Abstract
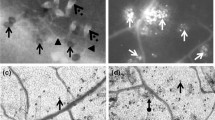
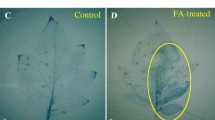
The generation and accumulation of hydrogen peroxide (H2O2) and superoxide anion (O .-2 ), as well as guaiacol peroxidase, catalase, polyphenol oxidase, β-1,3-glucanase and chitinase activity, were studied in leaves of resistant and susceptible tomato genotypes inoculated with Oidium neolycopersici. Plants of the resistant genotype CNPH 1287 (Solanum habrochaites sin. Lycopersicon hirsutum) and susceptible genotype Santa Cruz Kada (S. lycopersicum sin. Lycopersicon esculentum), with the seven-nine and five-seven leaves completely developed, respectively, were inoculated in the second, third and fourth true leaves. Leaves were collected at the time of inoculation and at 4, 8, 12, 24, 48, 72, 96 and 120 h post inoculation (hpi). The production and accumulation of H2O2 and O .-2 were evaluated in situ using diaminobenzidine and nitroblue tetrazolium, respectively. Starting at 24–48 hpi, high accumulation of H2O2 and O .-2 was detected, and epidermal cells demonstrated a hypersensitive response, especially in the inoculated leaves of the resistant plant (S. habrochaites). An increase in guaiacol peroxidase, catalase, polyphenol oxidase, β-1,3-glucanase and chitinase activity was mainly detected by 24 hpi in the resistant plant. An association between the production of reactive oxygen species and the activity of enzymes related to reactive oxygen species metabolism (guaiacol peroxidase, catalase), hydrolytic enzymes (β-1,3-glucanase and chitinase) and phenol metabolism enzymes (polyphenol oxidase), as well as hypersensitive response, was evident during the defence responses of the resistant plants when inoculated with O. neolycopersici.







Similar content being viewed by others
Abbreviations
- CAT:
-
Catalase
- ROS:
-
Reactive oxygen species
- GLU:
-
Glucanase
- GPOX:
-
Guaiacol peroxidase
- hpi:
-
Hours post-inoculation
- HR:
-
Hypersensitive response
- PPO:
-
Polyphenol oxidase
- CHI:
-
Chitinase
References
Bai Y, Huang CC, Van der Hulst R, Meijer-Dekens F, Bonnema G, Lindhout P (2005) QTLs for tomato powdery mildew resistance (Oidium lycopersici) in Lycopersicon parviflorum G1.1601 co-localize with two qualitative powdery mildew resistance genes. Mol Plant Microbe Interact 16:169–176
Baker CJ, Orlandi EW (1995) Active oxygen in plant pathogenesis. Annu Rev Phytopathol 33:299–321
Balbi-Peña MI, Schwan-Estrada KRF, Stangarlin JR, Tolentino Júnior JB (2010) Development of Oidium neolycopersici on Lycopersicon genotypes. Summa Phytopathol 36:35–39
Bolwell GP, Wojtaszek P (1997) Mechanisms for the generation of reactive oxygen species in plant-defence-broad perspective. Physiol Mol Plant Pathol 51:347–366
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brisson LF, Tenhaken R, Lamb C (1994) Function of oxidative cross-linking of cell wall structural proteins in plant disease resistance. Plant Cell 6:1703–1712
Ciccarese F, Amenduni M, Schiavone D, Cirulli M (1998) Occurrence and inheritance of resistance to powdery mildew (Oidium lycopersici) in Lycopersicon species. Plant Pathol 47:417–419
Dangl JL, Dietrich RA, Richberg MH (1996) Death don’t have no mercy: cell death programs in plant-microbe interactions. Plant Cell 8:1793–1807
Delledonne M (2005) NO news is good news for plants. Curr Opin Plant Biol 8:390–396
Duangmal K, Apenten RKO (1999) A comparative study of poliphenoloxidases from taro (Colocasia esculenta) and potato (Solanum tuberosum var. Romano). Food Chem 64:351–359
Gorjanovic SA (2009) Review: Biological and technological functions of barley seed pathogenesis-related proteins (PRs). J Inst Brew 115:334–360
Greenberg JT, Yao N (2004) The role and regulation of programmed cell death in plant–pathogen interactions. Cell Microbiol 6:201–211
Hammond-Kosack KE, Jones JDG (1996) Resistance gene-dependent plant defence responses. Plant Cell 8:1773–1791
Heath MC (2000) Hypersensitive response-related death. Plant Mol Biol 44:321–334
Heiser I, Osswald WF (2008) Reactive oxygen species and their function in plant-pathogen interactions. In: Pascholati SF, Leite B, Stangarlin JR, Cia P (eds) Plant-pathogen interaction: physiology, biochemistry and molecular biology. FEALQ, Piracicaba, pp 249–284
Heller J, Tudzynski P (2011) Reactive oxygen species in phytopathogenic fungi: signaling, development, and disease. Annu Rev Phytopathol 49:369–390
Huang C, Groot T, Meijer-Dekens F, Niks R, Lindhout P (1998) Hypersensitivity is the major mechanism of resistance to powdery mildew (Oidium lycopersicum) in Lycopersicon species. Eur J Plant Pathol 104:399–407
Huang C, Hoefs-Van De Putte PM, Haanstra-Van Der Meer JG, Meijer-Dekens F, Lindhout P (2000) Characterization and mapping of resistance to Oidium lycopersicum in two Lycopersicon hirsutum accessions: evidence for close linkage of two Ol-genes on chromosome 6 of tomato. Heredity 85:511–520
Hückelhoven R, Kogel KH (1998) Tissue-specific superoxide generation at interaction sites in resistant and susceptible near isogenic barley lines attacked by powdery mildew fungus (Erysiphe graminis f. sp. hordei). Mol Plant Microbe Interact 11:292–300
Hückelhoven R, Fodor J, Preis C, Kogel K (1999) Hypersensitive cell death and papilla formation in barley attacked by the powdery mildew fungus are associated with hydrogen peroxide but not with salicylic acid accumulation. Plant Physiol 119:1251–1260
Hückelhoven R, Fodor J, Trujillo M, Kogel K-H (2000) Barley Mla and Rar mutants compromised in the hypersensitive cell death response against Blumeria graminis f.sp. hordei are modified in their ability to accumulate reactive oxygen intermediates at sites of fungal invasion. Planta 212:16–24
Iiyama K, Lam TB-T, Stone BA (1994) Covalent cross-links in the cell wall. Plant Physiol 104:315–320
Jabs T, Tschöpe M, Colling C, Hahlbrock K, Scheel D (1997) Elicitor-stimulated ion fluxes and O -2 from the oxidative burst are essential components in triggering defense gene activation and phytoalexin synthesis in parsley. Proc Natl Acad Sci 94:4800–4805
Jones H, Whipps JM, Gurr SJ (2001) The tomato powdery mildew fungus Oidium neolycopersici. Mol Plant Pathol 2:303–309
Joosten MHAJ, De Wit PJGM (1989) Identification of several pathogenesis-related proteins in tomato leaves inoculated with Cladosporium fulvum (syn. Fulvia fulva) as 1,3-β-glucanases and chitinases. Plant Physiol 89:945–951
Kemp G, Botha A-M, Kloppers FJ, Pretorius ZA (1999) Disease development and β-1,3-glucanase expression following leaf rust infection in resistant and susceptible near-isogenic wheat seedlings. Physiol Mol Plant Pathol 55:45–52
Kini KR, Vasanthi NS, Shetty HS (2000) Induction of β-1,3-glucanase in seedlings of pearl millet in response to infection by Sclerospora graminicola. Eur J Plant Pathol 106:267–274
Koga H, Zeyen RJ, Bushnell WR, Ahlstrand GG (1988) Hypersensitive cell death, autofluorescence, and insoluble silicon accumulation in barley leaf epidermal cells under attack by Erysiphe graminis f. sp. hordei. Physiol Mol Plant Pathol 32:395–409
Lam E, Kato N, Lawton M (2001) Programmed cell death, mitochondria and the plant hypersensitive response. Nature 411:848–853
Lamb C, Dixon RA (1997) The oxidative burst in plant disease resistance. Annu Rev Plant Physiol Plant Mol Biol 48:251–275
Lawrence CB, Singh NP, Qiu J, Gardner RG, Tuzin S (2000) Constitutive hydrolytic enzymes are associated with polygenic resistance of tomato to Alternaria solani and may function as an elicitor release mechanism. Physiol Mol Plant Pathol 57:211–220
Lever M (1972) A new reaction for colorimetric determination of carbohydrates. Anal Biochem 47:273–279
Levine A, Tenhaken R, Dixon R, Lamb C (1994) H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell 79:583–593
Li L, Steffens JC (2002) Overexpression of polyphenol oxidase in transgenic tomato plants results in enhanced bacterial disease resistance. Planta 215:239–247
Li C, Bai Y, Jacobsen E, Visser R, Lindhout P, Bonnema G (2006) Tomato defense to the powdery mildew fungus: differences in expression of genes in susceptible, monogenic- and polygenic resistance responses are mainly in timing. Plant Mol Biol 62:127–140
Lindhout P, Pet G, Van Der Beek JG (1994) Screening wild Lycopersicon species for resistance to powdery mildew (Oidium lycopersicum). Euphytica 72:43–49
Lusso MFG, Pascholati SF (1999) Activity and isoenzymatic pattern of soluble peroxidases in maize tissues after mechanical injury or fungal inoculation. Summa Phytopathol 25:244–249
Mandal S, Mitra A, Mallick N (2008) Biochemical characterization of oxidative burst during interaction between Solanum lycopersicum and Fusarium oxysporum f. sp. lycopersici. Physiol Mol Plant Pathol 72:56–61
Mansfield J, Bennett M, Bestwick C, Woods-Tör A (1997) Phenotypic expression of gene-for-gene interaction involving fungal and bacterial pathogens: variation from recognition to response. In: Crute IR, Holub EB, Burdon JJ (eds) The gene-for-gene relationship in plant–parasite interactions. CAB International, Wallingford, pp 265–291
Matsuda Y, Mori Y, Sakano Y, Nishida M, Tarumoto K, Nonomura T, Nishimura H, Kusakari S, Toyoda H (2005) Screening of wild Lycopersicon species for resistance to japanese isolate of tomato powdery mildew Oidium neolycopersici. Breed Sci 55:355–360
Mauch F, Mauch-Mani B, Boller T (1988) Antifungal hydrolases in pea tissue. II. Inhibition of fungal growth by combination of chitinase and β-1,3-glucanase. Plant Physiol 88:936–942
Mellersh DG, Foulds IV, Higgens VJ, Heath MC (2002) H2O2 plays different roles in determining penetration failure in three diverse plant–fungal interactions. Plant J 29:257–268
Mieslerová B, Lebeda A, Kennedy R (2004) Variation in Oidium neolycopersici development on host and non-host plant species and their tissue defence responses. Ann Appl Biol 144:237–248
Mlíčková K, Luhová L, Lebeda A, Mieslerová B, Peč P (2004) Reactive oxygen species generation and peroxidase activity during Oidium neolycopersici infection on Lycopersicon species. Plant Physiol Biochem 42:753–761
Mohammadi M, Kazemi H (2002) Changes in peroxidases and polyphenol oxidases activities in susceptible and resistant wheat heads inoculated with Fusarium graminearum and induced resistance. Plant Sci 162:491–498
Piterková J, Petřivalský M, Luhová L, Mieslerová B, Sedlářová M, Lebeda A (2009) Local and systemic production of nitric oxide in tomato responses to powdery mildew infection. Mol Plant Path 10:501–513
Richard-Forget FC, Gauillard FA (1997) Oxidation of chlorogenic acid, catechins, and 4-methylcatechol in model solutions by combinations of pear (Pyrus communis cv. Williams) polyphenol oxidase and peroxidase: a possible involvement of peroxidase in enzymatic browning. J Agr Food Chem 45:2472–2476
Rivera ME, Codina JC, Olea F, De Vicente A, Pérez-García A (2002) Differential expression of β-1,3-glucanase in susceptible and resistant melon cultivars in response to infection by Sphaerotheca fusca. Physiol Mol Plant Pathol 61:257–265
Rodrigues FÁ, Jurick WM, Datnoff LE, Jones JB, Rollins JA (2005) Silicon influences cytological and molecular events in compatible and incompatible rice-Magnaporthe grisea interactions. Physiol Mol Plant Pathol 66:144–159
Romero D, Rivera ME, Cazorla FM, Codina JC, Fernández-Ortuño D, Torés JA, Pérez-García A, de Vicente A (2008) Comparative histochemical analyses of oxidative burst and cell wall reinforcement in compatible and incompatible melon–powdery mildew (Podosphaera fusca) interactions. J Plant Physiol 165:1895–1905
Romero-Puertas MC, Rodriguez-Serrano M, Corpas FJ, Del Rio LA (2004) Cadmium- induced subcellular accumulation of O -2 and H2O2 in pea leaves. Plant Cell Environ 27:1122–1134
Sedlářová M, Luhová L, Petřivalský M, Lebeda A (2007) Localisation and metabolism of reactive oxygen species during Bremia lactucae pathogenesis in Lactuca sativa and wild Lactuca spp. Plant Physiol Biochem 45:607–616
Shetty NP, Jørgensen HJL, Jensen JD, Collinge DB, Shetty HS (2008) Roles of reactive oxygen species in interactions between plants and pathogens. Eur J Plant Pathol 121:267–280
Thipyapong P, Hunt MD, Steffens JC (2004) Antisense downregulation of polyphenol oxidases results in enhanced disease susceptibility. Planta 220:105–117
Tománková K, Luhová L, Petřivalský M, Peč P, Lebeda A (2006) Biochemical aspects of reactive oxygen species formation in the interaction between Lycopersicon spp. and Oidium neolycopersici. Physiol Mol Plant Pathol 68:22–32
Torres MA (2010) ROS in biotic interactions. Physiol Plant 148:414–429
Torres MA, Jones JDG, Dangl JL (2006) Reactive oxygen species signaling in response to pathogens. Plant Physiol 141:373–378
Tuite J (1969) Plant pathological methods: fungi and bacteria. Purdue University, Lafayette
Van Kan JAL, Joosten MHAJ, Wagemakers CAM, Van Den Berg-Velthuis GCM, De Wit PJGM (1992) Differential accumulation of mRNAs encoding extracellular and intracellular PR proteins in tomato induced by virulent and avirulent races of Cladosporium fulvum. Plant Mol Biol 20:513–527
Van Loon LC (1997) Induced resistance in plants and the role of pathogenesis-related proteins. Eur J Plant Pathol 103:753–765
Wang C-F, Huang L-L, Buchenauer H, Han Q-M, Zhang H-C, Kang Z-S (2007) Histochemical studies on the accumulation of reactive oxygen species (O -2 and H2O2) in the incompatible and compatible interaction of wheat-Puccinia striiformis f. sp. tritici. Physiol Mol Plant Pathol 71:230–239
Wang C-F, Huang L-L, Zhang H-C, Han Q-M, Buchenauer H, Kang Z-S (2010) Cytochemical localization of reactive oxygen species (O -2 and H2O2) and peroxidase in the incompatible and compatible interaction of wheat - Puccinia striiformis f. sp. tritici. Physiol Mol Plant Pathol 74:221–229
Wirth SJ, Wolf GA (1990) Dye-labelled substrates for the assay and detection of chitinase and lysozyme activity. J Microbiol Meth 12:197–205
Yoshikawa M, Yamaoka N, Takeuchi Y (1993) Elicitors: their significance and primary modes of action in the induction of plant defense reactions. Plant Cell Physiol 34:1163–1173
Acknowledgments
The authors would like to thank Conselho Nacional de Desenvolvimento Científico e Tacnológico (CNPq) for the M.I. Balbi-Peña doctoral scholarship and the productivity scholarship provided to K.R.F. Schwan-Estrada and J. R. Stangarlin. The authors also would like to thank Dr. Leonardo Boiteux from the National Center for Vegetable Crops Research (CNPH), EMBRAPA, for performing the molecular identification of the fungus and providing the seeds used in this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Balbi-Peña, M.I., Schwan-Estrada, K.R.F. & Stangarlin, J.R. Differential occurrence of the oxidative burst and the activity of defence-related enzymes in compatible and incompatible tomato-Oidium neolycopersici interactions. Australasian Plant Pathol. 41, 573–586 (2012). https://doi.org/10.1007/s13313-012-0150-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13313-012-0150-6