Abstract
Refractory status epilepticus (RSE) is a life-threatening emergency with high mortality and poor functional outcomes in survivors. Treatment is typically limited to intravenous anesthetic infusions and multiple anti-seizure medications. While ongoing seizures can cause permanent neurological damage, medical therapies also pose severe and life-threatening side effects. We tested the feasibility of using high-definition transcranial direct current stimulation (hd-tDCS) in the treatment of RSE. We conducted 20-min hd-tDCS sessions at an outward field orientation, intensity of 2-mA, 4 + 1 channels, and customized for deployment over the electrographic maximum of epileptiform activity (“spikes”) determined by real-time clinical EEG monitoring. There were no adverse events from 32 hd-tDCS sessions in 10 RSE patients. Over steady dosing states of infusions and medications in 29 included sessions, median spike rates/patient fell by 50% during hd-tDCS on both automated (p = 0.0069) and human (p = 0.0277) spike counting. Median spike rates for any given stimulation session also fell by 50% during hd-tDCS on automated spike counting (p = 0.0032). Immediately after hd-tDCS, median spike rates/patient remained down by 25% on human spike counting (p = 0.018). Compared to historical controls, hd-tDCS subjects were successfully discharged from the intensive care unit (ICU) 45.8% more often (p = 0.004). When controls were selected using propensity score matching, the discharge rate advantage improved to 55% (p = 0.002). Customized EEG electrode targeting of hd-tDCS is a safe and non-invasive method of hyperacutely reducing epileptiform activity in RSE. Compared to historical controls, there was evidence of a cumulative chronic clinical response with more hd-tDCS subjects discharged from ICU.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Defined as a prolonged seizure that does not spontaneously terminate, status epilepticus (SE) is a life-threatening neurological emergency associated with high morbidity and mortality [1, 2]. Refractory SE (RSE) persists despite first-line benzodiazepines and second-line antiseizure medications (ASM) such as phenytoin, levetiracetam, and valproate [1, 3,4,5,6,7]. Third-line agents include intravenous anesthetic therapy (IVAT) such as propofol, midazolam, and ketamine to initiate and maintain a medically induced coma [3, 8, 9].
Despite prolonged stays in the intensive care unit (ICU), RSE carries a mortality rate of over 50%, with survivors having an over 75% chance of poor functional outcome [10,11,12,13,14,15]. Pharmacological RSE treatments carry a host of severe and life-threatening side effects such as respiratory depression, cardiac dysfunction, severe hypotension requiring vasopressor support, gut dysmotility, and infection [2, 3, 7, 16]. Once established treatments fail, additional management becomes a matter of trial and error [3, 7]. As a result, there is a current and substantial need for a novel non-pharmacological RSE treatment option.
Transcranial direct current stimulation (tDCS) is a form of brain stimulation that non-invasively delivers direct current via electrodes on the scalp [17,18,19]. tDCS has demonstrated the ability to induce cortical neuronal plasticity, and change cerebral blood velocity and cerebral oxygenation [18,19,20]. In humans, clinical experience indicates that tDCS is well-tolerated and safe [17,18,19]. When a cathode is placed over the epileptogenic region, tDCS reduces epileptiform activity on EEG, and seizures in a variety of epileptic syndromes, such as refractory focal epilepsy, early-onset epileptic encephalopathy, and electrical status epilepticus in sleep [18, 21, 22].
The most common tDCS application in epilepsy is to place the cathode above the area of the epileptogenic focus to maximize its inhibitory effect over the target area. However, there is no consensus on anode location. Anode placement in a susceptible brain region may induce unwanted excitatory stimulation, which may diminish treatment effects or even worsen the condition. High-definition tDCS (hd-tDCS) has been introduced to address this limitation by placing multiple anodes surrounding the cathode (or vice versa for excitatory stimulation) such that the anodal ends are dispersed. This electrode positioning strategy has been further improved using computer simulation to maximize current flow in the target coordinates while limiting its spread [23]. Another advantage of hd-tDCS is that it is already integrated with EEG; thus, real-time seizure monitoring is readily available.
To our knowledge, neither tDCS nor hd-tDCS have been assessed in RSE [24]. Therefore, we prospectively recruited critically ill RSE patients and quantified hyperacute changes in epileptiform activity (for convenience, “spikes”) during and after hd-tDCS on real-time EEG monitoring. We also compared clinical dispositions of study subjects against carefully selected historical controls.
Material and Methods
Inclusion and Exclusion Criteria
This study was approved by the University of Manitoba Research Ethics Board. An RSE patient was defined as (1) having had a seizure(s) refractory to first and second-line agents, (2) requiring at least 24 h of a third-line IVAT, and (3) failing at least one previous wean of an IVAT. Patients were prospectively recruited from the medical or surgical ICU at the Health Sciences Centre Winnipeg (HSCW) between October 2019 and November 2020 until the COVID-19 pandemic stopped recruitment. Inclusion criteria were patients (1) over age 18 years, (2) with access to concomitant EEG monitoring. Exclusion criteria were (1) hd-tDCS malfunction, (2) vagal nerve stimulator, (3) cardiac pacemaker, (4) implanted cardioverter-defibrillator, or (5) lack of baseline IVAT “steady-state” period.
Transcranial Direct Current Stimulation
A portable EEG-compatible hd-tDCS (Soterix Medical, New York, USA) delivered 2 milliamperes (mA) of constant DC programmed for 20 min via surface electrodes applied at the bedside. Five-electrode (4 channels, 1 ground) positions and current intensity were determined by computer simulation using HDTargets software (Soterix Medical Inc.) that resulted in maximum focal current on the target epileptogenic focus with outward field orientation [23]. When possible, stimulation was coordinated with the ICU team during periods of absent or stable IVAT dosing. All stimulation occurred with real-time EEG monitoring to determine location of hd-tDCS stimulation, and to detect electrographic seizure activation that would have immediately discontinued stimulation. Due to logistical limitations, the maximal number of repeated stimulations per patient was capped at 10.
EEG Monitoring and Analysis
When requested by the ICU team, EEG monitoring occurred for no more than 3 continuous hours per 24 h. EEG was sampled at 500 Hz using 19 surface 10–20 electrodes. Data were reviewed in Neuroworks 7.1 software (Natus, Oakville, Canada). In real time, electroencephalographers identified the most prominent brain region with epileptiform activity for hd-tDCS. When no focus was identifiable, presumably non-dominant right temporal stimulation over the T4 electrode was performed. All real-time acquired EEG data were stored in a database for subsequent offline analysis. EEG recordings were divided into “steady-state” periods of absent or stable IVAT infusion dosing before, during, and/or after hd-tDCS. hd-tDCS generates two distinct types of EEG artifact: (1) “ramping” when the device technically “ramps up” in the 30 seconds immediately before stimulation, and again when it technically “ramps down” in the 30 seconds immediately after stimulation, and (2) “stimulating”, which is a relatively minor focal electrode-based artifact predominantly over the target electrode during stimulation. While EEG interpretation is not possible within technical ramping artifact, interpretability remains preserved during stimulation itself. However, because the persistence of minor focal electrode artifact visually signifies the presence of active stimulation, blinded human interpretation was not possible. Instead, automated spike detection in quantitative EEG software (Persyst 14, Prescott, USA) quantified epileptiform activity in spikes/second [25]. While automated detections tend to be more liberal in identifying spikes when there is low or negligible spike presence [25], it is also non-inferior to humans in certain clinical settings [26, 27]. To assess automated detections, a board-certified epileptologist (MCN) independently quantified spikes during steady-state periods while blinded to automated detections.
Retrospective Chart Review
We reviewed electronic medical records for age, sex, duration, and timing of dosing for second-line ASM and third-line IVAT, ICU length of stay, RSE etiology, comorbidities, seizure type, history of seizures, level of consciousness at initiation of SE treatment, and disposition (i.e. discharge from ICU, survival at last follow-up). We included ASMs given to abort seizures before ICU admission. IVAT boluses given before ICU admission were considered second-line agents, unless IVAT were administered as an infusion in which case it was considered a third-line agent. For patients transferred in and out of the ICU frequently, ICU length of stay was counted from time of first arrival to time of last discharge. If a patient died on the ward shortly after transfer out of ICU, then their time on the ward was included in the ICU length of stay.
Candidate Historical Controls
From September 2009 to October 2019, we searched the EEG database for RSE patients (1) over age 18 years; (2) admitted to the HSCW medical ICU, surgical ICU, or intermediate ICU; and (3) with access to ≥ 3 consecutive ≥ 1-h EEG recordings. We performed chart review for the same clinical characteristics as study patients to calculate the Status Epilepticus Severity Score (STESS) and Epidemiology-based Mortality score in Status Epilepticus (EMSE) for study patients and all historical control candidates [28, 29]. We used Child–Pugh scores of A, B, and C to define mild, moderate, and severe liver diseases in EMSE, respectively [30, 31]. We also defined moderate and severe renal disease in EMSE as documented acute kidney injury, chronic kidney disease, and/or end-stage renal failure.
Study Outcomes
All outcomes were assessed in steady state. Primary outcomes were per-patient based: (1a) change in spike rate during hd-tDCS compared to baseline (“pre-to-stim change”), (1b) change in spike rate after hd-tDCS compared to baseline (“pre-post change”), (1c) ICU discharge rate, and (1d) hospital discharge rate. Secondary outcomes were per-stimulation session based: (2a) “pre-to-stim” change in spike rate and (2b) “pre-post change” in spike rate.
Statistical Analysis and Propensity Matching
All data were assessed for normality using the Shapiro-Wilk test. We compared spike rate changes using the Wilcoxon signed-rank test. We compared ICU and hospital discharge rates between historical controls and study subjects using Pearson’s chi-squared test. Cramér’s V denoted strengths of nominal association. We compared clinical characteristics between historical controls and subjects using Wilcoxon rank-sum, Student’s t, and Pearson’s chi-squared tests as appropriate. To select historical controls for propensity score matching (PSM), we generated PS based on covariates of age, sex, EMSE, and STESS. We aimed for a standardized difference of < 0.10 for covariates between treatment and control groups by incorporating interaction terms (STESS-EMSE product) and higher order variables (age squared) to achieve respective mean and median model biases of 4.1% and 2.1%. Using a multivariate logistic regression model, we matched each study subject to their 4 nearest historical control neighbors with replacement [32,33,34,35]. Model validation also compared clinical characteristics between propensity matched controls and study subjects using Wilcoxon rank-sum, Student’s t, and Pearson’s chi-squared tests as appropriate. We used Stata 14.2 (College Station, USA) for all statistical analyses.
Results
Study Population
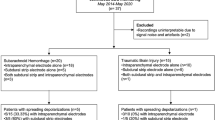
Informed consent was obtained for 11/17 RSE subjects who prospectively met inclusion criteria (Fig. 1). One was excluded due to technical hd-tDCS malfunction. Of 10 included patients (Table 1), median age was 66 years (IQR 62.3–71.5), 50% were female, 50% had a history of seizures before RSE (e.g., post-infectious, post-traumatic, juvenile myoclonic, idiopathic), median STESS was 5 (IQR 3–5), and median EMSE was 97 (IQR 82.8–122). In 8/10 patients, RSE etiologies were vascular (i.e. ischemic/hemorrhagic stroke, including right intracerebral or aneurysmal subarachnoid), infectious (i.e. cerebral abscess, empyema, sepsis), drug-related (with associated hyponatremia), or postoperative complication-related (i.e. left femoral endarterectomy, small bowel obstruction). RSE was unprovoked in 2/10 patients.
Of all 32 hd-tDCS sessions in 10 included patients, no adverse effects were reported. Three sessions could not be analyzed: 1/32 session due to hd-tDCS malfunction and 2/32 sessions in another patient due to lack of steady IVAT state. The 29 included sessions began 3–15 days into ICU admission (Table 2). In 16/29 sessions, steady states were defined by absence of IVAT. Steady-state durations in 13/29 sessions were composed of intravenous propofol, midazolam, and/or ketamine at the same infusion rate.
Of the 29 included sessions, all had characteristic generalized hd-tDCS “ramping up” and “ramping down” artifact for 30 seconds immediately before and after the stimulation period. No spike counting occurred during these ramping artifact periods. Only 1 session was contaminated by hd-tDCS ramping artifact that persisted through active stimulation in order to preclude interpretation of that session. Otherwise, the remaining 28 sessions in all 10 included patients only had minor focal hd-tDCS “stimulating” artifact predominantly over the EEG electrode targeted by hd-tDCS, but remained interpretable (Supplementary Fig. 1).
During hd-tDCS Spike Rate Change per Patient
On a per-patient basis, automated spike detection found significant “pre-to-stim” spike reduction in 10/10 patients (median absolute change −0.04 spikes/sec with IQR: −0.03 to −0.06, median relative change −45.9% with IQR −11.9 to −59%, z = −2.701, p = 0.0069; Fig. 2). Median pre-stimulation steady state/patient was 72.4 min (IQR 54.3–76.8). Patient #4 experienced +0.001 more spikes/sec during hd-tDCS (relative increase + 23.1%). Relatively, patient #6 experienced +1060.2% more median spikes (−84.3% in session #1, +2204.7% in session 2), but −0.03 median fewer spikes/sec (−0.08 fewer spikes/sec in session #1, +0.02 more spikes/sec in session #2).
Change in median automated spike rate per-patient during hd-tDCS. Spike rates: relative (purple, y-axis on left), absolute (orange, y-axis on right). Relative reduction of −100% denotes spike quiescence. Filled triangle indicates relative spike increase beyond 100%. Relative differences were exaggerated when a few rare spikes emerged from a baseline of quiescence. Note quiescence in patient #10. All but two patients experienced relative reductions. All but one patient experienced absolute reductions. Patient #4 experienced a relative median increase (+23.1%) corresponding to an absolute median increase of +0.001 spikes/sec. Patient #6 experienced a relative median increase (+1060.2%) corresponding to an absolute median decrease (−0.03 spikes/sec), which was driven by session 2/2 (relative +2204.7% increase but only +0.016 absolute increase in spikes/second). Although session 1/2 showed relative spiking reduction of −84.3%, this was overwhelmed by the relative rate in session 2/2
Independent human spike detection found no visible spikes in 3/10 patients. Another 1/10 patient (#10) was excluded due to diffuse “ramping up” and “ramping down” hd-tDCS technical-related artifact in 1 of their 2 sessions (session #1) (Fig. 3), which persisted throughout stimulation that precluded visual spike detection. Otherwise, EEG remained interpretable in 6/10 patients where minor hd-tDCS “stimulating” artifact was confined to the electrode targeted by hd-tDCS. Human detection found significant “pre-to-stim” spike reduction in these 6/10 patients (median absolute change −0.07 spikes/s with IQR: −0.02 to −0.18, median relative change −51.6% with IQR: −17.9 to −70.2%, z = −2.201, p = 0.0277; Fig. 4). Median pre-stimulation steady-state period/patient was 58.8 min (IQR 51.2–72.4). All 6/10 patients experienced absolute reduction in median spikes/second during hd-tDCS. Relatively, only 1/10 patient (#5) experienced +51.4% more median spikes (+178.4% in session #1, −75.5% in session #2), but −0.01 median spikes/s (+0.11 more spikes/sec in session #1, −0.13 fewer spikes/sec in session #2).
Two types of hd-tDCS artifact: “ramping” and “stimulating ” Referential montage EEG: low frequency filter 1 Hz, high-frequency filter 70 Hz, notch filter 60 Hz, sensitivity 7 μV/mm, timebase 30 mm/sec. Left and right panels. Examples of typical “ramping” artifact occurring 30 seconds immediately before hd-tDCS begins, and 30 seconds immediately after hd-tDCS ends, on every EEG. No spikes were counted during these technical ramping periods. Middle panel. Example of typical minor focal “stimulating” artifact after ramping-on artifact (left panel) abruptly ends and when actual hd-tDCS stimulation begins. After 20 min of active hd-tDCS stimulation ends, ramping-off artifact (right panel) abruptly begins. In this example, active hd-tDCS stimulation was targeted around the Pz electrode
Change in median human spike rate per-patient during hd-tDCS. Spike rates: relative (purple, y-axis on left), absolute (orange, y-axis on right). Relative reduction of −100% denotes spike quiescence (patient #6). All but one patient experienced relative reductions. All patients experienced absolute reductions. Patient #5 experienced a relative median increase (+51.4%) at the same time as an absolute median decrease (−0.01 spikes/sec)
During hd-tDCS Spike Rate Change per Session
On a per-session basis, automated spike detection found significant “pre-to-stim” spike reduction over 29/29 sessions from 10/10 patients (median absolute change −0.03 with IQR: − 0.0004 to −0.14, median relative change −47.2% with IQR −16.7 to −76.8%, z = −2.952, p = 0.0032; Fig. 5). Median pre-stimulation steady-state period per session was 63 min (IQR 47.7–73.8). In 7/29 sessions (three from patient #2), there were relative and absolute increased spiking (relative range +23.1 to +2204.7%, but absolute range just +0.001 to +0.25 more spikes/sec). In the greatest relative increase of +2204.7% from session #2 in patient #6, the corresponding absolute increase was just +0.016 more spikes/sec. In 2 sessions (session #3 of patient #1, session #2 of patient #10), there was absolute spike quiescence during hd-tDCS (i.e., 0 spikes/sec; Fig. 6).
Change in median automated spike rate per-session during hd-tDCS. Spike rates: relative (purple, y-axis on left), absolute (orange, y-axis on right). Relative reduction of −100% denotes spike quiescence. A filled triangle symbol indicates relative spike increase beyond 100%. Relative differences were exaggerated when a few rare spikes emerged from a baseline of quiescence. Note quiescence in session #3 of patient #1 and session #2 of patient #10. In session #2 of patient #6, relative spike rate increase was +2204.7% and absolute spike increase was +0.01 spikes/sec from a very low baseline of just 0.0007 to 0.0167 spikes/sec. In session #5 of patient #7, relative spike rate increase was +249.3% and absolute spike increase was + 0.25 spikes/sec
Example of longitudinal spike rate improvement over days with hd-tDCS in patient #10. Referential montage EEG: low-frequency filter 1 Hz, high-frequency filter 70 Hz, notch filter 60 Hz, sensitivity 7 μV/mm, timebase 30 mm/sec. (A) Baseline status epilepticus in the absence of intravenous anesthetic therapy (IVAT) after having failed a previous trial of weaning IVAT. (B) Steady-state pre-stimulation EEG (session #1 on day #6 of admission to the intensive care unit) with the presence of concomitant intravenous midazolam infusion. Boxes indicate residual spikes from status epilepticus breaking through effects of midazolam infusion. (C, D) Respective examples of steady-state during-stimulation and after-stimulation EEG (session #2 on day #7 of admission to the intensive care unit) with absence of spikes (“quiescence”) and absence of IVAT (i.e., midazolam), which had been successfully weaned between sessions #1 and #2. During-stimulation and after-stimulation EEG from session #1 are available in Supplementary Fig. 1J
Although human spike detection also confirmed a median “pre-to-stim” spike reduction over 15/32 sessions from 6/10 patients, this change was not statistically significant (p = 0.1118, median absolute change −0.13 spikes/sec with IQR −0.22 to +0.01, median relative change −38.5% with IQR −4.7 to −79.7%; Supplementary Fig. 2). However, there emerged absolute spike quiescence in 2 sessions during hd-tDCS (session #1 of patient #6, session #1 of patient #7).
After hd-tDCS Spike Rate Change per Patient
On a per-patient basis, automated spike detection found a median “pre-post” spike reduction from 10/10 patients, but this was not statistically significant (median absolute change −0.004 spikes/sec with IQR: −0.0146 to +0.011, median relative change −18.3% with IQR −25.1 to −1.4%, z = −0.561, p = 0.5751; Supplementary Fig. 3). Confirming spikes during steady states before and after stimulation in 7/10 patients, human detection found significant “pre-post” spike reduction (median absolute change −0.08 spikes/s with IQR: −0.06 to −0.10, median relative change −25.3% with IQR −15.5 to −42.6%, z = −2.366, p = 0.018; Fig. 7). Median pre-stimulation steady-state period per patient was 61.9 min (IQR 52.7–75.9). 7/10 patients experienced absolute reduction in median spikes/sec after hd-tDCS. Only 1/10 patient (#7) experienced a median relative +3.6% spike rate (session #1: +25.4%, session #3: −96.4%, session #4: −15.5%, session #7: +376.5%), but an absolute reduction of −0.08 fewer spikes/sec (session #1: +0.0048, session #3: −0.6764, session #4: −0.14436, session #5: +0.361632).
Change in median human spike rate per-patient after hd-tDCS. Spike rates: relative (purple, y-axis on left), absolute (orange, y-axis on right). All patients experienced absolute spike reduction. All but one patient experienced relative spike reduction. Patient #7 experienced a relative median increase (+3.6%) at the same time as an absolute median decrease (−0.08 spikes/sec)
After hd-tDCS Spike Rate Change per Session
On a per-session basis, neither automated spike detection of 29/29 sessions in 10/10 patients (p = 0.9094, median absolute change −0.0004 spikes/sec with IQR −0.02 to +0.02, median relative change −6.8% with IQR −48.7 to +21.5%; Supplementary Fig. 4), nor human spike detections in 16/29 sessions in 7/10 patients (p = 0.1961, median absolute change −0.07 spikes/sec with IQR −0.15 to +0.008, median relative change −21.8% with IQR −61.4 to +12.7%; Supplementary Fig. 5) found significant “pre-post” spike rate differences. However, absolute spike quiescence emerged after hd-tDCS in 2 sessions on automated counting (session #3 of patient #1, session #1 of patient #4). Furthermore, on subgroup analysis when only sessions in the absence of IVAT (16/29 sessions) were analyzed, there was significant spike reduction on automated spike counting (median absolute change −0.04 with IQR: −0.0008 to −0.19, median relative change −47.2% with IQR −28 to −72%, z = −2.499, p = 0.0125).
Differences in ICU Discharge Rate and Survival
Historically, we found 231 adult SRSE patients admitted over 10 years to an ICU, with ≥ 3 consecutive 1-h EEG recordings. In comparison, more study patients (n = 9/10) were successfully discharged from ICU (90% vs. 44.2%, χ [2] = 8.1083, p = 0.004), but hospital discharge rates did not differ (30% vs. 32%, χ2 = 0.0183, p = 0.893). Although study patients trended to being older (median 66 vs. 58 years, z = −1.891, p = 0.0587), there were no significant differences in sex (50% vs. 54.1% male, χ2 = 0.0653, p = 0.798), STESS (median 5 vs. 4, z = −0.079, p = 0.937), EMSE (median 97 vs. 89, z = −0.86, p = 0.39), maximal number of IVAT over ICU admission (median 2 vs. 2, z = 0.025, p = 0.9804), maximal number of ASM over ICU admission (median 3.5 vs. 3, z = −0.64, p = 0.522), or ICU admission length (median 12.5 vs. 12 days, z = −0.64, p = 0.522).
Propensity-Matched Historical Controls
A logistic regression model balancing covariates of age, sex, STESS, and EMSE generated PS for all 10 study subjects and 231 historical controls. Matching each subject to their 4-nearest neighbors with replacement yielded 38 PSM historical controls. The model yielded a significant 55% difference in favor of study subjects being discharged from the ICU (90% vs. 36.8%, χ2 = 9.9408, p = 0.002), with a standard error of 0.129 for this average treatment effect on the treated (i.e., discharge from ICU for those receiving hd-tDCS), and strong association (Cramér’s V = 0.4321).
The model also yielded a 7.5% difference in favor of study subjects over historical controls of being successfully discharged from hospital, with a standard error of 0.169 for this average treatment effect on the treated (i.e., discharge from hospital for those receiving hd-tDCS). However, this difference was not statistically significant (30% vs. 23.7%, χ2 = 0.1635, p = 0.686), with very weak association (Cramér’s V = 0.0592).
Compared to study patients, PSM historical controls did not differ in age (median 63 vs. 66 years, z = −0.395, p = 0.6931), sex (55.3% vs. 50% male, χ2 = 0.0881, p = 0.767), STESS (mean 4.1 vs. 4.1, t = 0.0139, p = 0.989), EMSE (mean 98.3 vs. 100.6, t = −0.226, p = 0.8222), maximal number of IVAT over ICU admission (mean 2.26 vs. 2.2, t = 0.1931, p = 0.8478), maximal number of ASM over ICU admission (mean 3.79 vs. 3.8, t = −0.0183, p = 0.9854), or ICU admission length (median 13 vs. 12 days, z = −0.025, p = 0.9797).
Discussion
This pilot study demonstrated the feasibility of using hd-tDCS in RSE patients in the ICU. There were no reported adverse events over 32 stimulation sessions in 10 patients. For any given patient, hd-tDCS significantly cut median spike rates by approximately 50% during stimulation (“pre-to-stim change”) on both independent automated and human spike counts, which demonstrates an impressive confluence of different methods yielding remarkably similar results. For any given stimulation session (irrespective of subject), hd-tDCS also significantly cut median spike rates by approximately 50% during stimulation on automated spike counting. These findings suggest that while the acute effect of hd-tDCS for an individual stimulation session may vary, the net effect of stimulations within a person is to overall decrease epileptiform activity.
Our findings add to the growing body of literature on the safety of tDCS in seizures and epilepsy [17, 36]. In the non-ICU outpatient setting, cathodal tDCS at 0.016–0.571 A/m2 for 20–40 min over 1–14 sessions has been used in 1–70 adult and pediatric patients with refractory epilepsy [37,38,39,40,41,42,43]. Most of these studies have shown acutely reduced interictal epileptiform activity by as much as 64.3% [37, 38]. This is comparable to our electrographic findings of median 50% spike reduction during hd-tDCS. Unlike other studies, ours is the first to deploy tDCS in critically ill RSE patients.
After hd-tDCS, median spikes per-patient remained significantly down by 25% from baseline (“pre-post change”) on human spike counting. However, there was no significant per-session pre-post difference (irrespective of subject) on either automated or human spike counting. Concurrent effects during tDCS are mainly explained by direct effects on neuronal membrane potential changes, while tDCS after effects require subsequent synaptic activities involving both glutamatergic and GABAergic transmission, as well as neuromodulators including dopamine, adenosine, serotonin, and acetylcholine [44, 45]. Since all RSE patients were heavily medicated, including with benzodiazepines and IVAT, the acute after-effects of hd-tDCS may have been blunted by these concurrent medications. Indeed, when the per-session pre-post changes were analyzed only in the absence of IVAT (16/29 sessions), there was significant spike reduction on automated spike counting (median absolute change −0.04 with IQR: −0.0008 to −0.19, median relative change −47.2% with IQR −28 to −72%, z = −2.499, p = 0.0125). These medication effects were absent in non-ICU outpatient studies, which have shown a relatively greater 57.6% spike reduction in a delayed 48-h fashion [37, 39].
Further to electrographic improvement, hd-tDCS subjects also demonstrated clinical improvement, with more subjects leaving the ICU alive than historical RSE controls. Compared to the entire cohort of 231 historical controls, subjects trended to being older. When this possible age difference was negated on propensity score matching, discharge rates improved from 45 to 55% more study patients leaving ICU alive. These findings infer RSE resolution to the point that IVAT was successfully discontinued, extubation was successful, and the patient was stable enough for safe transfer out of the ICU and onto the ward. This finding also supports a more delayed cumulative clinical benefit from hd-tDCS sessions in addition to real-time hyperacute improvements on EEG.
However, hd-tDCS did not significantly affect discharge from hospital — no matter if study subjects were compared to the entire historical cohort or to propensity matched controls — which implies that patients still succumbed to complications and/or the underlying condition for RSE after leaving ICU. This is not surprising because hd-tDCS would not have been expected to fundamentally alter underlying systemic disease states contributing to RSE in many study subjects, such as sepsis, hyponatremia, or postoperative complications. Nevertheless, these findings suggest that hd-tDCS can mitigate RSE as an additional complicating pathophysiological process in critically ill patients.
These improved clinical outcomes are consistent with most previous cathodal tDCS studies that have also shown clinical seizure reduction. In the largest multi-center, double blind, sham-controlled trial to date of 70 focal epilepsy patients, daily 20-min cathodal tDCS sessions for 2 weeks reduced seizures by 21.9–50.7% over 4 weeks, while twice-daily sessions for 2 weeks reduced seizures by 49.7–61.3% over 5 weeks [43]. This continuing clinical effect, even after stimulation sessions have ended, agrees with the delayed cumulative clinical benefit from hd-tDCS sessions in our RSE cohort; notably, there were 55% higher discharge rates from ICU where median stays were 1.8 weeks for all study subjects, but stimulation sessions only covered up to a median of 1.3 weeks of their stay.
Limitations
The main limitation of this study is lack of sham stimulation and blinding of assessors. While this would not have affected our primary outcomes of automated spike counting, hd-tDCS could have affected clinical outcomes by altering ICU team decision-making. The presence of hd-tDCS may have dissuaded withdrawal of care, given RSE’s high mortality rate and limited treatment options. However, ICU lengths of stay did not differ between study subjects and historical controls (either the whole cohort or propensity matched controls). Future hd-tDCS studies should be randomized controlled trials in which many more RSE patients across different centers using a standardized treatment protocol are randomly allocated to receive true or sham tDCS.
We also cannot rule out bias from human spike detection, which may account for the only major significant pre-post difference found in this study: on a per-patient basis when considering human spike detections. While there was also significant pre-post spike reduction on automated subgroup analysis of sessions without IVAT, no significant changes were found on the main automated pre-post analyses on either a per-patient or per-session basis. To minimize bias, future studies may consider blinding human reviewers by incorporating artificial “ramping” and “stimulating” hd-tDCS EEG artifacts into sham stimulation sessions. Ramping artifact is presumed due to the current generated by the hd-tDCS changing every 1 second during the “ramping up” and “ramping down” period, which can be easily simulated as a high-amplitude decrescendoing sailboat morphology 1-Hz artifact for 10 seconds before and after sham stimulation in future studies.
Including multiple centers would also allow multiple electroencephalographers to perform independent human spike counting. In this study, we mitigated the bias of a single electroencephalographer by incorporating Persyst 14. Although automated spike detection is susceptible to erroneous spike labeling (such as overcalling sharply contoured activity in burst suppression as spikes), its EEG analysis is also neutral and unbiased (allowing systematic sharpness comparisons within a burst suppressed EEG subject to hd-tDCS that human labelling could not). Despite these differences, there still was remarkable net convergence between human and automated median spike count changes during hd-tDCS even if there was methodological disagreement over individual spike labels. The greatest discordance between automated and human methods occurred for relative spike rates, for example, in patient #5 where relative median spiking decreased on automated counting, but increased on human counting, and in patient #6 where relative median spiking increased on automated counting, but decreased on human counting. However, both methods agreed on absolute median spiking decreases in both patients. Relative differences were often exaggerated when even just a few rare spikes emerged during or after hd-tDCS against a baseline background of relative quiescence.
This study is also limited by different numbers of hd-tDCS sessions between subjects. At either extreme, 1/10 patient received the maximal 10 sessions, while 2/10 patients received one session. While the net effect of stimulation was electro-clinical benefit over all patients, the ideal number of sessions in any given patient remains unknown. In the maximally stimulated patient (#2), the last session (#9) demonstrated respective pre-to-stim and pre-post spike rate increases of +772.6% and +1027.9% on human counting, and +70.2% and +308.6% on automated counting. This raises the question of whether 10 sessions were too many; however, session #4 in this patient had already demonstrated even higher respective pre-to-stim and pre-post increases of +90.3% and +424.1% on automated counting, while spike rates mostly decreased in remaining sessions. Furthermore, this patient fared well and survived at least 599 days in follow-up.
On the other hand, one patient (#4) had a single session because they improved, left ICU, and did not qualify for more sessions. Automated spike counting showed −100% quiescence after hd-tDCS. Months later on the ward, however, patient #4 died from a non-RSE cause. Another patient (#8) received a single session because stimulation started late (admission day #15) and they died in ICU from a non-RSE cause before another session could be performed. Although automated spike detection found a pre-post relative spike rate increase of +1193.3% and absolute increase of +0.28 spikes/sec, this was a burst suppressed EEG such that automated detections were representations of sharpness rather than epileptiform activity, given no human spike detections. Furthermore, real-time clinical electroencephalographers did not report activation or safety concerns throughout the EEG.
Conclusions
Customized hd-tDCS EEG electrode targeting at the ICU bedside in a prospective pilot cohort of RSE patients is safe, non-invasive, and effective at hyperacutely reducing epileptiform activity with possible sustained reduction in the immediate post-stimulation setting. When compared to historical controls, there was evidence of a delayed cumulative clinical response with significantly more hd-tDCS subjects discharged from ICU due to resolution of RSE.
References
Gugger JJ, Husari K, Probasco JC, Cervenka MC. New-onset refractory status epilepticus: a retrospective cohort study. Seizure. 2019;2020(74):41–8. https://doi.org/10.1016/j.seizure.2019.12.002.
Trinka E, Cock H, Hesdorffer D, et al. A definition and classification of status epilepticus - report of the ILAE Task Force on Classification of Status Epilepticus. Epilepsia. 2015;56(10):1515–23. https://doi.org/10.1111/epi.13121.
Glauser T, Shinnar S, Gloss D, et al. Evidence-based guideline: Treatment of convulsive status epilepticus in children and adults: report of the guideline committee of the American epilepsy society. Epilepsy Curr. 2016;16(1):48–61. https://doi.org/10.5698/1535-7597-16.1.48.
Leppik LE, Derivan AT, Homan RW, Walker J, Ramsay RE, Patrick B. Double-blind study of lorazepam and diazepam in status epilepticus. JAMA J Am Med Assoc. 1983;249(11):1452–4. https://doi.org/10.1001/jama.1983.03330350028021.
Pauletto G, Bax F, Gigli GL, et al. Status epilepticus mimicking stroke recurrence. Epilepsy Behav. 2020;104: 106509. https://doi.org/10.1016/j.yebeh.2019.106509.
Sculier C, Gaspard N. New onset refractory status epilepticus (NORSE). Seizure. 2018;2019(68):72–8. https://doi.org/10.1016/j.seizure.2018.09.018.
Krumholz A, Wiebe S, Gronseth GS, et al. Evidence-based guideline: management of an unprovoked first seizure in adults. Neurology. 2015;84(16):1705–13. https://doi.org/10.1212/WNL.0000000000001487.
Gaspard N, Foreman B, Judd LM, et al. Intravenous ketamine for the treatment of refractory status epilepticus: a retrospective multicenter study. Epilepsia. 2013;54(8):1498–503. https://doi.org/10.1111/epi.12247.
Claassen J, Hirsch LJ, Emerson RG, Bates JE, Thompson TB, Mayer SA. Continuous EEG monitoring and midazolam infusion for refractory nonconvulsive status epilepticus. Neurology. 2001;57(6):1036–42. https://doi.org/10.1212/WNL.57.6.1036.
Kantanen AM, Reinikainen M, Parviainen I, et al. Incidence and mortality of super-refractory status epilepticus in adults. Epilepsy Behav. 2015;49:131–4. https://doi.org/10.1016/j.yebeh.2015.04.065.
Madžar D, Knappe RU, Reindl C, et al. Factors associated with occurrence and outcome of super-refractory status epilepticus. Seizure. 2017;52:53–9. https://doi.org/10.1016/j.seizure.2017.09.003.
Kilbride RD, Reynolds AS, Szaflarski JP, Hirsch LJ. Clinical outcomes following prolonged refractory status epilepticus (PRSE). Neurocrit Care. 2013;18(3):374–85. https://doi.org/10.1007/s12028-013-9823-4.
Alvarez V, Drislane FW. Is favorable outcome possible after prolonged refractory status epilepticus? J Clin Neurophysiol. 2016;33(1):32–41. https://doi.org/10.1097/WNP.0000000000000223.
Shorvon S, Ferlisi M. The outcome of therapies in refractory and super-refractory convulsive status epilepticus and recommendations for therapy. Brain. 2012;135(8):2314–28. https://doi.org/10.1093/brain/aws091.
Cooper AD, Britton JW, Rabinstein AA. Functional and cognitive outcome in prolonged refractory status epilepticus. Arch Neurol. 2009;66(12):1505–9. https://doi.org/10.1001/archneurol.2009.273.
Vasile B, Rasulo F, Candiani A, Latronico N. The pathophysiology of propofol infusion syndrome: a simple name for a complex syndrome. Intensive Care Med. 2003;29(9):1417–25. https://doi.org/10.1007/s00134-003-1905-x.
Bikson M, Grossman P, Thomas C, et al. Safety of transcranial direct current stimulation: evidence based update 2016. Brain Stimul. 2016;9(5):641–61. https://doi.org/10.1016/j.brs.2016.06.004.
Karvigh SA, Motamedi M, Arzani M, Roshan JHN. HD-tDCS in refractory lateral frontal lobe epilepsy patients. Seizure. 2017;47:74–80. https://doi.org/10.1016/j.seizure.2017.03.005.
Hurley R, Machado L. Using tDCS priming to improve brain function: can metaplasticity provide the key to boosting outcomes? Neurosci Biobehav Rev. 2017;83(May):155–9. https://doi.org/10.1016/j.neubiorev.2017.09.029.
Lin Y, Wang Y. Neurostimulation as a promising epilepsy therapy. Epilepsia Open. 2017;2(4):371–87. https://doi.org/10.1002/epi4.12070.
Faria P, Fregni F, Sebastião F, Dias AI, Leal A. Feasibility of focal transcranial DC polarization with simultaneous EEG recording: preliminary assessment in healthy subjects and human epilepsy. Epilepsy Behav. 2012;25(3):417–25. https://doi.org/10.1016/j.yebeh.2012.06.027.
Meiron O, Gale R, Namestnic J, et al. High-definition transcranial direct current stimulation in early onset epileptic encephalopathy: a case study. Brain Inj. 2018;32(1):135–43. https://doi.org/10.1080/02699052.2017.1390254.
Datta A, Bansal V, Diaz J, Patel J, Reato D, Bikson M. Gyri-precise head model of transcranial direct current stimulation: improved spatial focality using a ring electrode versus conventional rectangular pad. Brain Stimul. 2009;2(4):201–7. https://doi.org/10.1016/j.brs.2009.03.005.
Stavropoulos I, Pak HL, Valentin A. Neuromodulation in super-refractory status epilepticus. J Clin Neurophysiol. 2021;38(6):494–502. https://doi.org/10.1097/WNP.0000000000000710.
Jing J, Sun H, Kim JA, et al. Development of expert-level automated detection of epileptiform discharges during electroencephalogram interpretation. JAMA Neurol. 2020;77(1):103–8. https://doi.org/10.1001/jamaneurol.2019.3485.
Joshi CN, Chapman KE, Bear JJ, Wilson SB, Walleigh DJ, Scheuer ML. Semiautomated spike detection software persyst 13 is noninferior to human readers when calculating the spike-wave index in electrical status epilepticus in sleep. J Clin Neurophysiol. 2018;35(5):370–4. https://doi.org/10.1097/WNP.0000000000000493.
Scheuer ML, Wilson SB, Antony A, Ghearing G, Urban A, Bagić AI. Seizure detection: interreader agreement and detection algorithm assessments using a large dataset. J Clin Neurophysiol. 2021;38(5):439–47. https://doi.org/10.1097/WNP.0000000000000709.
Rossetti AO, Logroscino G, Milligan TA, Michaelides C, Ruffieux C, Bromfield EB. Status Epilepticus Severity Score (STESS): a tool to orient early treatment strategy. J Neurol. 2008;255(10):1561–6. https://doi.org/10.1007/s00415-008-0989-1.
Leitinger M, Höller Y, Kalss G, et al. Epidemiology-Based Mortality Score in Status Epilepticus (EMSE). Neurocrit Care. 2015;22(2):273–82. https://doi.org/10.1007/s12028-014-0080-y.
Child CG III TJ. Surgery and portal hypertension. In: III CC, ed. The Liver and Portal Hypertension. WB Saunders. 1964:50.
Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MCWR. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60(8):646.
Austin PC. Some methods of propensity-score matching had superior performance to others: results of an empirical investigation and Monte Carlo simulations. Biometrical J. 2009;51(1):171–84. https://doi.org/10.1002/bimj.200810488.
Bascoul-Mollevi C, Gourgou-Bourgade S, Kramar A. Two-part statistics with paired data. Stat Med. 2005;24(9):1435–48. https://doi.org/10.1002/sim.1979.
Deb S, Austin PC, Tu JV, et al. A review of propensity-score methods and their use in cardiovascular research. Can J Cardiol. 2016;32(2):259–65. https://doi.org/10.1016/j.cjca.2015.05.015.
Schober P, Vetter TR. Statistical minute. Int Anesth Res Soc. 2019;129(2):2019.
San-Juan D. Cathodal transcranial direct current stimulation in refractory epilepsy. J Clin Neurophysiol. 2021;Publish Ah(00). https://doi.org/10.1097/wnp.0000000000000717.
Fregni F, Thome-Souza S, Nitsche MA, Freedman SD, Valente KD, Pascual-Leone A. A controlled clinical trial of cathodal DC polarization in patients with refractory epilepsy. Epilepsia. 2006;47(2):335–42. https://doi.org/10.1111/j.1528-1167.2006.00426.x.
Auvichayapat N, Rotenberg A, Gersner R, et al. Transcranial direct current stimulation for treatment of refractory childhood focal epilepsy. Brain Stimul. 2013;6(4):696–700. https://doi.org/10.1016/j.brs.2013.01.009.
Auvichayapat N, Sinsupan K, Tunkamnerdthai O, Auvichayapat P. Transcranial direct current stimulation for treatment of childhood pharmacoresistant lennox-gastaut syndrome: a pilot study. Front Neurol. 2016;7(MAY):1–8. https://doi.org/10.3389/fneur.2016.00066.
Assenza G, Campana C, Assenza F, et al. Cathodal transcranial direct current stimulation reduces seizure frequency in adults with drug-resistant temporal lobe epilepsy: a sham controlled study. Brain Stimul. 2017;10(2):333–5. https://doi.org/10.1016/j.brs.2016.12.005.
Tekturk P, Erdogan ET, Kurt A, et al. The effect of transcranial direct current stimulation on seizure frequency of patients with mesial temporal lobe epilepsy with hippocampal sclerosis. Clin Neurol Neurosurg. 2016;149:27–32. https://doi.org/10.1016/j.clineuro.2016.07.014.
San-Juan D, Espinoza López DA, Vázquez Gregorio R, et al. Transcranial direct current stimulation in mesial temporal lobe epilepsy and hippocampal sclerosis. Brain Stimul. 2017;10(1):28–35. https://doi.org/10.1016/j.brs.2016.08.013.
Yang D, Wang Q, Xu C, et al. Transcranial direct current stimulation reduces seizure frequency in patients with refractory focal epilepsy: a randomized, double-blind, sham-controlled, and three-arm parallel multicenter study. Brain Stimul. 2020;13(1):109–16. https://doi.org/10.1016/j.brs.2019.09.006.
Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000;527(3):633–9. https://doi.org/10.1111/j.1469-7793.2000.t01-1-00633.x.
Stagg CJ, Antal A, Nitsche MA. Physiology of transcranial direct current stimulation. J ECT. 2018;34(3):144–52. https://doi.org/10.1097/YCT.0000000000000510.
Acknowledgements
We thank Michelle-Lee Jones and Alexei Yankovsky for contributing to clinical EEG interpretation and the EEG technologists at the Health Sciences Centre Winnipeg for accommodating the project within their clinical workflow.
Funding
Canadian Institutes of Health Research project grant #178217 (MCN), Manitoba Medical Service Foundation grant #8–2019-98 (MCN), Canada Foundation for Innovation grant #35590 (JHK), and Natural Science and Engineering Research Council of Canada grant #RGPIN-2016–05964 (JHK).
Author information
Authors and Affiliations
Contributions
Disclosure forms provided by the authors are available with the online version of this article.
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ng, M.C., El-Alawi, H., Toutant, D. et al. A Pilot Study of High-Definition Transcranial Direct Current Stimulation in Refractory Status Epilepticus: The SURESTEP Trial. Neurotherapeutics 20, 181–194 (2023). https://doi.org/10.1007/s13311-022-01317-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13311-022-01317-5