem and leaf resistance against the fungal pathogen Botrytis cinerea in wild relatives of tomato. Eur J Plant Pathol 117:153–166. https://doi.org/10.1007/s10658-006-9081-9
Timmer LW, Garnsey SM, Graham JH (2000) Compendium of citrus diseases, Second Edition. second edn. APP Press, St. Paul
Veloso JS, Lima WG, Reis A, Doyle VP, Michereff SJ, Câmara MPS (2021) Factors influencing biological traits and aggressiveness of Colletotrichum species associated with cashew anthracnose in Brazil. Plant Pathol 70(1):167–180. doi:https://doi.org/10.1111/ppa.13276
Vitale A, Alfenas AC, de Siqueira DL, Magista D, Perrone G, Polizzi G (2020) Cultivar Resistance against Colletotrichum asianum in the World Collection of Mango Germplasm in Southeastern Brazil. Plants Today 9(2):182. doi:https://doi.org/10.3390/plants9020182
VSN_International (2020) Genstat for Windows 21st Edition.VSN International. Genstat.co.uk.Accessed 30/06/2022 2022
Weir BS, Johnston PR, Damm U (2012) The Colletotrichum gloeosporioides species complex. Stud Mycol 73:115–180. doi:https://doi.org/10.3114/sim0011
Wharton PS, Iezzoni A, Jones AL (2003) Screening Cherry Germ Plasm for Resistance to Leaf Spot. Plant Dis 87(5):471–477. doi:https://doi.org/10.1094/pdis.2003.87.5.471
Zainuri, Joyce DC, Wearing AH, Coates L, Terry L (2001) Effects of phosphonate and salicylic acid treatments on anthracnose disease development and ripening of ‘Kensington Pride’ mango fruit. Aust J Exp Agric 41(6):805–813
Acknowledgements
This project was funded by the State of Queensland acting through the Department of Agriculture and Fisheries. The research team are also grateful to the funding provided by Hort Innovation MG09003 ‘Mango Breeding Support’; MG13002 ‘Integrating genomics into an applied mango breeding program’. The authors would like to acknowledge the time, assistance and support provided in fruit harvest, experiment setup and data collection by colleagues, in particular Peter Trevorrow and Garry Minnis. The authors would also like to thank Gary Zill for freely sharing his knowledge, as always, on mango varieties and their disease tolerances. We appreciate Kaylene Bransgrove who has taken time to review the manuscript and provide feedback to improve the clarity and quality of the paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Grice, K.R.E., Bally, I.S.E., Wright, C.L. et al. Mango germplasm screening for the identification of sources of tolerance to anthracnose. Australasian Plant Pathol. 52, 27–41 (2023). https://doi.org/10.1007/s13313-022-00899-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13313-022-00899-0