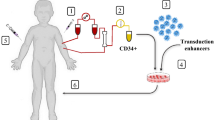
Abstract
β-Thalassemia is one of the most prevalent monogenic diseases usually caused by quantitative defects in the production of β-globin, a component of adult hemoglobin (α2β2), leading to severe anemia. Technological advances in genome sequencing, stem cell selection, viral vector development, transduction and gene-editing strategies now allow for efficient ex-vivo genetic manipulation of human hematopoietic stem cells that can lead to a meaningful clinical benefit in thalassemia patients. In this perspective, the status of the gene-therapy approaches available for transfusion-dependent thalassemia and early results of clinical trials are discussed. It is highly anticipated that gene therapies will soon become a treatment option for patients lacking compatible donors for hematopoietic stem cell transplant and will offer a suitable alternative for definitive treatment of β-thalassemia, even in young children.
Similar content being viewed by others
References
Giardine B, Borg J, Viennas E, et al. Updates of the HbVar database of human hemoglobin variants and thalassemia mutations. Nucleic Acids Res. 2014;42:D1063–9.
Rund D, Rachmilewitz E. Beta-thalassemia. N Engl J Med. 2005;353:1135–46.
Rachmilewitz EA, Giardina PJ. How I treat thalassemia. Blood. 2011;118: 3479–88.
Thein SL. Genetic modifiers of beta-thalassemia. Haematologica. 2005;90:649–60.
Danjou F, Anni F, Galanello R. Beta-thalassemia: From genotype to phenotype. Haematologica. 2011;96:1573–5.
Bernards R, Flavell RA. Physical mapping of the globin gene deletion in hereditary persistence of foetal haemoglobin (HPFH). Nucleic Acids Res 1980;8:1521–34.
Forget BG. Molecular basis of hereditary persistence of fetal hemoglobin. Ann NY Acad Sci. 1998;850:38–44.
Musallam KM, Rivella S, Vichinsky Rachmilewitz EA. Non-transfusion-dependent thalassemias. Haematologica. 2013; 98: 833–44.
Cao A, Galanello R. Beta-thalassemia. Genet Med. 2010; 12: 61–76.
Angelucci E, Matthes-Martin S, Baronciani D, et al. Hematopoietic stem cell transplantation in thalassemia major and sickle cell disease: indications and management recommendations from an international expert panel. Haematologica, 2014;99: 811–20.
Lucarelli G, Isgrò A, Sodani P, Gaziev J. Hematopoietic stem cell transplantation in thalassemia and sickle cell anemia. Cold Spring Harb Perspect Med. 2012;2: a011825.
Locatelli F, Kabbara N, Ruggeri A, et al. Outcome of patients with hemoglobinopathies given either cord blood or bone marrow transplantation from an HLA-identical sibling. Blood. 2013;122:1072–8.
Sodani P, Isgrò A, Gaziev J, et al. Purified T-depleted, CD34+ peripheral blood and bone marrow cell transplantation from haploidentical mother to child with thalassemia. Blood, 2010;115: 1296–302.
Bertaina A, Merli P, Rutella S, et al. HLA-haploidentical stem cell transplantation after removal of alphabeta+ T and B cells in children with nonmalignant disorders. Blood. 2014;124: 822–6.
Soni S, Breslin N, Cheerva A. Successful unrelated umbilical cord blood transplantation for class 3 beta-thalassemia major using a reduced-toxicity regimen. Pediatr Transplant. 2014;18:E41–3.
Kim A, Dean A. Chromatin loop formation in the beta-globin locus and its role in globin gene transcription. Mol Cells. 2012;34:1–5.
Lan X, Khandros E, Huang P, et al. The E3 ligase adaptor molecule SPOP regulates fetal hemoglobin levels in adult erythroid cells. Blood Adv. 2019;3:1586–97.
Masuda T, Wang X, Maeda M, et al. Transcription factors LRF and BCL11A independently repress expression of fetal hemoglobin. Science. 2016;351:285–9.
Sankaran VG, Menne TF, Xu J, et al. Human fetal hemoglobin expression is regulated by the developmental stage-specific repressor BCL11A. Science, 2008;322:1839–42.
Breda L, Carla Casu, Sara Gardenghi, et al. Therapeutic hemoglobin levels after gene transfer in beta-thalassemia mice and in hematopoietic cells of beta-thalassemia and sickle cells disease patients. PLoS One, 2012;7: e32345.
Bank A, Dorazio R, Leboulch P. A phase I/II clinical trial of beta-globin gene therapy for beta-thalassemia. Ann N Y Acad Sci. 2005;1054:308–16.
Thompson AA, Walters MC, Kwiatkowski J, et al. Gene therapy in patients with transfusion-dependent beta-thalassemia. N Engl J Med. 2018;378:1479–93.
Roselli EA, Mezzadra R, Frittoli MC, et al. Correction of beta-thalassemia major by gene transfer in haematopoietic progenitors of pediatric patients. EMBO Mol Med, 2010;2:315–28.
Yannaki E, Karponi G, Zervou F, et al. Hematopoietic stem cell mobilization for gene therapy: superior mobilization by the combination of granulocyte-colony stimulating factor plus plerixafor in patients with beta-thalassemia major. Hum Gene Ther. 2013;24: 852–60.
Yannaki E, Papayannopoulou T, Jonlin E, et al. Hematopoietic stem cell mobilization for gene therapy of adult patients with severe beta-thalassemia: results of clinical trials using G-CSF or plerixafor in splenectomized and nonsplenectomized subjects. Mol Ther. 2012;20:230–8.
Cavazzana M, Mavilio F. Gene Therapy for Hemoglobinopathies. Hum Gene Ther. 2018.
Dong A, Rivella S, Breda L. Gene therapy for hemoglobinopathies: Progress and challenges. Transl Res. 2013;161: 293–306.
Malik P, Arumugam PI. Gene therapy for beta-thalassemia. Hematology Am Soc Hematol Educ Program. 2005: p. 45–50.
Negre O, Eggimann AV, Beuzard Y, et al. Gene therapy of the beta-hemoglobinopathies by lentiviral transfer of the beta (A(T87Q))-globin gene. Hum Gene Ther. 2016;27:148–65.
Payen E, Colomb C, Negre O, Beuzard Y, Hehir K, Leboulch P. Lentivirus vectors in beta-thalassemia. Methods Enzymol. 2012;507:109–24.
Ginn SL, Liao SVL, Dane AP, et al. Lymphomagenesis in SCID-X1 mice following lentivirus-mediated phenotype correction independent of insertional mutagenesis and gammac overexpression. Mol Ther. 2010;18:965–76.
Hacein-Bey-Abina S, Garrigue A, Wang GP, et al. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J Clin Invest. 2008;118:3132–42.
Moiani A, Paleari Y, Sartori D, et al. Lentiviral vector integration in the human genome induces alternative splicing and generates aberrant transcripts. J Clin Invest. 2012;122: 1653–66.
Ronen K, Negre O, Roth S, et al. Distribution of lentiviral vector integration sites in mice following therapeutic gene transfer to treat beta-thalassemia. Mol Ther. 2011;19:1273–86.
Kanter J, Walters MC, Hsieh M, et al. Outcomes for initial patient cohorts with up to 33 months of follow-up in the Hgb-206 Phase 1 Trial. Blood. 2018;132:1080–80.
Thompson AA, Walters MC, Kwiatkowski JC, et al. Northstar-2: Updated safety and efficacy analysis of lentiglobin gene therapy in patients with transfusion-dependent β-thalassemia and non-â0/â0 genotypes. Blood. 2019;134:3543–3543.
Lal A, et al. Northstar-3: Interim results from a phase 3 study evaluating lentiglobin gene therapy in patients with transfusion-dependent β-thalassemia and either a β0 or IVS-I-110 mutation at both alleles of the HBB Gene. Blood. 2019;134:815–15.
Gilles AF, Averof M. Functional genetics for all: engineered nucleases, CRISPR and the gene editing revolution. Evodevo. 2014;5:43.
Palpant NJ, Dudzinski D. Zinc finger nucleases: Looking toward translation. Gene Ther. 2013;20:121–7.
Scharenberg AM, Duchateau P, Smith J. Genome engineering with TAL-effector nucleases and alternative modular nuclease technologies. Curr Gene Ther. 2013;13:291–303.
Hoban MD, Bauer DE. A genome editing primer for the hematologist. Blood. 2016;127:2525–35.
Rossin EJ, Wu DM. CRISPR-based gene editing: A guide for the clinician. Int Ophthalmol Clin. 2017;57:151–64.
Komaroff AL. Gene editing using CRISPR: Why the Excitement? JAMA. 2017;318:699–700.
Dever DP, Porteus MH. The changing landscape of gene editing in hematopoietic stem cells: A step towards Cas9 clinical translation. Curr Opin Hematol, 2017;24: 481–88.
Bauer DE, Kamran SC, Lessard S, et al. An erythroid enhancer of BCL11A subject to genetic variation determines fetal hemoglobin level. Science. 2013;342:253–7.
Psatha N, Reik A, Phelps S, et al. Disruption of the BCL11A erythroid enhancer reactivates fetal hemoglobin in erythroid cells of patients with beta-thalassemia major. Mol Ther Methods Clin Dev. 2018;10:313–26.
Bauer DE, Orkin SH. Hemoglobin switching’s surprise: the versatile transcription factor BCL11A is a master repressor of fetal hemoglobin. Curr Opin Genet Dev. 2015;33:62–70.
Sankaran VG, Xu JNY Orkin SH. Transcriptional silencing of fetal hemoglobin by BCL11A. Ann NY Acad Sci. 2010;1202:64–8.
Alhashem YN, Vinjamur DS, Basu M, et al. Transcription factors KLF1 and KLF2 positively regulate embryonic and fetal beta-globin genes through direct promoter binding. J Biol Chem. 2011;286:24819–27.
Ikonomi P, Noguchi CT, Miller W, et al. Levels of GATA-1/GATA-2 transcription factors modulate expression of embryonic and fetal hemoglobins. Gene. 2000;261:277–87.
Traxler EA, Yao Y, Wang YD, et al. A genome-editing strategy to treat beta-hemoglobinopathies that recapitulates a mutation associated with a benign genetic condition. Nat Med. 2016;22:987–90.
Lin MI, Wang J, Tan Y, et al. Re-creating hereditary persistence of fetal hemoglobin (HPFH) to treat sickle cell disease (SCD) and β-thalassemia. Blood. 2016;128:4708–4708.
Zhang XH, Tee LY, Wang XG, Huag QS, Yang SH. Off-target effects in CRISPR/Cas9-mediated genome engineering. Mol Ther Nucleic Acids. 2015;4: e264.
Lazzarotto CR, Nguyen NT, Tang X, et al. Defining CRISPR-Cas9 genome-wide nuclease activities with CIRCLE-seq. Nat Protoc. 2018;13:2615–2642.
Cho SW, Kim S, Kim Y, et al. Analysis of off-target effects of CRISPR/Cas-derived RNA-guided endonucleases and nickases. Genome Res. 2014;24:132–41.
Cradick TJ, Qiu P, Lee CM, Fine EJ, Bao G, et al. COSMID: A web-based tool for identifying and validating CRISPR/Cas offtarget sites. Mol Ther Nucleic Acids. 2014;3:e214.
Kim D, Bae S, Park J, et al. Digenome-seq: Genome-wide profiling of CRISPR-Cas9 off-target effects in human cells. Nat Methods. 2015;12:237–43.
Tsai SQ, Joung JK. Defining and improving the genome-wide specificities of CRISPR-Cas9 nucleases. Nat Rev Genet. 2016;17:300–12.
Long J, Hoban MD, Cooper AR, et al. Characterization of gene alterations following editing of the beta-globin gene locus in hematopoietic stem/progenitor cells. Mol Ther. 2018;26: 468–79.
Corbacioglu S, Chapin J, Chu-Osier N, et al. Efficacy results with a single dose of autologous crispr-cas9 modified cd34+ hematopoietic stem and progenitor cells in transfusion-dependent β-thalassemia and sickle cell disease. EHA Meeting Abstract. S280.
Marktel S, Scaramuzza, S, Cicalese MP, et al. Intrabone hematopoietic stem cell gene therapy for adult and pediatric patients affected by transfusion-dependent β-thalassemia. Nat Med. 2019;25:234–41.
Author information
Authors and Affiliations
Corresponding author
Additional information
Note
Supplementary material related to this study is available with the online version at www.indianpediatrics.net
Competing interests
The author is also employed by Crispr Therapeutics Inc. that sponsors the CTX001 thalassemia trial. Only publicly available information has been provided and the manuscript was not influenced in any way by this relationship. Part of the text in this manuscript was adapted for pediatrics audience from previously submitted reviews to other journals by the author.
Funding
None.
Rights and permissions
About this article
Cite this article
Soni, S. Gene Therapies for Transfusion-Dependent β-Thalassemia. Indian Pediatr 58, 667–674 (2021). https://doi.org/10.1007/s13312-021-2263-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13312-021-2263-x