Abstract
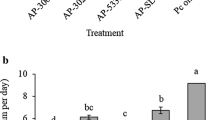
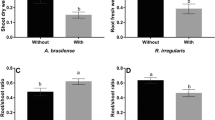
The noxious weed Allium triquetrum (Alliaceae) has invaded widespread areas in southern Australia, forming dense monocultures that threaten indigenous ground flora. Two soilborne biocontrol agents, the fungus Stromatinia cepivora and the bacterium Pectobacterium carotovorum subsp. carotovorum (Pcc) have previously been reported as killing A. triquetrum in gnotobiotic controlled conditions. This study aimed to find why glasshouse pot trials in complex potting media and field trials showed no effect of inoculation with either agent. Bacteria were consistently isolated from inside surface-sterilised bases and bulbs of A. triquetrum. Most bacteria from actively growing plants were closely related to Bacillus but one from dormant bulbs (RPTAtOch1) belonged to Ochrobactrum. Most bacteria reduced soft rot caused by Pcc in vitro by up to 100% when inoculated the day before Pcc. Co-cultivation with Pcc reduced its extracellular pectin lyase and polygalacturonase, which target plant cell walls. RPTAtOch1 was identified as O. quorumnocens by traditional physiological, biochemical and molecular tests, whole genome sequencing and Average Nucleotide Identity comparisons. Its draft genome consisted of 76 contigs, 70% of which were closest to isolate A44 of O. quorumnocens, which antagonises soft rotting of potato by Pcc by destroying its quorum-sensing lactones but, like RPTAtOch1, does not inhibit growth of Pcc. Also, endophytic bacteria inhibited germination of S. cepivora sclerotia and so prevented white rot. Thus, the failure in biocontrol of A. triquetrum by both S. cepivora and Pcc may be due, ironically, to biocontrol of the intended pathogens by endophytic bacteria inside the target weed.











Similar content being viewed by others
References
Abd-El-Khair H, Haggag Karima HE (2007) Application of some bactericides and bioagents for controlling the soft rot disease in potato. Res J Agric Biol Sci 3:463–473
Agriculture Victoria (2017) Angled onion. Accessed on 23 April 2019. http://agriculture.vic.gov.au/agriculture/pests-diseases-and-weeds/weeds/a-z-of-weeds/angled-onion
Bean D, Dudley T (2018) A synoptic review of Tamarix biocontrol in North America: tracking success in the midst of controversy. BioControl 63:361–376
Blood K (2009) Environmental weeds: a field guide for SE Australia. Bloomings Books, Melbourne
Borodina E, Kelly DP, Schumann P, Rainey FA, Ward-Rainey NL, Wood AP (2002) Enzymes of dimethylsulfone metabolism and the phylogenetic characterization of the facultative methylotrophs Arthrobacter sulfonivorans sp. nov., Arthrobacter methylotrophus sp. nov., and Hyphomicrobium sulfonivorans sp. nov. Arch Microbiol 177:173–183
Coil D, Jospin G, Darling AE (2015) A5-miseq: an updated pipeline to assemble microbial genomes from Illumina MiSeq data. Bioinform 31:587–589
Coley-Smith JR, Esler G (1983) Infection of cultivars of onion, leek, garlic and Allium fistulosum by Sclerotium cepivorum. Plant Pathol 32:373–376
Coley-Smith JR, Holt RW (1966) The effect of species of Allium on germination in soil of Sclerotium cepivorum Berk. Ann Appl Biol 58:273–278
Coley-Smith JR, Mitchell CM, Sansford CE (1990) Long-term survival of sclerotia of Sclerotium cepivorum. Plant Pathol 32:373–376
Costa SP, Angelim AL, Sousa MDVD, Melo VMM (2014) Vegetative cells of Bacillus pumilus entrapped in chitosan beads as a product for hydrocarbon biodegradation. Int Biodeter Biodegrad 87:122–127
Czajkowski R, Krzyżanowska DM, Karczewska J, Atkinson S, Przysowa J, Lojkowska E, Williams P, Jafra S (2011) Inactivation of AHLs by Ochrobactrum sp. A44 depends on the activity of a novel class of AHL acylase. Environ Microbiol 3:59–68
De Vos P, Garrity GM, Jones D, Krieg NR, Ludwig W, Rainey FA, Schleifer KH, Whitman WB (eds) (2009) Bergey’s manual of systematic bacteriology, 2nd edn. New York, Springer, Dordrecht, Heidelberg, London
Dong Y-H, Gusti AR, Zhang Q, Xu J-L, Zhang L-H (2002) Identification of quorum-quenching N-acyl homoserine lactonases from Bacillus species. Appl Environ Microbiol 68:1754–1759
Dong Y-H, Xu J-L, Li X-Z, Zhang L-H (2000) AiiA, an enzyme that inactivates the acylhomoserine lactone quorum-sensing signal and attenuates the virulence of Erwinia carotovora. PNAS 97:3526–3531
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evol 39:783–791
Fetzner S (2015) Quorum quenching enzymes. J Biotechnol 201:2–14
Flagan S, Ching WK, Leadbetter JR (2003) Arthrobacter strain VAI-A utilizes acyl-homoserine lactone inactivation products and stimulates quorum signal biodegradation by Variovorax paradoxa. Appl Environ Microbiol 69:909–916
Garge SS, Nerurkar AS (2017) Evaluation of quorum quenching Bacillus spp. for their biocontrol traits against Pectobacterium carotovorum subs. carotovorum causing soft rot. Biocatalysis Agric Biotechnol 9:48–57
Grady EN, MacDonald J, Liu L, Richman A, Yuan Z-C (2016) Current knowledge and perspectives of Paenibacillus: a review. Microb Cell Factories 15:203. https://doi.org/10.1186/s12934-016-0603-7
Grandclément C, Tannières M, Monéra S, Dessaux Y, Faure D (2016) Quorum quenching: role in nature and applied developments. FEMS Microbiol Rev 40:86–116
Graumann P, ed. (2012) Bacillus: cellular and molecular biology, 2nd edn. Caister Academic Press
Helman Y, Chernin L (2015) Silencing the mob: disrupting quorum sensing as a means to fight plant disease. Mol Plant Pathol 16:316–329
Huber B, Scholz HC, Kämpfer P, Falsen E, Langer S, Busse H-J (2010) Ochrobactrum pituitosum sp. nov., isolated from an industrial environment. International Journal of Syst Evol Microbiol 60:321–326
Imran A, Hafeez FY, Frühling A, Schumann P, Malik KA, Stackebrandt (2010) Ochrobactrum ciceri sp. nov., isolated from nodules of Cicer arietinum. Int J Syst Evol Microbiol 60: 1548–1553
Jafra S, Przysowa J, Czajkowski R, Michta A, Garbeva P, Van der Wolf JM (2006) Detection and characterization of bacteria from the potato rhizosphere degrading N-acyl-homoserine lactone. Can J Microbiol 52:1006–1015
Jukes TH, Cantor CR (1969) Evolution of protein molecules. In: Munro HN (ed) Mammalian protein metabolism. Academic Press, New York, pp 21–132
Kämpfer P, Buczolits S, Albrecht A, Busse H-J, Stackebrandt E (2003) Towards a standardized format for the description of a novel species (of an established genus): Ochrobactrum gallinifaecis sp. nov. Int J Syst Evol Microbiol 53:893–896
Kämpfer P, Huber B, Busse H-J, Scholz HC, Tomasco H, Hotzel H, Melzer F (2011) Ochrobactrum pecoris sp. nov., isolated from farm animals. Int J Syst Evol Microbiol 61:2278–2283
Kämpfer P, Kroppenstedt RM (1996) Numerical analysis of fatty acid patterns of coryneform bacteria and related taxa. Can J Microbiol 42:985–1005
Kämpfer P, Sessitsch A, Schloter M, Hubre B, Busse HJ, Scholz HC (2008) Ochrobactrum rhizosphaerae sp. nov. and Ochrobactrum thiophenivorans sp. nov. from the environment. Int J Syst Evol Microbiol 58:1426–1431
Kämpfer P, Steiof M, Dott W (1991) Microbiological characterization of a fuel-oil contaminated site including numerical identification of heterotrophic water and soil bacteria. Microbiol Ecol 21:227–251
Kim S-Y, Kim J-H, Kim S-C, Lee Y-B (2014) Occurrence of tetracyclines resistant bacteria in the soil applied with livestock manure compost. Korean J Environ Agric 22:409–413
Konstantinidis KT, Tiedje JM (2005) Genomic insights that advance the species definition for prokaryotes. Proc Natl Acad Sci U S A 102:2567–2572
Krzyżanowska DM, Maciag T, Ossowicki A, Rajewska M, Kaczyńskiz Z, Czerwickaz M, Rabalski L, Czaplewska P, Jafra S (2019) Ochrobactrum quorumnocens sp. nov., a quorum quenching bacterium from the potato rhizosphere, and comparative genome analysis with related type strains. PLOS ONE https://doi.org/10.1371/journal.pone.0210874
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874
Kuykendall LD, Roy MA, O’Neill JJ, Devine TE (1988) Fatty acids, antibiotic resistance, and deoxyribonucleic acid homology groups of Bradyrhizobium japonicum. Int J Syst Evol Microbiol 38:358–361
Lebuhn M, Achouak W, Schloter M, Berge O, Meier H, Barakat M, Hartmann A, Heulin T (2000) Taxonomic characterization of Ochrobactrum sp. isolates from soil samples and wheat roots, and description of Ochrobactrum tritici sp. nov. and Ochrobactrum grignonense sp. nov. Int J Syst Evol Microbiol 50:2207–2223
Liu H, Carvalhais LC, Crawford M, Singh E, Dennis PG, Pieterse CMJ, Schenk PM (2017) Inner plant values: diversity, colonization and benefits from endophytic bacteria. Front Microbiol 8: article 2552
Lopes R, Tsui S, Gonçalves PJRO, Vieira de Queiroz M (2018) A look into a multifunctional toolbox: endophytic Bacillus species provide broad and underexploited benefits for plants. World J Microbiol Biotechnol 34: article 94 (10 pp.)
Ma B, Hibbing ME, Kim H-S, Reedy RM, Yedidia I, Breuer J, Breuer J, Glasner JD, Perna NT, Kelman A, Charkowski AO (2007) Host range and molecular phylogenies of the soft rot enterobacterial genera Pectobacterium and Dickeya. Phytopathol 97:1150–1163
Mavromatis K, Lorito M, Woo SL, Bouriotis V (2003) Mode of action and antifungal properties of two cold-adapted chitinases. Extremophiles 7:385–390
McInroy JA, Kloepper JW (1994) Novel bacterial taxa inhabiting internal tissues of sweetcorn and cotton. In: Ryder MH, Stephens PM, Bowen GD (eds) Improving plant productivity with rhizosphere bacteria. CSIRO, Adelaide, pp 19–27
Molina L, Constantinescu F, Michel L, Reimmann C, Duffy B, Défago G (2003) Degradation of pathogen quorum-sensing molecules by soil bacteria: a preventative and curative biological control mechanism. FEMS Microbiol Ecol 1522:1–11
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Nedjma M, Hoffmann N, Belarbi A (2001) Selective and sensitive detection of pectin lyase activity using a colorimetric test: application to the screening of microorganisms possessing pectin lyase activity. Anal Biochem 291:290–296
Ngom A, Nakagawa Y, Sawada H, Tsukahara J, Wakabayashi S, Uchiumi T, Nuntagij A, Kotepong S, Suzuki A, Higashi S, Abe M (2004) A novel symbiotic nitrogen-fixing member of the Ochrobactrum clade isolated from root nodules of Acacia mangium. J Gen Appl Microbiol 50:17–27
Nguyen TH, Minh TQ, Pham XH, Nguyen TTT, Naruto J, Hoang HL (2018) Biological control of potato tuber soft rot using N-acyl-L-homoserine lactone-degrading endophytic bacteria. Curr Sci 115:1921–1927
Niu B, Paulson JN, Zheng X, Kolter R (2017) Simplified and representative bacterial community of maize roots. PNAS: E2450–E2459. Accessed on 2 May 2019. https://www.pnas.org/content/114/12/E2450
Park S-Y, Lee SJ, Oh T-K, Oh J-W, Koo B-T, Yum S-Y, Lee J-K (2003) AhlD, an N-acylhomoserine lactonase in Arthrobacter sp., and predicted homologues in other bacteria. Microbiol 149:1541–1550
Parsons WT, Cuthbertson EG (1992) Noxious weeds of Australia. Inkata Press, Melbourne, pp 85–86
Pitcairn MJ, (2018) Weed biological control in California, USA: review of the past and prospects for the future. BioControl 63(3):349–359
Pirhonen M, Flegol D, Heikinheimo R, Palva ET (1993) A small diffusible signal molecule is responsible for the global control of virulence and exoenzyme production in the plant pathogen Erwinia carotovora. EMBO J 12:2467–2476
Prasad S, Manasa P, Buddhi S, Singh SM, Shivaji S (2011) Antagonistic interaction networks among bacteria from a cold soil environment. FEMS Microbiol Ecol 76:376–385
Puri A, Padda KP, Chanway CP (2016) Seedling growth promotion and nitrogen fixation by a bacterial endophyte Paenibacillus polymyxa P2b-2R and its GFP derivative in corn in a long-term trial. Symbiosis 69:123–129
Qian Y, Kando CK, Thorsen L, Larsen N, Jespersen L (2015) Production of autoinducer-2 by aerobic endospore-forming bacteria isolated from the west African fermented foods. FEMS Microbiol Letts 362: article UNSP-fnv186
Queensland Government (2016) Weeds of Australia: Queensland biosecurity edition. https://keyserver.lucidcentral.org/weeds/data/media/Html/allium_triquetrum.htm. Accessed 13 June 2019
Rahman MM, Ali ME, Khan AA, Akanda AM, Uddin MK, Hashim U, AbdHamid SB (2012) Isolation, characterization, and identification of biological control agent for potato soft rot in Bangladesh. Sci World J: article ID 723293 (6 pp.)
Raoul des Essarts YR, Cigna J, Quêtu-Laurent A, Caron A, Munier E, Beury-Cirou A, Hélias V, Faure D (2016) Biocontrol of the potato blackleg and soft rot diseases caused by Dickeya dianthicola. Appl Environ Microbiol 82:268–278
Richter M, Rosselló-Móra R, Oliver Glöckner F, Peplies J (2016) JSpeciesWS: a web server for prokaryotic species circumscription based on pairwise genome comparison. Bioinformatics 32:929–931
Royal Botanic Gardens Victoria (2018) Allium triquetrum. Accessed 2 May 2019. https://vicflora.rbg.vic.gov.au/flora/taxon/88b31d41-7bd4-4583-ae99-56a728936720
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Schwarzländer M, Hinz HL, Winston RL, Day MD (2018) Biological control of weeds: an analysis of introductions, rates of establishment and estimates of success, worldwide. BioControl 63:319–331
Someya N, Ikeda S, Morohoshi T, Noguchi M, Yoshida T, Sawada H, Ikeda T, Tsuchiya K (2011) Diversity of culturable chitinolytic bacteria from rhizospheres of agronomic plants in Japan. Microbes Environ 26:7–17
Taiz L, Zeiger E (2002) Plant physiology, 3rd edn. Sinauer Associates Inc, Sunderland
Tamura K, Nei M (1993) Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol 10:512–526
Tehranchian P, Adair R, Lawrie AC (2012) Bulb rot in live Allium triquetrum by Pectobacterium carotovora ssp. carotovora. 18th Australian weeds conference, Melbourne, Australia, 8-11 October, 135-141. ISBN 978-0-646-58670-0
Tehranchian P, Adair RJ, Lawrie AC (2014) Potential for biological control of the weed angled onion (Allium triquetrum) by the fungus Stromatinia cepivora in Australia. Australas Plant Pathol 43:381–392. https://doi.org/10.1007/s13313-014-0279-6
Thompson JD, Higgins DG, Gibson TJ (1994) ClustalW: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 122:4673–4680
Tripathi AK, Verma SC, Chowdhury SP, Lebuhn M, Gattinger A, Schloter M (2006) Ochrobactrum oryzae sp. nov., an endophytic bacterial species isolated from deep-water rice in India. Int J Syst Evol Microbiol 56:1677–1680
Trivedi P, Spann T, Wang NA (2011) Isolation and characterization of beneficial bacteria associated with citrus roots in Florida. Microbial Ecol 62:324–336
Trognitz F, Hackl E, Widhalm S, Sessitch A (2016) The role of plant-mirobiome interactions in weed establishment and control. FEMS Microbiol Ecol 92, fiw138
Trujillo ME, Willems A, Abril A, Planchuelo A-M, Rivas R, Ludeña D et al (2005) Nodulation of Lupinus albus by strains of Ochrobactrum lupini sp. nov. Appl Environ Microbiol 71:1318–1327
US Food and Drug Administration (2017) BAM media M127: Potato dextrose agar. Accessed on 2 May 2019. https://www.fda.gov/food/laboratory-methods-food-safety/bam-media-m127-potato-dextrose-agar
Velize EA, Martinez-Hidalgo P, Hirsch AM (2017) Chtinase-producing bacteria and their role in biocontrol. AIMS Microbiol 3:689–705
Weisburg WG, Barns SM, Pelletier DA, Lane DJ (1991) 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol 173:697–703
Woo S-G, Ten LN, Park J, Lee M (2011) Ochrobactrum daejeonense sp. nov., a nitrate-reducing bacterium isolated from sludge of a leachate treatment plant. Int J Syst Evol Microbiol 61:2690–2696
Zurdo-Piñeiro JL, Rivas R, Trujillo ME, Vizcaino N, Carrasco JA, Chamber M et al (2007) Ochrobactrum cytisi sp. nov., isolated from nodules of Cytisus scoparius in Spain. Int J Syst Evol Microbiol 57:784–788
Acknowledgements
The authors thank Parks Victoria for funding part of this research on attempts to find biological control organisms for A. triquetrum, Prof. Andrew Smith for making Illumina sequencing possible for RPTAtOch1, and the Yarra Ranges Shire Council (especially Paul Smitka) for allowing the use of the Birdsland Reserve for field trials.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tehranchian, P., Adair, R.J., Van, T.T.H. et al. Biological Control of the Noxious Weed Angled Onion (Allium triquetrum) Thwarted by Endophytic Bacteria in Victoria, Australia. Australasian Plant Pathol. 49, 373–392 (2020). https://doi.org/10.1007/s13313-020-00710-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13313-020-00710-y