Abstract
Prion infections of the central nervous system (CNS) are characterized by initial reactive gliosis followed by overt neuronal death. Gliosis is likely to be caused initially by the deposition of misfolded, proteinase K-resistant, isoforms (termed PrPSc) of the normal cellular prion protein (PrPc) in the brain. Proinflammatory cytokines and chemokines released by PrPSc-activated glia and stressed neurons may also contribute directly or indirectly to the disease development by enhancing gliosis and inducing neurotoxicity. Recent studies have illustrated that early neuroinflammation activates nuclear factor of activated T cells (NFAT) in the calcineurin signaling cascade, resulting in nuclear translocation of nuclear factor kappa B (NF-κB) to promote apoptosis. Hence, useful therapeutic approaches to slow down the course of prion disease development should control early inflammatory responses to suppress NFAT signaling. Here we used a hamster model of prion diseases to test, for the first time, the neuroprotective and NFAT-suppressive effect of a second-generation semisynthetic tetracycline derivative, minocycline, versus a calcineurin inhibitor, FK506, with known NFAT suppressive activity. Our results indicate that prolonged treatment with minocycline, starting from the presymptomatic stage of prion disease was more effective than FK506 given either during the presymptomatic or symptomatic stage of prion disease. Specifically, minocycline treatment reduced the expression of the astrocyte activation marker glial fibrillary acidic protein and of the microglial activation marker ionized calcium-binding adapter molecule-1, subsequently reducing the level of proinflammatory cytokines interleukin 1β and tumor necrosis factor-α. We further found that minocycline and FK506 treatment inhibited mitogen-activated protein kinase p38 phosphorylation and NF-κB nuclear translocation in a caspase-dependent manner, and enhanced phosphorylated cyclic adenosine monophosphate response element-binding protein and phosphorylated Bcl2-associated death promoter levels to reduce cognitive impairment and apoptosis. Taken together, our results indicate that minocycline is a better choice for prolonged use in prion diseases and encourage its further clinical development as a possible treatment for this disease.
Similar content being viewed by others
Introduction
The transmissible spongiform encephalopathies (TSEs), or prion diseases, are fatal neurodegenerative disorders that include bovine spongiform encephalopathies in cattle; scrapie in sheep and goats; chronic wasting disease in elk and deer; and kuru, Creutzfeldt–Jakob disease (CJD), Gerstman–Sträussler–Scheinker syndrome, and fatal familial insomnia in humans [1, 2]. Prion diseases have been the object of growing interest since the discovery of the newest, most devastating form of TSEs in humans, termed variant CJD [3]. Prion diseases are characterized by the presence of PrPSc, an abnormal protease-resistant misfolded isoform of the cellular prion protein PrPc [3, 4]. PrPSc is highly pathogenic and neurotoxic owing to its β-sheet-rich conformation as compared with the predominantly α-sheet-rich structure of PrPc [5, 6]. Like several other neurodegenerative disorders, prion diseases are also associated with the accumulation and aggregation of misfolded/unfolded disease-specific proteins in the brain, leading to neuroinflammation and neurodegeneration. However, the exact molecular mechanisms behind neuronal inflammation and apoptosis are still unclear, and hence the development of an effective therapeutic strategy remains elusive [7].
T-cell activation contributes to disease pathogenesis by creating an inflammatory environment that leads to cellular damage and, ultimately, to cell death, as well as decreased immune function. Activated T cells increase neuroinflammation by activating microglia and, subsequently, the nuclear factor of activated T cells (NFAT) signaling cascade, leading to nuclear translocation of nuclear factor kappa B (NF-κB) [8]. Given its function as a key regulatory factor in T-cell activation, NFAT is 1 of the major targets of traditional immunosuppressive drugs in the immunophilin ligand class (ciclosporin A, FK506) [9]. Recently, modulation of T-cell activation and function has been proposed as 1 of the major mechanisms of action of nontraditional immunosuppressive drugs towards achieving the goal of reducing neuroinflammation and alleviating neurodegeneration.
Minocycline, a tetracycline derivative, is well known for its antimicrobial properties in many bacterial infections; however, the cytoprotective role mediated by minocycline is still unclear. Minocycline is broadly protective in neurologic disease models featuring inflammation and cell death, and is being evaluated in clinical trials of major neurodegenerative diseases or brain injury models [10–15]. Microglia, the brain innate immune macrophage-like cells, are activated by the oligomers and fibrils formed by amyloid proteins, including PrPSc, and are normally found clustered around amyloid plaques where they phagocytize and degrade plaques as an integral part of clearance mechanism [13]. The activation states of microglia are divided into 2 distinct phenotypes based on their relationship with inflammatory processes: M1 (proinflammatory phenotype) and M2 (anti-inflammatory phenotype). M1 microglia release proinflammatory and neurotoxic molecules that contribute towards cell death, whereas M2 microglia release anti-inflammatory molecules, including cytokines and neurotrophic factors that help in nervous system repair and regeneration processes [13, 16]. T cells are postulated to be a primary target of minocycline, involving the suppression of NFAT signaling leading to reduced NF-κB nuclear translocation in CD+ T cells, as recently reported [8].
Tacrolimus or FK506 is a well-known immunosuppressive drug, mainly used to combat allograft rejection in solid-organ transplant recipients. FK506 binds to the FK506-binding protein (FKBP) and the FK506/FKBP complex interacts with and inhibit calcineurin (CaN), a calcium- and calmodulin-dependent phosphatase, resulting in the inhibition of NFAT and thus suppression of the T-lymphocyte-mediated signal transduction pathway leading to interleukin (IL)-2 transcription [17]. Notably, FK506 has also been reported to afford neuroprotection in various animal models of central nervous system (CNS) injury [18–20], as well as several neurodegenerative disorders, including Alzheimer’s disease (AD), Parkinson’s disease, and prion diseases [1, 3, 21–23].
A novel therapeutic strategy towards achieving the goal of neuroprotection in neurodegenerative diseases should be focused on modulating microglial activity to promote their neuroprotective mechanisms and alleviate their neurotoxic mechanisms in early stages of neurodegeneration. With this goal in mind, the present study was designed to evaluate the neurotherapeutic efficacy of minocycline and FK506 in an animal model of prion diseases. Specifically, we explored the comparative immunomodulatory effect of minocycline and FK506 at a cellular level on the expression of different cell populations in the brain of prion-infected hamsters and evaluated their effect on prion-related cognitive impairments and survival.
Materials and Methods
Animal Ethics Statement
All experimental procedures with the laboratory animals used in this study were performed and approved according to the strict guidelines of Chinese Regulations of Laboratory Animals—The Guidelines for the Care of Laboratory Animals (Ministry of Science and Technology of People's Republic of China), and Laboratory Animal Requirements of Environment and Housing Facilities (GB 14925–2010, National Laboratory Animal Standardization Technical Committee). The license number related to the research protocol was 20110611–01, and the animal study proposal was approved by The Laboratory Animal Ethical Committee of China Agricultural University, Beijing, China. We aimed to minimize the suffering of the animals and to reduce the number of animals studied at minimum.
Animal Model of Prion Diseases
The infectious model of prion disease in hamsters is a very good model for TSE-related studies, as it reproduces many of the clinical, neuropathologic, and biochemical aspects of the disease in humans and other mammals in a relatively short incubation period as compared with other animal models [24]. Five-week-old Syrian golden hamsters (n = 60) were used in the current study. Each animal was housed in a separate cage. The animals were divided randomly into 5 groups: prion–vehicle group administered with 0.9% saline as a positive control group with only vehicle treatment once daily started at day 65 postinfection before the appearance of obvious clinical signs (group A); prion–FK506 early treatment started at day 65 postinfection with once-daily injection of FK506 before the appearance of obvious clinical signs (group B); prion–FK506 late treatment started at day 90 postinfection with once-daily injection of FK506, when the first visible signs of disease were observed in more than half of the animals (group C); prion–minocycline treatment started at day 65 postinfection with once-daily injection of minocycline before the appearance of obvious clinical signs (group D); and no prion–vehicle group administered with 0.9% saline as negative control group with only vehicle treatment given once daily on a daily basis (group E). All groups consisted of 12 animals. Normal saline (0.9%) was used a vehicle treatment in groups A–E to induce same level of stress in infected and noninfected animals. All the animals except those in group E were injected intraperitoneally (i.p) with 75 μl 10% brain homogenate prepared in phosphate buffered saline from terminally dead hamsters with 263 K strain of prion according to previous protocols [25, 26]. The animals showed first sign of disease after 80 ± 10 days postinfection. The signs were divided into 5 stages: 1) normal animal; 2) roughcoat on limbs with erect hairs; 3) extensive roughcoat, hunckback, circling, and visible motor abnormalities; 4) urogenital lesions; and 5) terminal stage of the disease in which the animal presented with cachexia and lies in the cage with little movement. The period between the appearance of the first disease symptom and death range between 10 to 30 days in animals without treatment. Treatment administration of FK506 and minocycline was carried out until animals died or were sacrificed for experiment.
FK506 Preparation and Administration
FK506 (Cat# F-4900) was purchased as white crystalline powder from LC Laboratories (Woburn, MA, USA). Purity was ≥ 99%. F506 stock solution (0.5 mg/ml) was prepared by dissolving the compound in saline (0.9% NaCl) containing 1.25% PEG40 Castor Oil (Cat#C9510; Solarbio Life Sciences China, Beijing, China), and 2% ethanol (Beijing Chemical Works, Beijing, China). Animals in group B and C were injected i.p. with 0.12 mg FK506 (5 mg/kg) dissolved in 100 ml of the vehicle solution mentioned above [3]. FK506 stock solution was stored frozen in a light-protected bottle.
Minocycline Preparation and Administration
Minocycline (M9511) was purchased as yellow crystalline powder from Sigma-Aldrich (St. Louis, MO, USA). Purity was ≥ 99%. Minocycline stock solution (0.5 mg/ml) was prepared by dissolving the compound in saline (0.9% NaCl) containing 1.25% PEG40 Castor Oil (Cat#C9510; Solarbio Life Sciences China) and 2% ethanol (Beijing Chemical Works). Animals in group D were injected i.p. with 0.36 mg minocycline (15 mg/kg) dissolved in 100 ml of the vehicle solution mentioned above according to previous experiments [27–29]. Minocycline stock solution was stored frozen in a light-protected bottle.
Reagents
The rabbit polyclonal anti-pan-CaN A antibody (2614; 1:1000; Cell Signaling Technology, Danvers, MA, USA). The rabbit polyclonal anti-CaN B antibody (AF1348; 0.25 μg/ml; R&D Systems, Minneapolis, MN, USA).The rabbit polyclonal anti-Bcl2-associated death promoter (BAD) antibody (9292; 1:1000), rabbit polyclonal antiphosphor-BAD antibody (9291; 1:1000), rabbit monoclonal anti-cyclic adenosine monophosphate response element-binding (CREB) antibody (9197; 1:1000), rabbit monoclonal antiphospho-CREB antibody (9198; 1:1000), rabbit polyclonal anti-p38 mitogen-activated protein kinase (MAPK) antibody (9212; 1:1000), rabbit polyclonal antiphospho-p38 MAPK antibody (9211; 1:1000), and rabbit polyclonal antinucleophosmin antibody (3542; 1:1000) were purchased from Cell Signaling Technology. The rabbit polyclonal anticaspase-12 antibody (55238-1-AP; 1:2000), rabbit polyclonal anticaspase-9 antibody (10380-1-AP; 1:2000), rabbit polyclonal anticaspase-3 p17-specific antibody (25546-1-AP; 1:1000), rabbit polyclonal anti-postsynaptic density protein 95 antibody (20665-1-AP; 1:800 Western Blot), rabbit polyclonal antisynaptophysin antibody (17785-1-AP; 1:800 WB), rabbit polyclonal p65, RELA antibody [10745-1-AP; 1:2000 WB (1:50 Immunohistochemistry)], rabbit polyclonal anti-glial fibrillary acidic protein (GFAP) antibody (16825-1-AP; 1: 50 IHC), rabbit polyclonal anti-NeuN antibody (23060-1-AP; 1:50 IHC), and rabbit polyclonal anti-ionized calcium-binding adapter molecule 1 (IBA-1) antibody (10904-1-AP; 1:50 IHC) were purchased from Proteintech Biotechnology (Chicago, IL, USA). The rabbit polyclonal antilamin A/C antibody (D120927; 1:50 IHC) was from BBI Life Sciences (Sangon Biotechnology, Shanghai, China). The mouse monoclonal antiprion-3 F4 antibody (SIG-39600; 1:1000; SIGNET-Covance, San Diego, CA, USA). The rabbit polyclonal β-tubulin antibody (10094-1-AP; 1:5000) and mouse monoclonal antiglyceraldehyde 3-phosphate dehydrogenase antibody (60004-1-lg; 1:1000) were from Proteintech Biotechnology. The rabbit polyclonal antirat β-actin antibody (Cat#AP0060; 1:1000) and goat antirabbit IgG (Heavy and Light Chains)–horseradish peroxidase (HRP) secondary antibody (Cat#BS13278; 1:5000) were purchased from Bioworld Technology (Nanjing, China). The rabbit antigoat IgG (H+L) (ZB-2306; 1:5000), Alexa Fluor 594-Conjugated AffiniPure Goat Anti-Rabbit IgG (H&L) (ZF-0516; 1:100), and goat antimouse IgG (H&L)–HRP secondary antibody (1:100) were purchased from Beijing ZSGB Biotechnology (Beijing, China). 4,6-Diamidino-phenylindole dihydrochloride and propidium iodide were purchased from Beyotime Biotechnology (Wuhan, China). DAB Horseradish Peroxidase Color Development Kit (P0202) (Beyotime Biotechnology). Reagents and apparatus used in immunoblotting assays were purchased from Bio-Rad (Richmond, CA, USA). The Colorimetric Mouse TNF-α ELISA kit (KE10002;) was from Proteintech. The IL-1 beta/IL-1 F2 ELISA kit (DY401-05) was from R&D Systems.
CaN Activity Assay
The phosphatase activity of CaN was measured using the Calcineurin Cellular Activity Assay kit (Cat#207007; Calbiochem, Darmstadt, Germany) as previously described [3]. The brain homogenates were prepared in the assay buffer and the residual phosphate was removed by passing through a desalting column. A final concentration of 1 μg/μl of the homogenate was used for the enzyme assay in presence of bovine calmodulin. The reaction mixture was incubated with a final concentration of 150 mM RII peptide (substrate) at 37 °C for 20 min and reactions were terminated by the addition of 100 ml malachite green reagent. Product formation was measured by recording the absorbance at 635 nm.
Animal Behavioral Studies
To investigate whether treatment with minocycline and FK506 alters clinical signs, we performed nesting behavior; open field; and novel object recognition tests. The nesting behavior was observed continuously for 13 weeks and scoring in a group was done according to a previous protocol, with little modification [30]. Briefly, partially shredded colored paper was placed on the opposite side of the normal nesting location within the cage, and nesting was scored the next day on the basis of the location of the shredded paper inside or near the nest. The quality of the nests was evaluated using a modified 5-point scale. Tissue not noticeably touched, moved, or shredded (>90% in same place as originally placed = 1 point); tissue partially touched, moved, or shredded (50–90% near the nest or inside nest = 2 points); mostly shredded or moved but not identifiable as a nest (>50% of the paper near the nest or inside the nest = 3 points), an almost intact nest (>90% of the paper near or inside the nest = 4 points); an intact nest (100% of the paper inside the nest = 5 points). Open-field tests were performed to monitor exploratory behavior and locomotor activity according to a previous experiment with a little modification [31]. Briefly, the animals were placed individually at the center of a 100 × 100 × 40-cm wooden box and left to move freely within it for 5 min. For all behavioral tests, data were gathered with a digital video camera (Sony W280) mounted on top of the open-field box. The box was divided into 15 equal horizontal and vertical lines and all activities during various time intervals were recorded. We measured and analyzed total distance covered, moving time, inactive time, and rearing activity time in 5-min test intervals. Testing was carried out in a temperature, noise, and light controlled room.
Novel object recognition tests were performed a day after the open-field test in a 100 × 100 × 40-cm wooden box [32]. Animals were pretrained to habituate to the box for 2 consecutive days, without objects present. For testing, animals were placed individually at the inlet made in side wall the of the box and the central part of the box was covered with different color paper each time to attract hamsters to the presence of 4 objects of 2 identical shapes (old objects) for 10 min. The identically shapes objects were placed side by side before the test. After that period, the box and objects were cleaned with 50% methanol solution. The animal was later (after 2 h) exposed to the identical objects placed across from each other and in center a dummy sheep was placed as a novel object, and the box and objects were cleaned again to continue with the next animal. The recognition index was calculated as the time spent exploring the new object divided by the time exploring old objects.
PrPSc Detection Assay
The presence and quantity of PrPSc in brain homogenates of sick animals was measured by a standard assay consisting of the ability of the misfolded protein to resist proteolytic degradation. Samples were incubated in the presence of proteinase K (50 μg/ml) for 1 h with shaking at 37 °C. The digestion was stopped by adding electrophoresis sample buffer and protease-resistant PrPSc was detected by Western blotting, as previously described [24]. Briefly, proteins were fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), electroblotted onto polyvinylidene fluoride membranes (Immobilon-PSQ, ISEQ00010, 0.2 μm). Blots were blocked by 5% nonfat milk in TBST (25 mMTris base, 137 mM sodium chloride, 2.7 mM potassium chloride and 0.05% Tween-20; pH7.4) for 1 h at room temperature, incubated with the indicated 3 F4 antibody at 4 °C overnight and the corresponding HRP-labeled secondary antibody for 50 min at 37 °C, and the signal detected using an enhanced chemiluminescence detection kit (Bio-Rad, Hercules, CA, USA).
Western Blotting
Frozen brain samples were homogenized in RIPA buffer containing a cocktail of protease inhibitors and were sonicated for 15 s, and then centrifuged at 20,000 × g for 5 min. The supernatants were collected and boiled for 10 min after addition of loading buffer (250 mM Tris-HCl pH 6.8, 10% SDS, 0.5% Bromophenol Blue, 50% glycerol, 0.5 M Dithiothreitol). Protein concentration in each sample was measured before adding loading buffer by using a Bicinchoninic Acid Assay (BCA) (CWBio, Shanghai, China) and 40 μg/well of protein extracts were subjected to SDS-PAGE, and Western blotting. Samples for PrPSc detections and other sample aliquots were separated via SDS-PAGE and the proteins were transferred to polyvinylidene fluoride membranes (Immobilon-PSQ, ISEQ00010, 0.2 μm). Blots were blocked by 5% nonfat milk in TBST (25 mMTris base, 137 mM sodium chloride, 2.7 mM potassium chloride and 0.05% Tween-20; pH 7.4) for 1 h at room temperature, incubated with the indicated primary antibody overnight at 4 °C and the corresponding HRP-labeled secondary antibody for 50 min at 37 °C, and the signal detected using an enhanced chemiluminescence detection kit (Bio-Rad).
Tissue Preparation and Immunohistochemistry Analysis
Histological studies were carried out to assess the effect of FK506 and minocycline treatment on brain damage. Brains from dead or sacrificed animal were removed surgically under aseptic conditions as quickly as possible. Terminally sick animals showing the 5 signs on the scale were anaesthetized with ketamine:zyline 3:1 (250 μl/100 g body weight); then whole brain was excised aseptically and cut sagitally into 2 halves—1 half was snap frozen in liquid nitrogen for Western blot and CaN assay, while the other half was fixed in 10% formaldehyde solution, embedded in paraffin, and cut into sections using a microtome (Leica RM2235; Leica, Buffalo Grove, IL, USA). Serial sections (from 2 μm to 5 μm thick) from each block were stained to assess spongiform degeneration, brain inflammation, neuronal degeneration, and neuronal loss. The following studies were done.
Brain Vacuolation
One of the neuropathologic hallmarks of prion diseases is the presence of spongiform degeneration in the brain. Vacuolation was assayed by staining of the tissue with hematoxylin and eosin. Then, the numbers of vacuoles were counted and scored on a scale from 1 to 4 in cerebellum, medulla, hippocampus thalamus, and cortex of each animal, as described [33].
Brain Inflammation
Astrocytosis was assessed by immunohistochemistry techniques utilizing antibodies against GFAP expressed in abundance in activated astrocytes, following a previously described protocol [33]. Staining for activated microglia was done with the IBA-1 antibody. IBA-1 is a 17-kDa interferon-γ-inducible calcium-binding protein, associated with chronic inflammatory processes, which has been previously used to assess microglial activation in patients with CJD [34]. Digital images were collected on Olympus DP72 microscope fitted with an apotome for optimal sectioning. To calculate the extent of astrocytosis and microglial activation, 5 brains from each group were visualized at 10×, 20×, and 40×. All the brain regions were carefully visualized and the stained area compared with the total tissue area was determined by using the Image-Pro Plus 6 program (Media Cybernetics, Rockville, MD, USA).
Neurodegeneration
To evaluate neuronal degeneration and death we used Fluoro-Jade C (23062; AAT Bioquest, Sunnyvale, Canada) staining to detect degenerated cells and NeuN, a specific marker for neurons, was used for immunohistochemistry analysis of living neurons. The Fluoro-Jade C staining was done following the protocol described by Schmued [35]. Briefly, 5-μm sections were cut from paraffin-embedded brains and spread on microscope slides and allowed to air dry, followed by mounting on microscope slides and placed in 70% ethanol. The sections were washed and oxidized by soaking in a solution of 0.06% KMnO4 for 15 min. After washing, they were stained with 0.001% Fluoro-Jade C (AAT Bioquest) in 0.1% acetic acid for 20 min. Slides were washed again and dried overnight at room temperature. Digital images were collected on an Olympus Fluoview FV1000 confocal microscope. Five 20× sections of cerebellum, medulla, thalamus, and cortex were collected per animal. Fluoro-Jade C-positive cells were counted from each field. The number of total neurons was counted after staining with the polyclonal anti-NeuN antibody (Proteintech) at 1:100 dilutions. NeuN is a specific neuronal marker for a DNA-binding protein present in the nucleus of postmitotic neurons [36]. Brain sections were mounted onto gelatin-coated coverslips and allowed to air dry. Air-dried sections were blocked and permeabilized in 0.1 Molar Phosphate Buffered Solution (M PB) with 0.3% TX-100 (Sigma-Aldrich) and 10% fetal calf serum for 1 h. Following permeabilization, the mouse polyclonal anti-NeuN antibody (Proteintech) was applied at a 1:100 dilution and incubated overnight at room temperature. After washing, the secondary antibody was applied for 1 h at 37 °C followed by 3 consecutive washes and color development via 3, 3'-Diaminobenzidine (DAB) application. Slides were visualized under the microscope by 2 different researchers blinded to the treatment, who counted the number of neurons in different brain areas.
Preparation of the Cytoplasmic and Nuclear Extracts
The brain tissue was homogenized with ice-cold PBS buffer using a dounce homogenizer until a clear suspension was visible with the naked eye; it was then centrifuged at 500 × g for 2 to 3 min. A cyctoplasmic and nuclear protein extraction kit (AR0106; Boster Biological Technology, Pleasanton, Canada) was used to prepare cytoplasmic and nuclear extracts. The supernatant was removed and the sediment volume was estimated. The sedimentary cells were resuspended in 1:10 of cytoplasmic extraction reagent, shaken and vortexed strongly for 15 s until the cell sediment was mixed with cytoplasmic extraction reagent and incubated for 10 to 30 min on ice, shaken at high speed for 5 s, centrifuged at 12,000 to 16,000 × g for 10 min at 4 °C. The supernatant was pipetted into precooled plastic tubes; the supernatant obtained was used as cytoplasmic extract. The sediment was suspended in a 1:2 ratio of nuclear extraction reagent. High-speed shaking was done for 15 s until the sediment mixed completely with nuclear extraction reagent. Then the sample was incubated in an ice bath for 30 min. Then samples were shaken at high speed for 10 to 20 s, centrifuged at 12,000 to 16,000 × g for 10 min at 4 °C. The supernatant was pipetted into precooled plastic tubes and the supernatant contained nuclear protein.
Statistical Analysis
All assays were performed on 3 separate occasions. Data are expressed as means ± SD. All comparisons for parametric data were made using 1-way analysis of variance followed by post-hoc Turkey’s multiple comparison test or 2-way analysis of variance followed by post-hoc Bonferroni multiple comparison test using the GraphPad Prism 5 software (GraphPad, La Jolla, CA, USA) The in vivo survival study was assessed with the log-rank (Mantel–Cox) test using GraphPad Prism 5 and Image-Pro Plus (Media Cybernetics, Rockville, MD, USA) was used for immunohistochemistry image analysis. A p-value < 0.05 was considered statistically significant.
Results
Minocycline-Enhanced Nesting Behavior, Locomotor Function, Novel Object Finding, and Prolonged Survival in Prion-Infected Hamsters
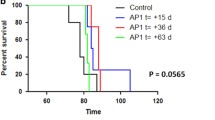
The nesting habit, exploratory behavior, and general locomotor activity are widely used tools in rodent behavioral science, where both the quality and quantity of the activity can be measured. To assess the effect of minocycline and FK506 on the nesting behavior of Syrian golden hamsters, we checked the nest quality of all experimental groups once weekly for 13 weeks until the appearance of clinical signs. Scoring of the nesting behavior was as previously described [30], with minor modifications. Briefly, shreddings of sheets of paper of various colors were placed in the animal cage and after 24 hours the nest quality and the presence of shredded paper in or around the nest was checked. The results showed that in the first 2 months of the study, there was no significant difference in the nesting behavior among any of the experimental groups, except the prion–vehicle group (group A) and no prion-vehicle group (group E; Fig. 1a, b). However, during the last 2 weeks of the third month, the nesting behavior of the prion–vehicle (group A) and prion–FK506 (group B) group was dramatically reduced as the disease progressed, while the prion–minocycline group (group D) had intact nesting behavior throughout the 13-week trial period and beyond (Fig. 1a, b). To investigate the locomotor and novel object exploring behavior, each animal was individually placed into an open-field test arena and observed for 5 min. Our results showed that there was no significant difference between groups over the first 2 months postinfection. There was no significant difference in moving and inactive times during the third month of testing period, while a significant difference was observed in rearing and novel object exploring times in the prion–minocycline (group D) and prion–FK506 group (group B; Fig. 1c; Videos S1–S8). To further investigate locomotor function, we measured the total distance covered by individual animals during the 5-min test period. We found a significant difference in total distance covered between the prion–minocycline group (group D) and prion–FK506 group (group B; Fig. 1d). The life expectancy measurement is a reliable indicator of the efficacy of any therapeutic drug. Our results showed that the prion-minocycline group (group D) had significantly prolonged survival compared with all other prion-infected groups, including prion–FK506 early treatment at 65 days (group B) or prion–FK506 late treatment started at 85 days postinfection (group C; Fig. 1e). We further confirmed that prion–FK506 late treatment was more effective than prion–FK506 early treatment (Fig. 1e, f). The health status of an animal can be judged by assessing daily weight gain and feed and water consumption. Our results showed that the weight of animals in all experimental groups increased until week 9, followed by a decline after week 10 postinfection (Fig. S1A). A more pronounced weight loss was observed in the prion–FK506 group (group B), followed by the prion–vehicle group (group A) and prion–minocycline group (group D) respectively (Fig. S1A). We further confirmed that the loss of animal body weight was directly proportional to feed and water consumption, which underwent a sudden decline after week 10 of infection except in the no prion-vehicle group (group E), which consumed amount of food and water proportionate with recorded increase in animals weight (Fig. S1B, C).
Minocycline-enhanced nesting behavior, locomotor function, novel object finding, and prolonged survival in prion infected hamsters. (a) Representative pictures of the nesting behavior observed in prion-infected and noninfected groups; top panel shows the nesting plan, where colored shredded paper was placed in top portion of the cage, while the bottom four images show the nesting behavior when the shredded paper was moved to or near the nest after overnight exposure. (b) Nesting score based on nest quality and movement of shredded paper from its initial location. The graph represents the data of 3 animals per group. Data were analyzed by 2-way analysis of variance (ANOVA) with Bonferroni’s post-test (***p < 0.001). (c) Graph shows moving, inactive, rearing activity, and novel object exploration duration in experimental groups relative to 5-min test time; number of animals tested per group was 5. Data were analyzed by 2-way ANOVA with Bonferroni’s post-test (***p < 0.001). (D) Total distance covered relative to 5-min test time in experimental groups; number of animal tested per group was 5. Data were analyzed using 1-way ANOVA with Tukey’s multiple comparison post-test (***p < 0.0001). (E) Survival analysis in different groups using the log-rank (Mantel–Cox test); each group comprised 12 animals. (F) Graph showing the infective period in all the groups. The number of animals per group used for statistical analysis was 12 and the data were analyzed by 1-way ANOVA test with Tukey’s multiple comparison post-test (***p < 0.0001). ns = nonsignificant
Minocycline Partially Reduced the Activation of CaN in Prion-Infected Hamsters
Accumulation of misfolded proteins in the endoplasmic reticulum (ER), leading to dysregulated calcium homeostasis is observed in all protein misfolding neurodegenerative diseases [37, 38]. To investigate whether hyperactivation of CaN is dependent on the level of PrPSc in the brain homogenates obtained from the different group of animals, brain homogenates were treated with Proteinase K (PK) (50 μg/ml for 1 h at 37 °C) and evaluated by Western blot. We found no difference in the level of PrPSc in any of the infected groups of hamsters (Fig. 2a). These data suggest that minocycline and FK506 act downstream to PrPSc aggregation and have no effect on misfolding of prion. Cytoplasmic calcium concentrations modulate CaN activity after the accumulation of misfolded proteins [3]. We checked CaN activity at 2 different time points in our experimental model of prion disease, on day 85 postinfection, just before the appearance of first visible clinical signs, and on day 125 of infection. Our results showed that CaN activity was significantly elevated in the prion-vehicle group (group A) compared with other groups (Fig. 2b), and that CaN levels further increased with the progression of disease. Notably, there was no increase in CaN activity in the prion–minocycline group (group D), suggesting partial reduction in misfolded PrPSc and reduced neuroinflammation in this group of animals (Fig. 2b). Our Western blot results detecting the catalytic subunit of CaN, CaN A, as well as the regulatory subunit of CaN, CaN B, were consistent with the CaN assay results, showing hyperactivated CaN in the prion–vehicle group (group A) compared with the rest of the groups (Fig. 2c–f).
Minocycline (Mino/Minocyc) partially reduced the activation of calcineurin (CaN) in prion-infected hamsters. (a) Representative blots of 2 animals per group PK– or PK+. Eight animals from each group were used for Western blot analysis. (b) CaN activity in response to the accumulation of misfolded proteins: day 85 and day 125 results of all groups; number of animals tested per group was 3 for each experiment. Data were analyzed by 2-way analysis of variance (ANOVA) with Bonferroni’s post-test (***p < 0.001). (C) Representative blots of 2 animals per group for the regulatory unit of CaN expression. (d) Statistical analysis of CaN B expression in brain homogenates from different groups; number of animals used for statistical analysis per group was 5; and the data were analyzed by 1-way ANOVA with Tukey’s multiple comparison post-test (***p < 0.0001). (e) Representative blots from 2 animals per group for the catalytic unit of CaN expression. (f) Statistical analysis of CaN A expression in brain homogenates from different groups; number of animals used for statistical analysis per group was 5; and the data were analyzed by 1-way ANOVA with Tukey’s multiple comparison post-test (***p < 0.0001)
Minocycline Efficiently Reduced Astroglyosis in Prion-Infected Hamsters
To assess the effect of minocycline on prion-induced neurodegeneration, formalin-fixed, paraffin-embedded brain sections from 5 animals per group were stained and analyzed. Spongiform degeneration, brain vacuolation, and astroglyosis are hallmarks of prion diseases [39]. Accordingly, the medulla, cerebellum, thalamus, and cortex were analyzed for astrocytes and microglial activation. There was a highly significant difference in astrocytes activation in prion-infected groups compared with noninfected groups (Fig. 3a, b). The prion–vehicle group (group A) showed the most astrocytes activation in all 4 observed regions of brain, while cerebellum, thalamus, and cortex of prion–minocycline (group D) and prion–FK506 late (group C) had less activated astrocytes than prion–vehicle (group A) and prion–FK506 early (group C) treatment (Fig. 3a, b), suggesting that although both drugs reduced number of astrocytes in the prion-infected animals, the reduction was partial compared with animals in the no prion–vehicle group (group E) that had almost no activated astrocytes.
Minocycline efficiently reduced astroglyosis in prion-infected hamsters. (a) Representative immunohistochemistry pictures of medulla, cerebellum, thalamus, and cortex stained with glial fibrillary acidic protein antibody for activated astrocytes. (b) Graphical presentation of activated astrocytes in medulla, cerebellum, thalamus, and cortex of 5 animals per group; data were analyzed by 2-way analysis of variance (ANOVA) with Bonferroni’s post-test (***p < 0.001). (c) Representative immunohistochemistry pictures of medulla, cerebellum, thalamus, and cortex stained with ionized calcium-binding adapter molecule 1 antibody for activated microglia. (d) The graphical presentation of activated microglia in medulla, cerebellum, thalamus, and cortex of 5 animals per group. Data were analyzed by 2-way ANOVA with Bonferroni’s post-test (***p < 0.001)
Microglia, the resident immune cells of the CNS, play an important role in CNS homeostasis during development, adulthood, and aging. Microglial function is tightly regulated by the CNS microenvironment, and increasing evidence suggests that disturbances, such as neurodegeneration and aging, can have profound consequences for microglial phenotype and function [40]. We observed in all 4 brain regions that microglial activation was significantly reduced in the prion–minocycline group (group D)and prion–FK506 late group (group C) as compared with the prion–vehicle group (group A) and the prion–FK506 early group (group B) (Fig. 3c, d), suggesting an effect of drug treatment on prion-driven microglial activation.
Minocycline Rescues Prion-Infected Hamsters From Spongiform Degeneration
Spongiform degeneration and brain vacuolations are the hallmarks of prion infection [39]. We observed vacuolations mostly in medulla and cerebellum of prion-infected animals (Fig. 4a, b). Our results showed a significantly higher number of vacuoles in the medulla of the prion–vehicle group (group A) and, to a minor extent, in the prion–FK506 late treated group (group C), whereas the prion–FK506 early (group B) and prion–minocycline (group D) groups had significantly less vacuolation (Fig. 4a). The number of vacuoles in cerebellum was also higher in the prion–vehicle (group A) and prion–FK506 late (group C) groups, but noticeably less in the prion–FK506 early (group B) and the prion–minocycline group (group D) animals. Less vacuolation was observed in thalamus of the prion–minocycline group (group A), with no significant difference in vacuolation profiles of cortex in all infected hamsters (Fig. 4a, b). These data suggest that minocycline and FK506 treatment administered during the presymptomatic stage of prion disease significantly reduce brain vacuolation compared with FK506 treatment started after the insurgence of the clinical symptoms of the disease.
Minocycline rescues prion-infected hamsters from spongiform degeneration. (a) Representative hematoxylin and eosin stained pictures from different regions of brain for estimating vacuolation profile in different experimental groups. (b) Graphical presentation of the vacuolation profile in medulla, cerebellum, thalamus, and cortex from 5 animals analyzed in each set. Data represent the average ± SE of the extent of vacuolation. Data were analyzed by 2-way analysis of variance with Bonferroni’s post-test (***p < 0.001). ns = nonsignificant
Minocycline Rescues Prion-Infected Hamsters From Synaptic Dysfunction and Neurodegeneration
Excessive activation of CaN in neurons results in early, reversible impairment and late, irreversible cell damage. Consequently, reversal of neuronal injury is only possible in the early stages of disease. If treatment is delayed, proapoptotic Bcl-2 family proteins are activated, leading to apoptosis and neurodegeneration [3, 4]. Sustained long-term ER stress leads to early neurodegenerative anomalies such as synaptic dysfunction and hampered axonal transport, which are followed by neuronal death [41, 42]. To study the number of CNS neurons in our experimental conditions, we fixed 5 brains from each group and immunohistochemical analysis was performed using the well-established neuronal marker NeuN [36]. Our results in the thalamus region showed a significantly higher number of neurons in prion–minocycline group (group D) and prion-FK506 late group (group C) as compared with other infected groups (Fig. 5a). Indeed, hamsters in the prion–minocycline group (group D), and prion–FK506 late treatment group (group C) had almost double the number of neurons as compared with prion–vehicle group (group A) animals (Fig. 5a, b), suggesting that treatment with minocycline in the presymptomatic stage or with FK506 after the appearance of first visible signs of prion infection is more effective than FK506 given early, before the appearance of clinical symptoms. Although our neuron analysis shows a significantly higher number of neurons in the prion–minocycline group (group D) and prion–FK506 late group (group C); however, the number of neurons was significantly less than in the no prion–vehicle group (group E) animals, suggesting partial protection with minocycline and FK506 treatment (Fig. 5b).
Minocycline (Mino) rescues prion-infected hamsters from synaptic dysfunction and neurodegeneration. (a) Representative immunohistochemistry pictures of thalamus stained with NeuN antibody to visualize living neurons. (b) Graphical representation of number of neurons per mm2 in thalamus of 5 animals per group. Data were analyzed by 1-way analysis of variance (ANOVA) with Tukey’s multiple comparison post-test (***p < 0.0001). (c) Representative Fluoro Jade-C-stained pictures of degenerating neurons in thalamus of different experimental groups. (d) Graphical representation of the number of degenerating neurons per mm2 in thalamus of 5 animals per group. Data were analyzed by 1-way ANOVA with Tukey’s multiple comparison post-test (***p < 0.0001). (e) Representative Western blots of 2 animals each from every group for protein expression of postsynaptic density protein (PSD)-95 and synaptophysin levels in comparison to beta tubulin. (f) Data showing the levels of PSD-95 and synaptophysin in brain homogenates of 5 animals each per group. Data were analyzed by 2-way ANOVA with Bonferroni’s post-test (***p < 0.001). ns = nonsignificant
In order to further characterize neuronal damage in our experimental groups, we used a well-established method to stain the brain sections with Fluoro-Jade, which specifically stains degenerating neurons [35]. In the thalamus, we observed a significantly lower number of Fluoro-Jade-positive cells in the prion–minocycline (group D) and prion-FK506 late (group C) groups compared with other infected groups (Fig. 5c, d). However, the efficacy of the treatments was moderate as the number of Fluoro-Jade-positive cells remained significantly higher in all of the prion-infected groups compared with the noninfected vehicle group (group E; Fig. 5d).
To evaluate the effect of our treatments on synaptic dysfunction we carried out Western blot analysis for the synaptic proteins postsynaptic density protein-95 and synaptophysin in the brain homogenates. Our results suggest a significant protection of synaptic proteins with minocycline treatment followed by FK506 treatment in prion-infected hamsters (Fig. 5e, f).
Minocycline Modulates the Caspase-Dependent MAPK Pathway
While the exact molecular mechanisms leading to neurodegeneration in protein misfolding disorders, including prion disease, are still unclear, compelling evidence suggests that 1 event that appears to be common to all neurodegenerative disorders is early neuroinflammation that contributes towards later neurodegeneration [7, 13, 43]. Stimulated microglia release several proinflammatory and neurotoxic molecules, including tumor necrosis factor (TNF)-α, IL-1β, IL-6, nitric oxide, eicosanoids, proteinases, and reactive oxygen species [44, 45]. To assess the effect of minocycline treatment on neuroinflammation, we determined the levels of IL-1β and TNF-α in brain homogenates of prion-infected hamsters. We observed a significant increase in IL-1β and TNF-α levels in infected hamsters compared with noninfected animals; however, prion–minocycline animals (group D) had significantly lower IL-1β levels and TNF-α levels compared with prion–FK506 early (group B) and prion–FK506 late animals (group C; Fig. 6a). Notably, IL-1β levels in the prion–minocycline group (group D) were not significantly different from those of the uninfected control animals, whereas TNF-α levels were only slightly, albeit significantly increased (Fig. 6b). These data suggest that minocycline effectively reduced proinflammatory cytokines levels in the brain of prion-infected hamsters compared with FK506 treatment.
Minocycline (Mino) modulates the caspase-dependent mitogen-activated protein kinase (MAPK) pathway. (a) Level of interleukin (IL)-1β in brain homogenates of 3 animals per group detected using enzyme-linked immunosorbent assay (ELISA). Data were analyzed by 1-way analysis of variance (ANOVA) with Tukey’s multiple comparison post-test (***p < 0.0001). (b) Level of tumor necrosis factor (TNF)-α in brain homogenates of 3 animals per group detected using ELISA. Data were analyzed by 1-way ANOVA with Tukey’s multiple comparison post-test (***p < 0.0001). (c) Representative Western blots of 2 animals each from every group for protein expression of MAPK phosphorylated p38 level and total MAPK p38 level in comparison with β-tubulin (third panel). (d) Expression level of MAPK phosphorylated p38 in brain homogenates of 5 animals each per group. Data were analyzed by 1-way ANOVA test with Tukey’s multiple comparison post-test (***p < 0.0001). (e) Representative Western blots of 2 animals per group for caspase-12, caspase-9, and caspase-3. (f) Levels of activated caspase-12, caspase-9, and caspase-3 in brain homogenates of 5 animals each per group. Data were analyzed by 2-way ANOVA with Bonferroni’s post-test (***p < 0.001). ns = nonsignificant
Activated microglial cells control the release of proinflammatory mediators through activation of the MAPK signaling pathways [46]. To gain a better insight into the molecular mechanisms mediating the beneficial effects of minocycline and FK506, we therefore determined the level of total MAPK p38 and MAPK phosphorylated p38 in brain homogenates from our experimental groups. Our results showed a significant difference in phosphorylated MAPK p38 level between prion–vehicle (group A) and prion treatment groups (groups B–D); furthermore, animals in the prion–FK506 early treatment group (group B) had significantly higher expression of phosphorylated MAPK p38 compared with those in the prion–FK506 group (group C), whereas animals in the prion–minocycline group (group D) and the no prion–vehicle group (group A) showed further reduced expression of phosphorylated MAPK p38 (Fig. 6c, d), suggesting that treatment with minocycline in the presymptomatic stage and with FK506 in the symptomatic stage of the prion infection were more effective than FK506 treatment administered during the presymptomatic stage of prion disease.
Compelling evidence suggests that aggregation of misfolded/unfolded proteins leads to ER stress and a subsequent increase in cytoplasmic Ca2+ followed by Ca2+ uptake by mitochondria, resulting in cytosolic cyctochrome C release, triggering activation of caspases, and resulting in apoptotic cell death [47–50]. To investigate whether phosphorylated MAPK p38 triggers caspase-mediated apoptotic cell death in response to prion infection and the effect of minocycline and FK506, we determined caspase-12, caspase-9, and caspase-3 levels in the brain homogenates from all of the experimental groups. We observed significantly higher levels of caspase-12, caspase-9, and caspase-3 activation in the prion–vehicle group (group A) compared with all other groups of animals (Fig. 6e, f). These data suggest that a caspase-mediated phosphorylated MAPK p38 pathway mediates neurodegeneration induced by prion, and this phenomenon was significantly reduced by minocycline and FK506 treatment.
Minocycline Effectively Leads to Reduced NF-κB p65 Nuclear Translocation
MAPK phosphorylation result in translocation of NF-κB from cytoplasm to nucleus. NF-κB transcription factors are present in the cytosol in an inactive state complexed with inhibitory kappa B proteins. MAPK phosphorylation leads to phosphorylative activation of inhibitory kappa B and its subsequent proteosome-mediated degradation, resulting in the release and nuclear translocation of active NF-κB [44, 51]. To determine the nuclear translocation of NF-κB we prepared cyctoplasmic and nuclear extract from brain homogenates of all experimental groups and subjected them to Western blot analysis. We found increased NF-κB levels in the nuclear fraction of prion–vehicle group (group A) compared with all of the other groups of animals (Fig. 7a, b). Furthermore prion–minocycline (group D) and prion-FK506 (groups B and C) animals had almost twice the levels of NF-κB in the cyctoplasm than prion–vehicle animals (group A), similar to the levels found in the no prion–vehicle group (group E). These results indicate that minocycline and FK506 significantly reduced activation of NF-κB, and its subsequent nuclear translocation. Similar results were obtained when nuclear translocation of NF-κB was determined by confocal microscopy in formalin-fixed brain sections (Fig. 7c), which showed a significant accumulation of NF-κB around 4,6-diamidino-phenylindole-stained nuclei in prion–vehicle group animals (group A), and to a lesser extent in prion–FK506 early (group B) and prion–FK506 late (group C) group animals. Furthermore the prion–minocycline group (group D) had dispersed NF-κB in the cytoplasm, similar to that observed in no prion–vehicle group animals (group E; Fig. 7c), indicating a beneficial effect of minocycline in preventing prion-driven NF-κB nuclear translocation.
Minocycline (Mino) effectively leads to reduced nuclear factor kappa B (NF-κB) p65 nuclear translocation. (a) Representative Western blot of 2 animals from every group for the expression of NF-κB p65 in cytoplasmic extracts (upper panel) in comparison with β-actin levels and nuclear extracts (lower panel) in comparison with nucleophosmin levels. (b) The data showing NF-κB p65 expression levels in cytoplasm and nucleus of different experimental groups are based on 5 animals per group. Data were analyzed by 2-way analysis of variance with Bonferroni’s post-test (***p < 0.001). (c) Representative pictures of confocal microscopy for nuclear translocation of NF-κB in different groups, First panel stained for nucleus with 4,6’-diamidino-phenylindole (DAPI; blue), second panel stained for NF-κB p65 (red), third panel is a merger of first and second panel, and the last panel shows the high magnification of the merged image. NPM = Nucleophosmin
Minocycline Increases Cognition and Survival via CREB and BAD Phosphorylation
BAD is a proapoptotic member of the Bcl2 family of proteins known to modulate apoptosis. Dephosphorylation of BAD leads to its dissociation from scaffolding 14-3-3 proteins and subsequent interaction of BAD with and inhibition of the mitochondrial membrane-associated protein Bcl-xL or other Bcl2 family proteins, which results in the release of cytochrome c from the mitochondria, caspase activation, and neuronal apoptosis. CREB is a transcription factor that plays a critical role in synaptic function and memory formation. Inhibition of CREB translocation into the nucleus suppresses gene expression and induces synaptic dysfunction and memory loss [52–54]. To investigate the effect of minocycline on phosphorylation of BAD and CREB, 3 animals from each group were sacrificed at the time when hamsters injected with prion alone reached stage 5 of the clinical phase of the disease. The brain was collected and 1 hemisphere was kept frozen for later protein extractions and Western blotting while the other half was formalin fixed for histological analysis. Western blot analysis of 2 representative animals per group is depicted in Fig. 7, and showed that there is a significant difference in the levels of pBAD and pCREB in the prion–vehicle group (group A) that is partially alleviated by treatment with minocycline or FK506, with minocycline being more effective than FK506 (Fig. 8a–d).
Minocycline (Mino) increases cognition and survival via cyclic adenosine monophosphate response element-binding (CREB) and Bcl-associated death promoter (BAD) phosphorylation. (a) Representative Western blot panel of 2 animals from each group for total CREB and phosphorylated CREB levels. (b) Representative Western blot panel of 2 animals from each group for total BAD and phosphorylated BAD levels. (c) Protein expression level of phosphorylated CREB in different groups based on 5 animals data per group. Data were analyzed by 1-way analysis of variance (ANOVA) with Tukey’s multiple comparison post-test (***p < 0.0001). (d) Protein expression level of phosphorylated BAD in different groups based on data from 5 animals per group. Data were analyzed by 1-way ANOVA test with Tukey’s multiple comparison post-test (***p < 0.0001). (e) Representative pictures of immunohistochemical analysis for the expression of lamin A/C protein in different groups. (f) Protein expression levels of lamin A/C for apoptosis analysis in different experimental groups based on 5 animals per group. Data were analyzed by 1-way ANOVA with Tukey’s multiple comparison post-test (***p < 0.0001)
Further investigation of cell degeneration was conducted by determining lamin A/C immunohistochemical staining in brain sections. A significantly higher number of lamin A/C-positive cells were observed in prion–FK506 late treatment animals (group C) compared with other infected groups. Animals in the prion–vehicle group (group A) had the fewest lamin A/C-positive cells, almost half of the positive cells seen in the prion–FK506 early (group B) and prion–minocycline group (group C) animals (Fig. 8e, f) The no prion–vehicle group (group E) had the highest number of lamin A/C-positive cells, showing effective, albeit incomplete, protection afforded by all treatments.
Discussion
TSEs are a group of terminal neurodegenerative disorders characterized by progressive deterioration and motor paralysis, leading to complete disability in affected humans and animals [3]. Currently, there is no effective treatment for prion diseases, despite the extensive research effort that has been devoted to this disorder over the last several years [3, 7]. The accumulation of misfolded β-sheet-rich PrPSc in the ER, leading to ER stress, is the central event in all TSEs. Sustained long-term ER stress leads to early neurodegenerative anomalies such as synaptic dysfunction, hampered axonal transport, neuroinflammation, followed by neuronal degeneration and, ultimately, apoptosis. The continuous conversion of nontoxic α-sheet-rich cellular prion (PrPc) to highly neurotoxic β-sheet-rich scrapie prion (PrPSc) makes it difficult to develop an effective therapy for prion diseases. Accordingly, there is ample consensus that the most effective therapeutic strategy would be a combinatory approach based on stopping the conversion of cellular PrPc into the neurotoxic PrPSc, on the one hand, while rescuing neurons from early synaptic dysfunction and neuroinflammation to later neurodegeneration and apoptosis on the other.
While several compounds have shown efficacy in reducing PrPSc levels in in vitro models and increasing survival in in vivo models of prion diseases [55], no effective treatment is yet available for prion diseases, partly owing to the fact that many of these compounds either cannot cross the blood–brain barrier or cause severe toxic effects [56]. Our treatment approach was based on targeting either misfolded prion proteins or their downstream signaling to prevent early synaptic dysfunction, neuroinflammation, and neurodegeneration, both in the presymptomatic and symptomatic phases of prion disease. Our data showed that accumulation of misfolded PrPSc leads to ER stress-mediated alterations in calcium homeostasis and hyperactivation of CaN, a Ca2+/calmodulin-dependent serine/threonine protein phosphatase that has been implicated in T-cell activation through the induction of NFAT [8, 21, 57]. These data thus suggest that downregulation of CaN activity might be a promising therapeutic target for prion diseases. This possibility is further encouraged by the fact that effective and well-characterized CaN inhibitors such as tacrolimus (FK506) or ciclosporin are available and approved for clinical use in humans [1, 58].
Neuroinflammation is an important target for early therapeutic intervention in prion diseases. Minocycline, a tetracycline derivative, has potent anti-inflammatory, antiapoptotic, and neuroprotective properties [59]. Minocycline was chosen here not only because of its safety and blood–brain barrier crossing potential, but also for its reported plaque-reducing effects in AD and atherosclerotic models [60, 61].
Our results showed a significantly beneficial effect of minocycline on prion-driven behavioral abnormalities compared with FK506 treatment. We observed intact nesting behavior for the first 2 months postinfection in all experimental groups, except the prion–vehicle group (group A) that showed impaired nesting behavior by the second month compared with the no prion–vehicle animals (group E). By the third month, the impairment in nesting behavior of prion–vehicle animals (group A) was further exacerbated and was completely rescued by minocycline treatment (Fig. 1b). We observed that nesting behavior was directly proportional to the extent of disease progression and as the prion–vehicle (group A) and prion–FK506 early (group B) group animals did not show prolonged survival, their nesting behavior declined quickly compared with the prion–minocycline group. Other behavioral tests, including motor activity, rearing, novel object finding, exploring time, and average distance covered in an open-field test also showed significant differences between the minocycline and FK506-treated animals in that minocycline counteracted the behavioral deficits induced by prion infection, whereas FK506 was ineffective (Fig. 1c, d). Our results of better cognition and memory in animals in the prion–minocycline group (group D) are in accordance with previous reports [12, 14, 16, 62]. Interestingly, our data showed that reduced CaN activity in the FK506 early or late treatment groups (groups B and C, respectively) and minocycline group (group D) were independent of PrPSc levels in prion-infected hamsters, as illustrated by the fact that accumulation of the misfolded protein in the brain of prion-vehicle group (group A) animals was similar to those in the prion–minocycline (group D) and prion–FK506 groups (groups B and C). De Luigi et al. [29] have previously shown the beneficial effects of tetracycline, doxycycline and minocycline in hamsters infected with the 263 K strain of prion, either intramuscularly or subcutaneously. A single intramuscular dose of doxycycline 1 h after infection in the same site of inoculation prolonged median survival by 64%. Intraperitoneal doses of tetracyclines every 2 days for 40 or 44 days increased survival time by 25% (doxycycline), 32% (tetracycline), and 81% (minocycline) after intramuscular infection, and 35% (doxycycline) after subcutaneous infection. Our results of prolonged survival are consistent with the work of De Luigi et al. [29]: we found better results in infection of 263 K strain and subsequent treatment via the i.p. route. Overall, our findings on minocycline-treated animals are also consistent with previous work by Noble et al. [59] in AD models, and by Shahzad et al. [63] in atherosclerosis models, whereas our findings in FK506-treated animals are consistent with the work by Mukherjee et al. [3] in a prion-infected mice model where they showed no change in PrPSc level after FK506 treatment.
Inflammation is a common event in several neurodegenerative diseases and has been recently implicated as a key mechanism responsible for the progressive nature of these disorders. As such, controlling inflammation is regarded as a possible effective therapeutic approach [13, 28]. Consistent with previous reports [3, 28], our data showed a significant increase in astrocyte activation in prion–vehicle animals (group A) compared with the control group (group E) and further reveal that this phenomenon can be prevented by minocycline or FK506 treatment. Microglia, the brain innate immune cells, are activated in response to the accumulation of misfolded proteins resulting in neuroinflammation and subsequent neurodegeneration [13]. Our results showed a significant suppression of microglial activation in both the prion–minocycline (group D) and prion–FK506 late (group C) groups, indicating a beneficial effects of both drugs. These results are in accordance with the previous works showing anti-inflammatory functions of minocycline and FK506 in unrelated in vitro and in vivo models [13, 64–68].
The neuroprotective effect of a test treatment is ultimately assessed either by direct measurement of the number of living neurons or indirectly by measuring the number of degenerating neurons. Our results showed that hamsters in the prion–minocycline group (group D) and the prion–FK506 late group (group C) had more living neurons and fewer degenerating neurons in their CNS than both prion–vehicle and prion–FK506 early treatment groups (groups A and B, respectively). The observed neuroprotective effect of FK506 late treatment is consistent with previous work describing effective protection of neurons by FK506 after the appearance of clinical signs in prion-infected mice [1, 3]. We observed a progressive decline in body weight after 3 weeks of treatment in the prion–FK506 early treatment animal group (group B), even when half of the recommended dose was used. These finding suggests possible cytotoxicity due to prolonged treatment of FK506 in young animals [63, 69, 70]. The protective role of minocycline on prion-induced neurological dysfunction observed in our study is novel. Further, minocycline appears to be safe in comparison with FK506 as it did not affect body weight in treated animals. However, the survival of animals receiving minocycline after the appearance of clinical signs did not differ from that of animals in the prion–vehicle and prion-FK506 early groups (groups A and B, respectively), suggesting that late treatment with minocycline may be ineffective (data not shown). These findings suggest that the mechanisms of protection from prion with minocycline may involve reducing early neuroinflammation and subsequent neurodegeneration, which would be consistent with previous literature evidence [28, 64, 71, 72].
Recent studies have indicated a key role of the MAPK pathway in neuroinflammation and axonal degeneration [65, 73]. Microglial activation leads to an increase of proinflammatory cytokines such as IL-1β and TNF-α via the MAPK cascade [65, 74]. Our results showed higher levels of IL-1β and TNF-α in the prion–vehicle group (group A) than in the minocycline and FK506-treated groups. MAPK p38 expression was also elevated in the prion–vehicle group (group A) compared with the other treatment groups. These data suggest that minocycline and FK506 both reduce neuroinflammation via the MAPK pathway, which is also in line with previous reports [43, 65, 66, 75]. Minocycline treatment was comparatively better then FK506 treatment in reducing neuroinflammation and this could be one possible reason for prolonged survival in the prion–minocycline group (group D). Caspase-mediated apoptotic signaling has been well documented in prion and other neurodegenerative diseases [47, 48, 50, 76]. Our data showed significantly elevated caspase-12, caspase-9, and caspase-3 levels in the prion–vehicle group (group A) compared with the minocycline (group D) and FK506-treated groups (groups B and C). While these data confirmed the occurrence of a caspase-mediated apoptotic cascade in prion diseases, they also showed that this neurodegenerative phenomenon is effectively blocked by the minocycline and FK506 treatments. These results are in accordance with previous work in other systems reporting caspase inhibition by FK506 and minocycline [77–81].
Activation of proinflammatory mediators by the transcription factor NF-κB is a primary event in inflammation [4, 43]. Here we showed by Western blot analysis of representative cytoplasmic and nuclear extracts obtained from brain homogenates that animals in the prion–vehicle group (group A) had a significantly higher amount of NF-κB in nuclei compared with the minocycline- (group D) and FK506-treated groups (groups B and C; Fig. 7b). Further analysis of NF-κB nuclear localization using confocal microscopy showed a significantly higher amount of NF-κB protein clustering around nuclei in the prion–vehicle group (group A), and to a lesser extent in the prion–FK506 early (group B) and prion–FK506 late (group C) groups. However, animals in the prion–minocycline group (group D) showed the least amount of nuclear translocation of NF-κB (Fig. 7c), which is consistent with previous reports showing reduced nuclear translocation of NF-κB induced by minocycline and FK506 [8, 65, 82].
When we determined the effect of minocycline and FK506 treatment on known downstream targets of hyperactivated CaN, such as pCREB and pBAD, we found that the decrease in pCREB and pBAD induced by prion was effectively prevented by both minocycline and FK506, with the treatment with FK506 administered after the appearance of the clinical signs being more effective than FK506 given during the preclinical phase of prion infection (Fig. 8c, d). The neuroprotective effect of minocycline and FK506 was further confirmed by immunohistochemical analysis of apoptotic cells using lamin A/C staining. We observed a higher number of surviving neural cells in animals treated with FK506 and minocycline compared with those in the prion-vehicle group (group A). These results are also in accordance with previously published work showing protection of minocycline in AD and stroke animal models and of FK506 in prion models [3, 83, 84].
In conclusion, we have shown here for the first time that minocycline, given during the presymptomatic phase of prion infection, effectively protects from all of the neurodegenerative events and behavioral deficits that are normally associated with the clinical manifestation of prion in hamsters. We further confirmed, in a hamster model of prion disease, the effectiveness of FK506 treatment given after the appearance of clinical signs. Taken together, our results suggest that both minocycline and FK506 should be considered for clinical development of a combinatory therapeutic strategy that effectively counteracts the detrimental outcomes of prion infections during both the preclinical and clinical phases of the disease.
Change history
10 August 2020
The Editor-in-Chief has retracted this article [1] because of significant concerns regarding a number of figures presented in this work, which question the integrity of the data.
References
Nakagaki, Takehiro; Satoh., et al., FK506 reduces abnormal prion protein through the activation of autolysosomal degradation and prolongs survival in prion-infected mice. Autophagy, 2013. 9(9): p. 1386-94.
Puig Berta, Hermann C. Altmeppen, Sarah Ulbrich et al., Secretory pathway retention of mutant prion protein induces p38-MAPK activation and lethal disease in mice. Scientific Reports, 2016. 6(24970): p. 1-14.
Mukherjee Abhisek, Diego Morales-Scheihing, Dennisse Gonzalez-Romero ., et al., Calcineurin inhibition at the clinical phase of prion disease reduces neurodegeneration, improves behavioral alterations and increases animal survival. PLoS Pathog, 2010. 6(10): p. e1001138.
Mukherjee, A. and C. Soto, Role of calcineurin in neurodegeneration produced by misfolded proteins and endoplasmic reticulum stress. Curr Opin Cell Biol, 2011. 23(2): p. 223-30.
Song ZhiQi, Ting Zhu, Xiangmei Zhou et al., REST alleviates neurotoxic prion peptide-induced synaptic abnormalities, neurofibrillary degeneration and neuronal death partially via LRP6-mediated Wnt-β-catenin signaling. Oncotarget, 2016. 7(11): p. 12035-12052.
Stanley, Pruisiner., Prions. proc of Nat Acad of Sci, 1998. 95: p. 13363–13383.
Moreno Julie A. , Mark Halliday, Colin Molloy et al., Oral Treatment Targeting the Unfolded Protein Response Prevents Neurodegeneration and Clinical Disease in Prion-Infected Mice. Science Translational Medicine, 2013. 5(206): p. 206ra138,1-10.
Szeto Gregory L. , Joel L. Pomerantz, David R.M. Graham, Janice E. Clements, Minocycline suppresses activation of nuclear factor of activated T cells 1 (NFAT1) in human CD4+ T cells. J Biol Chem, 2011. 286(13): p. 11275-82.
Midtvedt K., Therapeutic Drug Monitoring of Cyclosporine. Transplantation Proceedings, 36 (Suppl 2S), 430S–433S (2004), 2004. 36(Suppl 2S): p. 430s-433s.
Brundula Veronika, N. Barry Rewcasle, Luanne M. Metz, Claude C. Bernard, V. Wee Yong, Targeting leuko- cyteMMPsand transmigration: minocycline as a potential therapy for multiple sclerosis. Brain, 2002. 125: p. 1297-1308.
Zhu Shan, Irina G. Stavrovskaya, Martin Drozda et al., Minocycline inhibits cytochrome c release and delays progression of amyotrophic lateral sclerosis in mice. Nature 2002. 417: p. 74-78.
Jiang Ying Jiang, Yingying Liu, Cansheng Zhu et al., Minocycline enhances hippocampal memory, neuroplasticity and synapse-associated proteins in aged C57 BL/6 mice. Neurobiol Learn Mem, 2015. 121: p. 20-9.
El-Shimy Ismail Amr , Olla Ahmad Heikal, Nabila Hamdi, Minocycline attenuates A oligomers-induced pro-inflammatory phenotype in primary microglia while enhancing A fibrils phagocytosis. Neuroscience Letters, 2015. 609: p. 36–41.
Ahmad Mohammad, Abdul Rahim Zakaria., Khalid M. Almutairi, Effectiveness of minocycline and FK506 alone and in combination on enhanced behavioral and biochemical recovery from spinal cord injury in rats. Pharmacology, Biochemistry and Behavior 2016. 145: p. 45-54.
Mishra Manoj Kumar, Aniraj Basu, Minocycline neuroprotects, reduces microglial activation, inhibits caspase 3 induction, and viral replication following Japanese encephalitis. J Neurochem, 2008. 105(5): p. 1582-95.
Choi Yoori, Hye-Sun Kim, Ki Young Shin et al., Minocycline Attenuates Neuronal Cell Death and Improves Cognitive Impairment in Alzheimer’s Disease Models. Neuropsychopharmacology, 2007. 32: p. 2393–2404.
Cardenas Maria E., R. Scot Muir, Tamara Breuder, Joseph Heitman, Targets of immunophilin-immunosuppressant complexes are distinct highly conserved regions of calcineurin A. The EMBO Journal, 1995. 14 (12): p. 2772-2783.
Wu Qiaoli, Guodong Liu, Lixia Xu et al., Repair of Neurological Function in Response to FK506 Through CaN/NFATc1 Pathway Following Traumatic Brain Injury in Rats. Neurochem Research, 2016: p. DOI 10.1007/s11064-016-1997-7.
Yousuf Seema, Fahim Atif, Varun Kesherwani, Sandeep Kumar Agrawal, Neuroprotective effects of Tacrolimus (FK-506) and Cyclosporin (CsA) in oxidative injury. Brain and Behavior, 2011. 1(2): p. 87-94.
Sharifi Zahra-Nadia , Farid Abolhassani, Mohammad Reza Zarrindast, ShabnamMovassaghi, Nasrin Rahimian, GholamrezaHassanzadeh, Effects of FK506 on Hippocampal CA1 Cells Following Transient Global Ischemia/Reperfusion in Wistar Rat. Stroke Research and Treatment, 2011. 2012(809417): p. 1-8.
Luo, J., Lixin Sun, Xian Lin et al., A calcineurin- and NFAT-dependent pathway is involved in alpha-synuclein-induced degeneration of midbrain dopaminergic neurons. Hum Mol Genet, 2014. 23(24): p. 6567-74.
Seonil Kima, Caroline J. Violette, Edward B. Ziff, Reduction of increased calcineurin activity rescues impaired homeostatic synaptic plasticity in presenilin 1 M146V mutant. Neurobiol Aging, 2015. 36(12): p. 3239-46.
Taglialatela, Giulio, Cristiana Rastellini, Luca. Cicalese, Reduced Incidence of Dementia in Solid Organ Transplant Patients Treated with Calcineurin Inhibitors. J Alzheimers Dis, 2015. 47(2): p. 329-33.
Xie Wu-Ling, Qi Shi, Jin Zhang et al., Abnormal activation of microglia accompanied with disrupted CX3CR1/CX3CL1 pathway in the brains of the hamsters infected with scrapie agent 263K. J Mol Neurosci, 2013. 51(3): p. 919-32.
Bolton David, C., Prion distribution in hamster lung and brain following intraperitoneal inoculation. Journal of General Virology, 1998. 79: p. 2557-2562.
Chen Baian, Claudio Soto, Rodrigo Morales, Peripherally administrated prions reach the brain at sub-infectious quantities in experimental hamsters. FEBS Letters, 2014. 588: p. 795-800.
Arisi Gabriel Maisonnave, Maira Licia Foresti, Andrés Montanez, Lee A. Shapiro, Minocycline Ameliorates Neuronal Loss after Pilocarpine-Induced Status epilepticus. Journal of Neurological Disorders & Stroke, 2014. 2(3)(1055): p. 1-8.
Cheng, Shanshan, Jinxing Hou, Chen Zhang et al., Minocycline reduces neuroinflammation but does not ameliorate neuron loss in a mouse model of neurodegeneration. Sci Rep, 2015. 5: p. 10535.
De Luigi Ada , Laura Colombo, Luisa Diomede et al., The efficacy of tetracyclines in peripheral and intracerebral prion infection. PLoS One, 2008. 3(3): p. e1888.
Schmitz Matthias , Saima Zafar, Christopher J Silva & Inga Zerr, Behavioral abnormalities in prion protein knockout mice and the potential relevance of PrPC for the cytoskeleton. Prion, 2014. 8(6): p. 381-386.
Tapia-Rojas Cheril , Carolina B. Lindsay, Carla Montecinos-Oliva et al., Is L-methionine a trigger factor for Alzheimer’s-like neurodegeneration?: Changes in Aβ oligomers, tau phosphorylation, synaptic proteins, Wnt signaling and behavioral impairment in wild-type mice. Molecular Neurodegeneration, 2015. 10(62): p. 1-17.
Inestrosa Nibaldo C. , Francisco J. Carvajala, Juan M. Zolezzi et al., Peroxisome Proliferators Reduce Spatial Memory Impairment, Synaptic Failure, and Neurodegeneration in Brains of a Double Transgenic Mice Model of Alzheimer’s Disease. Journal of Alzheimer’s Disease 2013. 33 p. 1–5.
Castilla Joaquın , Dennisse Gonzalez-Romero, Paula Saa , Rodrigo Morales, Jorge De Castro, Claudio Soto, Crossing the Species Barrier by PrPSc Replication In Vitro Generates Unique Infectious Prions. Cell 2008. 134: p. 757–768.
Deininger M. H., T. Weinschenk., R. Meyermann and H. J. Schluesener, The allograft inflammatory factor-1 in Creutzfeldt–Jakob disease brains. Neuropathology and Applied Neurobiology 2003. 29: p. 389–399.
Schmued Larry C. , Chris C. Stowersb, Andrew C. Scalleta, Lulu Xu, Fluoro-Jade C results in ultra high resolution and contrast labeling of degenerating neurons. Brain Research, 2005. 1035: p. 24-31.
Mullen Richard J. , Charles R. BuckK, Alan M. Smith, NeuN, a neuronal specific nuclear protein in vertebrates Development, 1992. 116: p. 201-211.
Soto, C., unfolding the role of protein misfolding in neurodegenerative diseases. Nature Reviews Neuroscience, 2003. 4: p. 49-60.
Freemana Oliver J. , Giovanna R. Mallucci Mallucci, The UPR and synaptic dysfunction in neurodegeneration. 2016.
Soto Claudio, Nikanj Satani, The intricate mechanisms of neurodegeneration in prion diseases. Trends Mol Med, 2011. 17(1): p. 14-24.
Perry V. Hugh & Jessica Teeling, Microglia and macrophages of the central nervous system: the contribution of microglia priming and systemic inflammation to chronic neurodegeneration. semin immunopathology, 2013. 35: p. 601–612.
Shah SZA, Deming Zhao., Sher Hayat Khan and Lifeng Yang, Unfolded Protein Response Pathways in Neurodegenerative Diseases. Journal of Molecular Neuroscience, 2015. 57: p. 529–537.
Moreno Julie A. , Helois Radford, Diego Peretti et al., Sustained translational repression by eIF2α-P mediates prion neurodegeneration. Nature, 2012. 485(7399): p. 507-511.
Sama, M.A., Diana M. Mathis, Jennifer L. Furman et al., Interleukin-1beta-dependent signaling between astrocytes and neurons depends critically on astrocytic calcineurin/NFAT activity. J Biol Chem, 2008. 283(32): p. 21953-64.
Santa-Cecilia Flavia V., Benjamin Socias, Mohand O. Ouidja et al., Doxycycline Suppresses Microglial Activation by Inhibiting the p38 MAPK and NF-kB Signaling Pathways. Neurotoxicity Research, 2016. 29: p. 447–459.
Henry Christopher J. , Yan Huang, Angela M. Wynne, Jonathan P. Godbout, Peripheral lipopolysaccharide (LPS) challenge promotes microglial hyperactivity in aged mice that is associated with exaggerated induction of both pro-inflammatory IL-1b and anti-inflammatory IL-10 cytokines. Brain Behav Immun, 2008. 23(3): p. 309–317.
Kang Seong-Mook , Sandeep Vasant Morea, Ju-Young Park et al., A novel synthetic HTB derivative, BECT inhibits lipopolysaccharide-mediated inflammatory response by suppressing the p38 MAPK/JNK and NF-κB activation pathways. Pharmacological Reports, 2014. 66: p. 471-479.
Ferreiro Elisabete , Rosa Resende, Rui Costa, Catarina R. Oliveira, and Cláudia M.F. Pereira, An endoplasmic-reticulum-specific apoptotic pathway is involved in prion and amyloid-beta peptides neurotoxicity. Neurobiology of Disease 2006. 23: p. 669-678.
Ferreiro Elisabete , Rui Costa, Sueli Marques, Sandra Morais Cardoso, Catarina R. Oliveira and Claudia M. F. Pereira, Involvement of mitochondria in endoplasmic reticulum stress-induced apoptotic cell death pathway triggered by the prion peptide PrP106–126. Journal of Neurochemistry, 2008b. 104 p. 766–776.
Ferreiro Elisabete , Catarina R. Oliveira, and Cláudia M.F. Pereira, The release of calcium from the endoplasmic reticulum induced by amyloid-beta and prion peptides activates the mitochondrial apoptotic pathway. Neurobiology of Disease 2008a. 30: p. 331-3423.
Szegezdi Eva, Una Fitgerald and Afshin Samali, Caspase-12 and ER stress mediated apoptosis. Ann. N.Y. Acad. Sci, 2003. 1010: p. 186-194.
Karin Michael and Yinon Ben-Neriah Ben-Neriah, phosphorylation meets ubiquitination: The Control of NF-jB Activity. Annual Review of Immunology, 2000. 18: p. 621-663.
Groth Rachel D. and Paul G. Mermelstein, Brain-Derived Neurotrophic Factor Activation of NFAT (Nuclear Factor of Activated T-Cells)-DependentTranscription: A Role for the Transcription Factor NFATc4 in Neurotrophin-Mediated Gene Expression. The Journal of Neuroscience, 2003. 23(22): p. 8125-8134.
Datta Sandeep Robert , Alex Katsov, Linda Hu et al., 14-3-3 Proteins and Survival Kinases Cooperate to Inactivate BAD by BH3 Domain Phosphorylation. Molecular Cell, 2000. 6: p. 41-51.
Wang Hong-Gang, Nuzhat Pathan, Iryna M. Ethell et al., Ca21-Induced Apoptosis Through Calcineurin Dephosphorylation of BAD. science, 1999. 284: p. 339-343.
Trevitt Clare R. and John Collinge., A systematic review of prion therapeutics in experimental models. brain, 2006. 129: p. 2241–2265.
Haïk Stéphane, Gabriella Marcon, Alain Mallet et al., Doxycycline in Creutzfeldt-Jakob disease: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet Neurology, 2014: p. 150-158.
Abdul Hafiz Muhammad, Michelle A. Sama, Jennifer L. Furman et al., Cognitive decline in Alzheimer's disease is associated with selective changes in calcineurin/NFAT signaling. J Neurosci, 2009. 29(41): p. 12957-69.
Sim Valerie L., Prion Disease: Chemotherapeutic Strategies. Infectious Disorders – Drug Targets, 2012. 12: p. 144-160.
Noble Wendy, Claire Garwood, John Stephenson, Anna M. Kinsey, Diane P. Hanger, Minocycline reduces the development of abnormal tau species in models of Alzheimer's disease. FASEB J. , 2008. 23: p. 739–750.
Noble Wendy, Claire J. Garwood and Diane P. Hanger, Minocycline as a potential therapeutic agent in neurodegenerative disorders characterised by protein misfolding. Prion, 2009. 3(2): p. 78-83.
Shahzad Khurrum , Madhusudhan Thati, Hongjie Wang et al., Minocycline reduces plaque size in diet induced atherosclerosis via p27Kip1. Atherosclerosis, 2011. 219 p. 74–83.
Fan, L. , Tian-Long Wang, Y.C. Xu, Y.H. Ma, W.G. Ye, Minocycline may be useful to prevent/treat postoperative cognitive decline in elderly patients. Med Hypotheses, 2011. 76(5): p. 733-6.
Garwood Claire J., Jonathan D. Cooper, Diane P. Hanger and Wendy Noble, Anti-Inflammatory Impact of Minocycline in a Mouse Model of Tauopathy. Frontiers in Psychiatry Neurodegeneration, 2010. 1(136): p. 1-10.
Cuenda Ana and Simon Rousseau, p38 MAP-Kinases pathway regulation, function and role in human diseases. Biochimica et Biophysica Acta 1773 (2007) 1358–1375
Perren Anke Van der , Francesca Macchi a, Jaan Toelen et al., FK506 reduces neuroinflammation and dopaminergic neurodegeneration in an a-synuclein-based rat model for Parkinson’s disease. Neurobiology of Aging, 2015. 36: p. 1559-1568.
Fields, J.A., Cassia Overk, Anthony Adame et al., Neuroprotective effects of the immunomodulatory drug FK506 in a model of HIV1-gp120 neurotoxicity. J Neuroinflammation, 2016. 13(1): p. 120.
Setkowicz Zuzanna, Maria Caryk, Milena Szafraniec, Anna Zmudzinska & Krzysztof Janeczko, Tacrolimus (FK506) and cyclosporin A reduce macrophage recruitment to the rat brain injured at perinatal and early postnatal periods. Neurol Res, 2009. 31(10): p. 1060-7.
Singh Lavleen , Geetika Singh, Alok Sharma, Aditi Sinha, Arvind Bagga, A.K. Dinda, A comparative study on renal biopsy before and after long-term calcineurin inhibitors therapy: an insight for pathogenesis of its toxicity☆. Human Pathology, 2014. 46: p. 34-39.
Rauch M.C, A. San Martín, D. Ojeda et al., Tacrolimus causes a blockage of protein secretion which reinforces its immunosuppressive activity and also explains some of its toxic side-effects. Transplant Immunology, 2009. 22: p. 72-81.
Saganova Kamila, Judita Orindacova, Igor Sulla Jr, Peter Filipcik, Dasa Cizkova, Ivo Vanicky, Effects of long-term FK506 administration on functional and histopathological outcome after spinal cord injury in adult rat. Cell Mol Neurobiol, 2009. 29(6-7): p. 1045-51.
Scholz, Rebecca, MarkusSobotka, Albert Caramoy,Thomas Stempfl, Christoph Moehle and Thomas Langmann, Minocycline counter-regulates proinflammatory microglia responses in the retina and protects from degeneration. Journal of Neuroinflammation, 2015. 12(209): p. 1-14.
Du Yansheng, Zhizhong Ma, Suizhen Lin, et al., Minocycline prevents nigrostriatal dopaminergic neurodegeneration in the MPTP model of Parkinson's disease. Proc Natl Acad Sci U S A, 2001. 98(25): p. 14669-74.
Yang Jing , Zhuhao Wu, Nicolas Renier et al., Pathological Axonal Death through a MAPK Cascade that Triggers a Local Energy Deficit. Cell Calcium, 2014. 160: p. 161–176.
Tikka Tiina , Bernd L. Fiebich, Gundars Goldsteins,Riitta Keinanen and Jari Koistinaho, Minocycline, a Tetracycline Derivative, Is Neuroprotective against Excitotoxicity by Inhibiting Activation and Proliferation of Microglia. The Journal of Neuroscience,, 2001. 21(8): p. 2580-2588.
Wang, N., X. MI, B. Gao et al., Minocycline inhibits brain inflammation and attenuates spontaneous recurrent seizures following pilocarpine-induced status epilepticus. Neuroscience, 2015. 287: p. 144-56.
Nakagawa Toshiyuki , Hong Zhu, Nobuhiro Morishima, Caspase-12 mediates endoplasmicreticulum-speci®c apoptosis and cytotoxicity by amyloid-b. Nature, 2012. 403(6): p. 98-103.
Grosskreutz Cynthia L, Virve A. Hanninen, Mina B. Pantcheva, Wei Huang, Nathaniel R. Poulin, Adam P. Dobberfuhl, FK506 blocks activation of the intrinsic caspase cascade after optic nerve crush. Experimental Eye Research 2004. 80: p. 681–686.
Nottingham Stephanie, Pamela Knapp and Joe Springer, FK506 Treatment Inhibits Caspase-3 Activation and Promotes Oligodendroglial Survival Following Traumatic Spinal Cord Injury. Experimental Neurology 2002. 177: p. 242–251.
Chen minghua, Victor O. Ona., Mingwei Li et al., Minocycline inhibits caspase-1 and caspase-3 expression and delays mortality in a transgenic mouse model of Huntington disease. Nature Medicine, 2000. 6: p. 797-801.
Heo Kyoung, Yang-Je Cho, Kyoung-Joo Cho et al., Minocycline inhibits caspase-dependent and -independent cell death pathways and is neuroprotective against hippocampal damage after treatment with kainic acid in mice. Neuroscience Letters 2006. 398: p. 195–200.
Festoff Barry W, Syed Ameenuddin, Paul M. Arnold, Andrea Wong, Karen S. Santacruz and Bruce A. Citron, Minocycline neuroprotects, reduces microgliosis, and inhibits caspase protease expression early after spinal cord injury. Journal of Neurochemistry, 2006. 97: p. 1314–1326.
Su Landi, Jing Ji, Jieyu Bian, Yuxuan Fu, Yingbin Ge, Zhilan Yuan, Tacrolimus (FK506) prevents early retinal neovascularization in streptozotocin-induced diabetic mice. International Immunopharmacology, 2012. 14: p. 606-612.
Zhao Yu , Ming Xiao., Wenbo He, Zhiyou Cai, Minocycline upregulates cyclic AMP response element binding protein and brain-derived neurotrophic factor in the hippocampus of cerebral ischemia rats and improves behavioral deficits. Neuropsychiatric Disease and Treatment, 2015. 11: p. 507-516.
Burgos-Ramos E, L. Puebla-Jiménezl and E. Arilla-Ferreiro, minocycline provides protection against -amyloid(25-35)-induced alterations of the somatostatin signaling pathway in the rat temporal cortex. Neuroscience, 2008. 154: p. 1458–1466.
Acknowledgments
We thank Professor Paul Barrow, Professor Suo Xun, Dr. Abhisek Mukherjee, Dr. Chaosi Li, Dr. Ruichao Yue, Dr Wei Yang, and Dr. Chunfa Liu for their valuable suggestions and critical reading of this manuscript. Shah S.Z.A, D. Zhao, and G. Taglialatela designed the experimental model. Shah S.Z.A. and S.H. Khan performed all the major experiments. T. Hussain, H. Dong, and M. Lai helped in performing the experiments and purchase of reagents. G. Taglialatela, X. Zhou, and L. Yang critically reviewed the manuscript before final submission. The authors declare that they have no competing conflict of interests. This work was supported by the Natural Science Foundation of China (Project No. 31172293), Ministry of Agriculture of China, 948 projects (2014-S9), The Foundation of Chinese Ministry of Science and Technology (Project No. 2015BAI07B02), and Chinese Universities Scientific Fund (Project No. 15055331).
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Author information
Authors and Affiliations
Corresponding author
Additional information
Syed Zahid Ali Shah and Deming Zhao contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
Prion infection leads of loss of weight and reduced feed and water intake as the disease progress. (A) The data showing weekly weight gain of individual animals in different experimental groups. Weekly weight gain was calculated upto 14th week post infection, the data was analyzed by two way ANOVA followed by Bonferroni post test (P<0.001) Note: FK506 started showing significant difference in weight loss after 8th week while other groups shown significant weight loss after 11th week post infection. (B) The data showing weekly feed consumed by individual animals in different experimental groups upto 14th week post infection, the data was analyzed by two way ANOVA followed by Bonferroni post test (P<0.001) Note: All groups shown significant difference in food consumed after 11th week post infection as compared to No prion-vehicle group. (C) The data showing weekly water consumed by individual animals in different experimental groups upto 13th week post infection, the data was analyzed by two way ANOVA followed by Bonferroni post test (P<0.001) Note: All groups shown significant difference in water consumed after 12 weeks post infection as compared to No prion-Vehicle group. (GIF 190 kb)
Fig. S2
Diagrammatic presentation of different stages of prion infection for possible therapeutic intervention. Note: In our hamster model of prion, we observed early clinical signs from 80±10 days post infection and animals without treatment survived for 3 to 4 weeks after initial signs. (GIF 108 kb)
Videos S1–S4
Clinical signs of prion infection in prion-vehicle, prion–FK506, prion–minocycline, and no prion–vehicle group on day 86 postinfection (WMV 5573 kb)
Videos S5–S8
Novel objects finding exploration in prion-vehicle, prion–FK506, prion–minocycline, and no prion–vehicle group (WMV 13677 kb)
ESM 1
(PDF 1224 kb)
About this article
Cite this article
Shah, S.Z.A., Zhao, D., Taglialatela, G. et al. RETRACTED ARTICLE: Early Minocycline and Late FK506 Treatment Improves Survival and Alleviates Neuroinflammation, Neurodegeneration, and Behavioral Deficits in Prion-Infected Hamsters. Neurotherapeutics 14, 463–483 (2017). https://doi.org/10.1007/s13311-016-0500-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13311-016-0500-0