Abstract
Despite extensive research, amyotrophic lateral sclerosis (ALS) remains a progressive and invariably fatal neurodegenerative disease. Limited knowledge of the underlying causes of ALS has made it difficult to target upstream biological mechanisms of disease, and therapeutic interventions are usually administered relatively late in the course of disease. Genetic forms of ALS offer a unique opportunity for therapeutic development, as genetic associations may reveal potential insights into disease etiology. Genetic ALS may also be amenable to investigating earlier intervention given the possibility of identifying clinically presymptomatic, at-risk individuals with causative genetic variants. There is increasing evidence for a presymptomatic phase of ALS, with biomarker data from the Pre-Symptomatic Familial ALS (Pre-fALS) study showing that an elevation in blood neurofilament light chain (NfL) precedes phenoconversion to clinically manifest disease. Tofersen is an investigational antisense oligonucleotide designed to reduce synthesis of superoxide dismutase 1 (SOD1) protein through degradation of SOD1 mRNA. Informed by Pre-fALS and the tofersen clinical development program, the ATLAS study (NCT04856982) is designed to evaluate the impact of initiating tofersen in presymptomatic carriers of SOD1 variants associated with high or complete penetrance and rapid disease progression who also have biomarker evidence of disease activity (elevated plasma NfL). The ATLAS study will investigate whether tofersen can delay the emergence of clinically manifest ALS. To our knowledge, ATLAS is the first interventional trial in presymptomatic ALS and has the potential to yield important insights into the design and conduct of presymptomatic trials, identification, and monitoring of at-risk individuals, and future treatment paradigms in ALS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite decades of research, there remains a significant unmet need for effective treatments for amyotrophic lateral sclerosis (ALS). The reasons are manifold, but two key considerations have emerged [1]. First, limited knowledge about the underlying etiology of ALS has made it challenging to select therapeutic strategies that target upstream biological mechanisms of disease. Second, therapeutic intervention often occurs relatively late in the disease course, in part due to the typically long latency from symptom onset to diagnosis [2,3,4]. However, advances in understanding the genetics of ALS yield potential opportunities to address these challenges. For the subset of patients in whom the genetic cause of ALS is known, therapeutics can be developed to target the underlying genetic defect or a biological mechanism that is proximate in pathophysiology. Moreover, it is possible to identify clinically unaffected individuals with a markedly elevated risk for ALS, and to contemplate early, even presymptomatic, therapeutic intervention with the goal of delaying (or preventing) the appearance of clinically manifest disease, or attenuating disease course following clinical onset [5]. Though precedent in neurodegenerative diseases such as spinal muscular atrophy suggests that intervention prior to significant motor neuron loss could result in improved response to genetically targeted therapies [6], doing so requires an understanding of who to treat and when to treat them, especially since the age at which clinical disease emerges is highly variable in ALS [1, 7].
Approximately 2% of ALS cases are attributed to variants in the superoxide dismutase 1 (SOD1) gene (SOD1-ALS) [8]. More than 200 SOD1 variants associated with ALS have been reported [9], with a high degree of variability in penetrance and rapidity of progression [10,11,12,13,14]. Though the mechanisms by which variants in the SOD1 gene cause degeneration of motor neurons in ALS are not fully understood, these variants are thought to confer a toxic gain of function [8, 15,16,17,18,19]. Tofersen, an antisense oligonucleotide designed to reduce synthesis of SOD1 protein via ribonuclease H-dependent degradation of SOD1 mRNA [16, 20, 21], is an investigational drug under development for the treatment of SOD1-ALS. In clinical studies (NCT02623699, NCT03070119) designed to evaluate tofersen in symptomatic patients with SOD1-ALS, intrathecal administration of tofersen 100 mg led to robust reductions in total cerebrospinal fluid (CSF) SOD1 protein, a marker of target engagement, and plasma NfL, a marker of neuronal degeneration [22, 23]. Data from these studies suggest that earlier initiation of tofersen may have a greater impact on preservation of clinical function [22, 23].
Consistent with other neurodegenerative diseases, there is emerging evidence for a presymptomatic phase of ALS — i.e., when the underlying disease process has begun, but clinical manifestations of the disease have not yet appeared [5, 24]. The Pre-Symptomatic Familial ALS (Pre-fALS) study, a longitudinal natural history and biomarker study of unaffected individuals at elevated genetic risk for ALS (NCT00317616), was initiated in 2007 with the goals of defining and characterizing the presymptomatic phase of disease, identifying biomarkers of incipient clinical disease, and acquiring the necessary data to facilitate the design of early intervention or disease prevention trials [25]. In the subset of Pre-fALS participants with a SOD1 variant associated with rapid disease progression (e.g., p.Ala5Val [A5V; A4V]) and in whom clinically manifest ALS emerged (i.e., phenoconversion) during follow-up, increased levels of serum neurofilament, most notably neurofilament light chain (NfL), were observed 6–12 months prior to phenoconversion [26, 27]. These data support the potential utility of blood-based measurement of NfL as a biomarker of early disease in SOD1-ALS to aid in predicting the timing of phenoconversion [25,26,27].
The ATLAS study (NCT04856982) is designed to evaluate the impact of initiating tofersen in presymptomatic carriers of SOD1 variants associated with high or complete penetrance and rapid disease progression who also have biomarker evidence of disease activity (elevated plasma NfL level). Specifically, ATLAS will test the hypothesis that presymptomatic initiation of tofersen will delay the emergence of clinically manifest ALS and/or reduce the loss of function over time as compared to initiation of treatment after emergence of clinically manifest ALS. ATLAS will also expand our understanding of the field of ALS biomarkers and the natural history of disease, particularly its earlier stages.
Methods
Overview
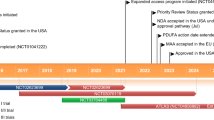
ATLAS is a global, randomized, placebo-controlled, Phase 3 study with a natural history run-in and open-label extension (Fig. 1). Participants in ATLAS must be at least 18 years of age, carry a SOD1 variant prespecified in the protocol or approved for inclusion by an independent Mutation Adjudication Committee based on the evidence of high or complete penetrance and association with rapid disease progression, be clinically presymptomatic for ALS, and have a plasma NfL level below the predefined threshold of 44 pg/mL (Siemens Healthineers assay; Malvern, PA, USA).
Part A is a natural history run-in during which participants will be monitored monthly for changes in plasma NfL levels and/or emergence of clinically manifest ALS. Considering feedback from the ALS community, Part A has been designed to minimize burden on study participants, with the opportunity for most assessments to be conducted at home (e.g., monthly blood draws to monitor plasma NfL). If at any point in Part A, a participant is found to have an elevation in plasma NfL level that exceeds the predefined threshold (plasma NfL level ≥ 44 pg/mL and an increase of ≥ 10 pg/mL from Part A baseline), no alternative cause unrelated to ALS disease activity for the elevation is identified, and the participant remains clinically presymptomatic, they will have the opportunity to screen for Part B. To reduce potential bias, masked NfL reports will inform only whether a participant has exceeded the predefined threshold, thus ensuring that both participants and investigators remain blinded to specific plasma NfL levels. We anticipate that enrollment in Part A will be completed within ~ 13 months: approximately 15 participants per month for the first 4 months and approximately 10 participants per month for the following 9 months. Enrollment in Part A will determine enrollment in Part B, in which approximately 28 participants will enroll within ~ 4.25 years from study start.
Part B is a randomized, double-blind, and placebo-controlled period in which presymptomatic participants with elevated NfL levels are randomized 1:1 to tofersen or placebo. In Part B, dynamic randomization has been implemented based on SOD1 variant type (e.g., p.Ala5Val [A5V; A4V] vs. other), last plasma NfL level prior to randomization (< 50 or ≥ 50 pg/mL), and age (< 30 vs. ≥ 30 years). Given the prognostic value of NfL, randomization will be stratified based on whether the last plasma NfL level prior to randomization is above or below 50 pg/mL, the estimated median NfL level at Part B baseline. Moreover, since the Pre-fALS participants who developed clinically manifest ALS were largely A5V carriers at least 30 years of age, we elected to stratify randomization on the basis of these factors rather than exclude other variant types or adults (≥ 18 years) under the age of 30. Dynamic randomization helps to ensure that a participant’s treatment assignment depends on their stratification factors, as well as the stratification factors and treatment assignments of previously randomized participants. This approach works to minimize imbalances across all stratification factors (weighted in order of importance as listed above) instead of constructing mutually exclusive strata. Participants who develop clinically manifest ALS in Part B will have the opportunity to receive open-label tofersen in Part C of the study. Together, Parts B and C may last up to ~ 2 years.
Participants who develop clinically manifest ALS prior to Part B randomization may enroll in Part D. Originally designed with a placebo-control (2:1 randomization), Part D was transitioned to open-label following review of the results of the Phase 3 VALOR study (233AS101 Part C). While no other changes to the ATLAS study protocol were deemed necessary, the VALOR data appear to reinforce the importance of early therapeutic intervention as the foundational principle behind ATLAS [23]. A schedule of assessments for study Parts A, B, C, and D can be found in Supplemental Tables 1, 2, 3, and 4, respectively.
Endpoints
The ATLAS study was designed to evaluate whether presymptomatic initiation of tofersen (upon elevation of plasma NfL level) can delay the emergence of clinically manifest ALS and/or reduce the loss of function over time as compared with delayed initiation of tofersen (upon emergence of clinically manifest ALS) (Table 1). To evaluate the delay in emergence of clinical onset, the following efficacy measures will be assessed in the Part B population: proportion of participants in whom clinically manifest ALS emerges within 12 months and 24 months of randomization (i.e., Part B baseline) and time from Part B baseline to emergence of clinically manifest ALS. Whether presymptomatic tofersen initiation reduces the loss of function compared with delayed initiation of tofersen will also be assessed by comparing those who receive tofersen in Part B with those who receive tofersen for the first time in Part C. Changes in clinical outcome measures from Part C and Part D baseline to end of study will also be analyzed in Part C and Part D participants, respectively.
For safety endpoints, the incidence of adverse events (AEs) and serious AEs (SAEs) during the treatment period are included (Parts B, C, D). Other exploratory analyses may include pharmacokinetic assessments, biomarkers, digital assessments, and health-related quality of life measures.
Statistical Analysis
A Bayesian decision rule will be used for the primary efficacy analysis. The number of participants with emergence of clinically manifest ALS by the end of month 12 in the placebo- and tofersen-treated groups is assumed to follow two independent binomial distributions and Jeffreys prior [28]; a noninformative prior of beta (0.5, 0.5) is used. The alternate hypothesis is that the proportion of participants with emergence of clinically manifest ALS by the end of month 12 for the tofersen-treated arm is less than that for the control arm. Based on emergent trial data, the posterior probability that the proportion of participants with emergence of clinically manifest ALS by the end of month 12 for the tofersen-treated arm is less than that for the control arm, will be calculated. If the posterior probability is at least 0.9, the alternative hypothesis is supported. Figure 2 below illustrates the decision rules with the green region representing situations where the alternative hypothesis is supported. The proportion of participants in each arm with emergence of clinically manifest ALS within 24 months of Part B baseline (Day 1) will also be reported, along with corresponding 95% confidence intervals (CIs), as a key secondary endpoint. Kaplan–Meier survival curves of the time to emergence of clinically manifest ALS will be presented and used to estimate the median time to event and corresponding 95% CI. The change in ALSFRS-R over time from Part B baseline will be summarized descriptively in the combined Part B and Part C dataset. The estimated proportion of participants with the event of death or permanent ventilation and the estimated proportion of deaths since Part B baseline (Day 1) will be reported, along with corresponding 95% CIs obtained from Kaplan–Meier analyses based on time to death/permanent ventilation and time to death.
Neurofilament as a Presymptomatic Biomarker of Disease Activity
NfL and phosphorylated neurofilament heavy chain (pNfH) levels were evaluated in serum and plasma samples collected from Pre-fALS participants with SOD1 variants. Trends across matrices, analytes, and assays were generally similar, illustrating a rise in neurofilament levels prior to phenoconversion (Fig. 3). For the ATLAS study, the plasma NfL assay by Siemens Healthineers was selected as the platform for NfL analysis based on analytical performance and utilization of a fully automated instrument that reduces sample-to-sample variability, avoids plate bias, and permits real-time testing to enable individual study participant-level treatment decisions. The Siemens Healthineers assay is a 2-site sandwich immunoassay using direct chemiluminometric technology.
Trends across matrices (serum and plasma), analytes (NfL and pNfH), and platforms (Quanterix Simoa, Siemens Healthineers, ProteinSimple Ella, Euroimmun) of NF prior to phenoconversion. Dotted horizon lines indicate the 95th percentile for the combined control and presymptomatic carrier group. Biological samples and clinical data were from the Pre-fALS (Pre-Symptomatic Familial ALS) study
A trajectory model, modified from the nonlinear Emax model, was built to predict the time from plasma NfL elevation to phenoconversion using samples from Pre-fALS phenoconverters with SOD1 variants associated with rapid disease progression (Fig. 4). A separate model fitted to the presymptomatic carriers was utilized to generate informative priors for select parameters that quantify NfL level before phenoconversion in the trajectory model for phenoconverters. This fitted trajectory model was used to identify an absolute NfL threshold (44 pg/mL) that minimized the false-positive rate (e.g., the incidence of individuals who met the NfL threshold but did not go on to develop clinically manifest ALS within 12 months) to less than 5% and enabled adequate time for sample processing, screening in Part B, and randomization prior to phenoconversion. Recognizing the potential effect of aging on NfL levels and the absence of an upper age limit for study eligibility, a second criterion of an increase in plasma NfL of at least 10 pg/mL from Part A baseline was introduced. Once a participant’s NfL level meets the dual criteria (≥ 44 pg/mL and ≥ 10 pg/mL increase in NfL from Part A baseline), the investigator will use their clinical judgment to confirm that there are no alternative (non-ALS) causes for the NfL elevation. Based on this NfL trajectory model, the average time from reaching the NfL threshold to emergence of clinically manifest ALS is estimated to be 3–4 months. As such, with ~ 1 month needed for sample processing, screening, and randomization during Part B screening, it is estimated that untreated participants would remain presymptomatic in Part B for an average of ~ 2–3 months before the emergence of clinically manifest ALS in this study.
Fitted neurofilament trajectory model (using Siemens Healthineers assay results) to inform the NfL threshold in ATLAS. The black curve represents the fitted model (with 95% prediction bands for the sigmoidal fit shown in gray shading), while the colored lines represent the data. The dotted horizon line indicates the predefined threshold of 44 pg/mL. Biological samples and clinical data were from the Pre-fALS (Pre-Symptomatic Familial ALS) study
SOD1 Variant Selection
ATLAS is enrolling carriers of gene variants associated with high or complete penetrance and rapidly progressive disease. The selection of this population is largely driven by the fact that, in Pre-fALS, the richest phenoconverter data to date are from individuals with rapidly progressive variants (mostly p.Ala5Val [A5V; A4V]). Data from this subgroup were therefore used to model the presymptomatic changes in NfL and to determine the study’s predefined NfL threshold for Part B. This model was then used to estimate the expected phenoconversion rate within 12 months after reaching the predefined NfL threshold and hence inform the ATLAS study’s sample size and duration. The ATLAS study was designed assuming that other variants associated with rapidly progressive disease (beyond p.Ala5Val [A5V; A4V]) would similarly be associated with elevations in NfL level in the 6–12 months prior to the emergence of clinically manifest disease.
Informed by the scientific literature, genetic databases, and clinical experience, a subset of 17 SOD1 variants was prespecified for inclusion in the protocol: p.Ala5Thr (A4T, A5T), p.Ala5Val (A4V, A5V), p.Cys7Phe (C6F, C7F), p.Cys7Gly (C6G, C7G), p.Asp102Gly (D101G, D102G), p.Asp102His (D101H, D102H), p.Gly115Ala (G114A, G115A), p.Gly42Ser (G41S, G42S), p.Gly86Arg (G85R, G86R), p.Gly86Ser (G85S, G86S), p.Gly94Ala (G93A, G94A), p.His44Arg (H43R, H44R), p.Leu107Phe (L106F, L107F), p.Leu107Val (L106V, L107V), p.Leu39Val (L38V, L39V), p.Arg116Gly (R115G, R116G), and p.Val149Gly (V148G, V149G). Recognizing the evolving understanding of penetrance and disease progression across SOD1 variants, and the heterogeneity observed across families carrying a given variant, an independent Mutation Adjudication Committee was formed to adjudicate eligibility of SOD1 variants not specified in the protocol based on available evidence from the participant’s pedigree, scientific literature, and other databases to evaluate the SOD1 variant criteria of association with high/complete penetrance and rapid disease progression.
Clinically Manifest ALS
ATLAS is designed to evaluate whether initiation of treatment in the presymptomatic stage of disease can delay the onset of clinically manifest ALS. Based on the operational definition of phenoconversion in the Pre-fALS study, clinically manifest ALS in ATLAS is defined as the presence of clinical symptoms or signs that, in the context of an integrated clinical assessment completed by a trained neurologist, definitely indicate ALS (Fig. 5) [5, 24]. Suspicion for clinically manifest ALS may be raised if a participant reports symptoms or signs during a scheduled study visit, if a participant reports symptoms or signs outside of a study visit, and/or if the investigator identifies signs of upper motor neuron (UMN) or lower motor neuron (LMN) dysfunction on examination during a study visit in Parts A or B. The participant will then undergo an integrated clinical evaluation performed by the investigator, termed the “Assessment for Clinically Manifest ALS.” The Assessment for Clinically Manifest ALS may include clinical history, neurological examination, and diagnostic testing (e.g., electromyography/nerve conduction studies, imaging, and/or laboratory tests), per the investigator’s clinical judgment and standard of care for neurological disorders. The investigator may then determine whether clinically manifest ALS has emerged. Recognizing the potential covert pressure from study participants who may wish to transition from Part B to Part C with the attendant potential bias for declaring that clinically manifest ALS has emerged, we have established an independent Endpoint Adjudication Committee (EAC) to confirm the presence of clinically manifest ALS and establish the timing of its emergence (i.e., phenoconversion). The EAC will blindly review the same source documentation (i.e., clinical history, neurological examination, and results of diagnostic testing) that were available to the investigator and communicate their determination within 12–20 days, permitting timely enrollment into Part C or Part D, as appropriate. Each potential instance of clinically manifest ALS will be independently adjudicated by each of three EAC members, who will be required to reach consensus on whether the case meets the definition of clinically manifest ALS.
The date of emergence of clinically manifest ALS will be defined as the date when the participant first experienced the relevant clinical symptom or, in the absence of symptoms, the date when neurological examination first detected evidence of UMN and/or LMN signs indicative of ALS, whichever is sooner.
Genetic Testing and Counseling, and Psychosocial Support
In the ATLAS study, participants undergo centralized SOD1 genetic testing to determine or confirm their SOD1 variant. Recognizing the complexities of predictive genetic testing and the potential for emotional, psychosocial, legal, and financial implications [25, 29,30,31], a robust genetic testing and counseling infrastructure has been developed for this study.
During screening for enrollment in Part A of the study, genetic counseling sessions are required before a DNA sample is collected for testing and at the time that the results are communicated. These sessions, which focus primarily on genetic testing, also include in-depth discussions about NfL — specifically, the uncertain but potential implications of above-threshold NfL levels for the risk of imminent clinical disease. Given the potential for continued psychosocial and emotional impact, especially after receiving a positive SOD1 result and anticipating or learning about a NfL level increase, additional counseling is available as needed throughout the study. Genetic counseling will be performed by qualified individuals as per local standard clinical practice; acknowledging that exact qualifications for providing genetic counseling differs around the world [32]. In addition to these qualifications, genetic counseling providers are required to complete study-specific centralized training. Resources, such as ad hoc consultation with genetic experts, are also available to those at study sites who are providing genetic counseling. For participants and their families, the study website and brochure are available as supplemental materials to help them understand the genetic testing and counseling processes even before enrolling in the study. Moreover, and as with any clinical trial, investigators are responsible for the overall wellbeing of study participants. This includes, but is not limited to, the assessment of psychosocial readiness to enroll in Part A and to undergo genetic and biomarker testing, oversight and monitoring throughout all parts of the study, and support or referral to an appropriate mental health provider as needed based on each study participant’s individual circumstance.
Ethics and Protections
The protection of participants’ privacy — in particular, their SOD1 variant genetic analysis results and their NfL levels — is paramount. If disclosed, positive SOD1 test results and elevated NfL levels could be used in discriminatory ways, with potential implications for both the study participant and their biological relatives in many spheres of life, including finances, insurance (disability, long-term, life, etc.), eligibility for educational and recreational activities, family planning, and employment status [30, 33, 34]. For these reasons, ATLAS has implemented multiple approaches to maximize participant privacy. In the USA, a Food and Drug Administration-issued Certificate of Confidentiality provides a legal layer of privacy protection for participants by prohibiting the forced disclosure of identifiable, sensitive research information to anyone not affiliated with the study. US participants also receive protection through the Genetic Information Non-Discrimination Act. Outside the USA, there are numerous protections, including specific genetic nondiscrimination laws, voluntary moratoriums on the use of genetic information by life insurance companies, and broader data protection regimes that offer varying levels of protection to participants (Fig. 6). Study sites are encouraged to keep ATLAS study data, particularly sensitive genetic information and NfL levels, strictly separate from participants’ medical records, and to limit access to data through user-specific authorization where possible. Furthermore, all study sites have been educated about genetic privacy and data management at the ATLAS investigator meetings and reinforced at site initiation visits. Finally, through the informed consent process, potential participants are educated about the benefits, risks, and burdens of presymptomatic genetic testing in addition to how the data will be analyzed, stored, and shared.
Country-specific privacy and antidiscrimination laws. This figure does not and is not intended to provide a complete or comprehensive description of all country-specific relevant laws, regulations, and practices. This is a starting place to learn the risks and legal protections that individuals undergoing SOD1 genetic testing may face and is limited to the countries planned to participate in ATLAS
Discussion
The ATLAS study incorporates unique design elements to evaluate whether presymptomatic initiation of tofersen can delay the emergence of clinically manifest ALS and/or reduce the loss of clinical function over time. ATLAS is the first interventional trial in ALS that uses neurofilament as an enrichment marker for study eligibility and a trigger for initiation of study treatment. The design of ATLAS was built upon the premise that elevations in NfL precede the emergence of clinically manifest ALS, as observed in rapidly progressive SOD1 variant carriers in the Pre-fALS study. The utility of this critical observation from the Pre-fALS study underscores the importance and value of high-quality natural history data in advancing therapy development for rare diseases [35].
ATLAS has the potential to aid in the identification and understanding of early markers of disease activity beyond NfL. The collection of CSF (at baseline for Part A and prior to every dose in Parts B/C/D) and urine/blood (annually in Part A; quarterly in Parts B/C/D) will enable the identification of other potential fluid markers of disease activity. Similarly, comprehensive electromyography (EMG) at screening for Part A and Part B may inform the timing of NfL elevation relative to the emergence of EMG abnormalities and the relative sensitivity of these two biomarkers. Through these biomarker assessments, ATLAS will help to inform the conduct of presymptomatic trials in other subsets of the ALS population.
A list of variants associated with high/complete penetrance and rapid disease progression was defined in the protocol based on the totality of information ascertained in the literature, genetic databases, and clinical experience. However, characterization of SOD1 variants is continuously evolving due to limited reporting and heterogeneity across carriers. To account for this, an independent Mutation Adjudication Committee was formed to adjudicate eligibility of non-protocol-defined SOD1 variants based on the most up-to-date information at the time. The use of clinically manifest ALS as a primary endpoint is not without challenges, given potential perverse incentive to receive open-label tofersen in Part C; it is for this reason that we have established an independent EAC to confirm the emergence of clinically manifest ALS.
Recognizing the potentially serious and life-long implications of positive genetic testing results on study participants and their families, the study also necessitated the development of a supportive infrastructure to inform, support, and protect prospective participants. Informed by experience from the Pre-fALS study, ATLAS aims to take into account two key considerations: (1) when and how participants are informed of their genetic risk, and (2) how participants’ privacy, especially regarding their SOD1 genetic variant and NfL biomarker status, can be protected. For both study sites and participants alike, education, training, and resources are essential to help address these concerns. Specifically, the ATLAS study has implemented genetic testing and counseling processes along with firewalls and strategies to protect participant privacy in the context of a clinical research study.
Conclusions
The ATLAS study (NCT04856982) is designed to evaluate the impact of initiating tofersen in presymptomatic carriers of SOD1 variants associated with high or complete penetrance and rapid disease progression who also have biomarker evidence of disease through elevated plasma NfL. Recognizing that therapeutic intervention in earlier phases of ALS has the potential to yield the greatest clinical benefit, ATLAS, together with data from the VALOR study and its open-label extension study, will inform the optimal timing of treatment initiation in SOD1-ALS. As the first interventional trial in presymptomatic ALS, ATLAS has the potential to yield important insights into the design and conduct of presymptomatic trials, identification, and monitoring of at-risk individuals, and future treatment paradigms in ALS and other neurodegenerative diseases.
Change history
29 September 2022
A Correction to this paper has been published: https://doi.org/10.1007/s13311-022-01286-9
References
Kiernan MC, Vucic S, Cheah BC, Turner MR, Eisen A, Hardiman O, et al. Amyotrophic lateral sclerosis. Lancet. 2011;377(9769):942–55.
Galvin M, Ryan P, Maguire S, Heverin M, Madden C, Vajda A, et al. The path to specialist multidisciplinary care in amyotrophic lateral sclerosis: a population- based study of consultations, interventions and costs. PLoS One. 2017;12(6):e0179796.
Nzwalo H, de Abreu D, Swash M, Pinto S, de Carvalho M. Delayed diagnosis in ALS: the problem continues. J Neurol Sci. 2014;343(1–2):173–5.
Williams JR, Fitzhenry D, Grant L, Martyn D, Kerr DA. Diagnosis pathway for patients with amyotrophic lateral sclerosis: retrospective analysis of the US Medicare longitudinal claims database. BMC Neurol. 2013;13(1):160.
Benatar M, Turner MR, Wuu J. Defining pre-symptomatic amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2019;20(5–6):303–9.
De Vivo DC, Bertini E, Swoboda KJ, Hwu WL, Crawford TO, Finkel RS, et al; Nurture Study Group. Nusinersen initiated in infants during the presymptomatic stage of spinal muscular atrophy: interim efficacy and safety results from the phase 2 NURTURE study. Neuromuscul Disord. 2019;29(11):842–56.
Logroscino G, Traynor BJ, Hardiman O, Chiò A, Mitchell D, Swingler RJ, et al. Incidence of amyotrophic lateral sclerosis in Europe. J Neurol Neurosurg Psychiatry. 2010;81(4):385–90.
Bunton-Stasyshyn RK, Saccon RA, Fratta P, Fisher EM. SOD1 function and its implications for amyotrophic lateral sclerosis pathology: new and renascent themes. Neuroscientist. 2015;21(5):519–29.
Amyotrophic Lateral Sclerosis online Database. SOD1 gene summary. Available at: https://alsod.ac.uk/output/gene.php/SOD1. Accessed 22 July 2021.
Bali T, Self W, Liu J, Siddique T, Wang LH, Bird TD, et al. Defining SOD1 ALS natural history to guide therapeutic clinical trial design. J Neurol Neurosurg Psychiatry. 2017;88(2):99–105.
Cudkowicz ME, McKenna-Yasek D, Sapp PE, Chin W, Geller B, Hayden DL, et al. Epidemiology of mutations in superoxide dismutase in amyotrophic lateral sclerosis. Ann Neurol. 1997;41(2):210–21.
Li HF, Wu ZY. Genotype-phenotype correlations of amyotrophic lateral sclerosis. Transl Neurodegener. 2016;5:3.
Prudencio M, Hart PJ, Borchelt DR, Andersen PM. Variation in aggregation propensities among ALS-associated variants of SOD1: correlation to human disease. Hum Mol Genet. 2009;18(17):3217–26.
Yamashita S, Ando Y. Genotype-phenotype relationship in hereditary amyotrophic lateral sclerosis. Transl Neurodegener. 2015;4:13.
Ilieva H, Polymenidou M, Cleveland DW. Non-cell autonomous toxicity in neurodegenerative disorders: ALS and beyond. J Cell Biol. 2009;187(6):761–72.
McCampbell A, Cole T, Wegener AJ, Tomassy GS, Setnicka A, Farley BJ, et al. Antisense oligonucleotides extend survival and reverse decrement in muscle response in ALS models. J Clin Invest. 2018;128(8):3558–67.
Saccon RA, Bunton-Stasyshyn RK, Fisher EMC, Fratta P. Is SOD1 loss of function involved in amyotrophic lateral sclerosis? Brain. 2013;136(Pt 8):2342–58.
Sau D, De Biasi S, Vitellaro-Zuccarello L, Riso P, Guarnieri S, Porrini M, et al. Mutation of SOD1 in ALS: a gain of a loss of function. Hum Mol Genet. 2007;16(13):1604–18.
Ekhtiari Bidhendi E, Bergh J, Zetterström P, Forsberg K, Pakkenberg B, Andersen PM, et al. Mutant superoxide dismutase aggregates from human spinal cord transmit amyotrophic lateral sclerosis. Acta Neuropathol. 2018;136(6):939–53.
Bennett CF, Krainer AR, Cleveland DW. Antisense oligonucleotide therapies for neurodegenerative diseases. Annu Rev Neurosci. 2019;42:385–406.
Shen X, Corey DR. Chemistry, mechanism and clinical status of antisense oligonucleotides and duplex RNAs. Nucleic Acids Res. 2018;46(4):1584–600.
Miller T, Cudkowicz M, Shaw PJ, Andersen PM, Atassi N, Bucelli RC, et al. Phase 1–2 trial of antisense oligonucleotide tofersen for SOD1 ALS. N Engl J Med. 2020;383(2):109–19.
Miller T, Cudkowicz M. In American Neurological Association Annual Meeting (Virtual, 2021 Oct 17–19).
Benatar M, Wuu J, McHutchison C, Postuma RB, Boeve BF, Petersen R, et al. Preventing amyotrophic lateral sclerosis: insights from pre-symptomatic neurodegenerative diseases. Brain. 2022;145(1):27–44.
Benatar M, Wuu J. Presymptomatic studies in ALS: rationale, challenges, and approach. Neurology. 2012;79(16):1732–9.
Benatar M, Wuu J, Lombardi V, Jeromin A, Bowser R, Andersen PM, et al. Neurofilaments in pre-symptomatic ALS and the impact of genotype. Amyotroph Lateral Scler Frontotemporal Degener. 2019;20(7–8):538–48.
Benatar M, Wuu J, Andersen PM, Lombardi V, Malaspina A. Neurofilament light: a candidate biomarker of presymptomatic amyotrophic lateral sclerosis and phenoconversion. Ann Neurol. 2018;84(1):130–9.
Jeffreys H. Theory of Probablility. Oxford Classic Texts in the Physical Sciences. 1939.
Fanos JH, Gronka S, Wuu J, Stanislaw C, Andersen PM, Benatar M. Impact of presymptomatic genetic testing for familial amyotrophic lateral sclerosis. Genet Med. 2011;13(4):342–8.
Benatar M, Stanislaw C, Reyes E, Hussain S, Cooley A, Fernandez MC, et al. Presymptomatic ALS genetic counseling and testing: experience and recommendations. Neurology. 2016;86(24):2295–302.
Roberts JS, Patterson AK, Uhlmann WR. Genetic testing for neurodegenerative diseases: ethical and health communication challenges. Neurobiol Dis. 2020;141:104871.
Abacan M, Alsubaie L, Barlow-Stewart K, Caanen B, Cordier C, Courtney E, et al. The global state of the genetic counseling profession. Eur J Hum Genet. 2019;27(2):183–97.
Chiò A, Battistini S, Calvo A, Caponnetto C, Conforti FL, Corbo M, et al; ITALSGEN Consortium. Genetic counselling in ALS: facts, uncertainties and clinical suggestions. J Neurol Neurosurg Psychiatry. 2014;85(5):478–85.
Fanos JH, Gelinas DF, Miller RG. “You have shown me my end”: attitudes toward presymptomatic testing for familial amyotrophic lateral sclerosis. Am J Med Genet A. 2004;129A(3):248–53.
US Food and Drug Administration. Rare diseases: natural history studies for drug development. Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/rare-diseases-natural-history-studies-drug-development. Accessed 30 Nov 2021.
Acknowledgements
The authors would like to thank the ALS community, Pre-fALS participants, and study team at the University of Miami, and ATLAS investigators and site staff. Yien Liu, PhD, from Excel Scientific Solutions provided writing assistance in the development of the first and subsequent drafts based on input from authors, and Cara Dickinson and Jackie Parker from Excel Scientific Solutions copyedited and styled the manuscript per journal requirements. The authors had full editorial control of the paper and provided their final approval of all content.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Funding
Biogen provided funding for medical writing support in the development of this report. The Pre-fALS study is supported by NIH (R01 NS105479).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jeong Heo was affiliated with Massachusetts College of Pharmacy and Health Sciences, Boston, MA 02115, USA at the time the study was designed.
Janice Wong was affiliated with Biogen, 225 Binney Street, Cambridge, MA 02142, USA at the time the study was designed.
The original version of this article was revised: Captions for Figure 1 and Figure 5 in this article were formatted in a confusing manner in this article as originally published and have been corrected.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Benatar, M., Wuu, J., Andersen, P.M. et al. Design of a Randomized, Placebo-Controlled, Phase 3 Trial of Tofersen Initiated in Clinically Presymptomatic SOD1 Variant Carriers: the ATLAS Study. Neurotherapeutics 19, 1248–1258 (2022). https://doi.org/10.1007/s13311-022-01237-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13311-022-01237-4