Abstract
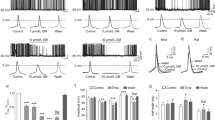
Inhibitors of voltage-gated sodium channels (Nav) have been used as anticonvulsants since the 1940s, while potassium channel activators have only been investigated more recently. We here describe the discovery of 2-amino-6-trifluoromethylthio-benzothiazole (SKA-19), a thioanalog of riluzole, as a potent, novel anticonvulsant, which combines the two mechanisms. SKA-19 is a use-dependent NaV channel blocker and an activator of small-conductance Ca2+-activated K+ channels. SKA-19 reduces action potential firing and increases medium afterhyperpolarization in CA1 pyramidal neurons in hippocampal slices. SKA-19 is orally bioavailable and shows activity in a broad range of rodent seizure models. SKA-19 protects against maximal electroshock-induced seizures in both rats (ED50 1.6 mg/kg i.p.; 2.3 mg/kg p.o.) and mice (ED50 4.3 mg/kg p.o.), and is also effective in the 6-Hz model in mice (ED50 12.2 mg/kg), Frings audiogenic seizure-susceptible mice (ED50 2.2 mg/kg), and the hippocampal kindled rat model of complex partial seizures (ED50 5.5 mg/kg). Toxicity tests for abnormal neurological status revealed a therapeutic index (TD50/ED50) of 6–9 following intraperitoneal and of 33 following oral administration. SKA-19 further reduced acute pain in the formalin pain model and raised allodynic threshold in a sciatic nerve ligation model. The anticonvulsant profile of SKA-19 is comparable to riluzole, which similarly affects NaV and KCa2 channels, except that SKA-19 has a ~4-fold greater duration of action owing to more prolonged brain levels. Based on these findings we propose that compounds combining KCa2 channel-activating and Nav channel-blocking activity exert broad-spectrum anticonvulsant and analgesic effects.








Similar content being viewed by others
References
Bialer M, White HS. Key factors in the discovery and development of new antiepileptic drugs. Nat Rev Drug Discov 2010;9:68-82.
Bialer M, Johannessen SI, Levy RH, Perucca E, Tomson T, White HS. Progress report on new antiepileptic drugs: a summary of the Eleventh Eilat Conference (EILAT XI). Epilepsy Res 2013;103:2-30.
Wickenden AD, Krajewski JL, London B, et al. N-(6-Chloro-pyridin-3-yl)-3,4-difluoro-benzamide (ICA-27243): A novel, selective KCNQ2/Q3 potassium channel activator. Mol Pharmacol 2008;73:977-986.
Roeloffs R, Wickenden AD, Crean C, et al. In vivo profile of ICA-27243 [N-(6-chloro-pyridin-3-yl)-3,4-difluoro-benzamide], a potent and selective KCNQ2/Q3 (Kv7.2/Kv7.3) activator in rodent anticonvulsant models. J Pharmacol Exp Ther 2008;326:818-828.
Dalby-Brown W, Jessen C, Hougaard C, et al. Characterization of a novel high-potency positive modulator of K(v)7 channels. Eur J Pharmacol 2013;709:52-63.
Biervert C, Schroeder BC, Kubisch C, et al. A potassium channel mutation in neonatal human epilepsy. Science 1998;279:403-406.
Mizoule J, Meldrum B, Mazadier M, et al. 2-Amino-6-trifluoromethoxy benzothiazole, a possible antagonist of excitatory amino acid neurotransmission. I. Anticonvulsant properties. Neuropharmacology 1985;24:767-773.
Rogawski MA. Epilepsy. In: Neurotherapeutics: Emerging Strategies Edited by Pullan L, Patel J. Totowa, NJ: Humana Press; 1996;193-273.
Gordon P, Corcia P, Meininger V. New therapy options for amyotrophic lateral sclerosis. Expert Opin Pharmacother 2013;14:1907-1917
Song JH, Huang CS, Nagata K, Yeh JZ, Narahashi T. Differential action of riluzole on tetrodotoxin-sensitive and tetrodotoxin-resistant sodium channels. J Pharmacol Exp Ther 1997;282:707-714.
Grunnet M, Jespersen T, Angelo K, et al. Pharmacological modulation of SK3 channels. Neuropharmacology 2001;40:879-887.
Duprat F, Lesage F, Patel AJ, Fink M, Romey G, Lazdunski M. The neuroprotective agent riluzole activates the two P domain K(+) channels TREK-1 and TRAAK. Mol Pharmacol 2000;57:906-912.
Sankaranarayanan A, Raman G, Busch C, et al. Naphtho[1,2-d]thiazol-2-ylamine (SKA-31), a new activator of KCa2 and KCa3.1 potassium channels, potentiates the endothelium-derived hyperpolarizing factor response and lowers blood pressure. Mol Pharmacol 2009;75:281-295.
Adelman JP, Maylie J, Sah P. Small-conductance Ca2+-activated K+ channels: form and function. Ann Rev Physiol 2012:74:245-269.
Wulff H, Kolski-Andreaco A, Sankaranarayanan A, Sabatier JM, Shakkottai V. Modulators of small- and intermediate-conductance calcium-activated potassium channels and their therapeutic indications. Curr Med Chem 2007;14:1437-1457.
White HS, Woodhead JH, Wilcox KS, Stables J, Kuferberg H, Wolf HH. Discovery and preclinical development of antiepileptic drugs. In:. Levy RH, Mattson RH, Meldrum B, Perucca E (eds) Antiepileptic drugs, 5th edn. Lippincott Williams & Wilkins, Philadelphia, PA, 2002, pp. 36-48.
Kokate TG, Svensson BE, Rogawski MA. Anticonvulsant activity of neurosteroids: correlation with gamma-aminobutyric acid-evoked chloride current potentiation. J Pharmacol Exp Ther 1994;270:1223-1229.
Frings H, Frings M. Development of strains of albino mice with predictable susceptibilities to audiogenic seizures. Science 1953;117:283-284.
Cao Z, Hammock BD, McCoy M, Rogawski MA, Lein PJ, Pessah IN. Tetramethylenedisulfotetramine alters Ca2+ dynamics in cultured hippocampal neurons: mitigation by NMDA receptor blockade and GABA(A) receptor-positive modulation. Toxicol Sci 2012;130:362-372.
Barton ME, Klein BD, Wolf HH, White HS. Pharmacological characterization of the 6 Hz psychomotor seizure model of partial epilepsy. Epilepsy Res 2001;47:217-227.
Bialer M, Twyman RE, White HS. Correlation analysis between anticonvulsant ED50 values of antiepileptic drugs in mice and rats and their therapeutic doses and plasma levels. Epilepsy Behav 2004;5:866-872.
McNamara CR, Mandel-Brehm J, Bautista DM, et al. TRPA1 mediates formalin-induced pain. Proc Natl Acad Sci USA 2007;104;13525-13530.
Pedarzani P, McCutcheon JE, Rogge G, et al. Specific enhancement of SK channel activity selectively potentiates the afterhyperpolarizing current I(AHP) and modulates the firing properties of hippocampal pyramidal neurons. J Biol Chem 2005;280:41404-41411.
Porter RJ, Dhir A, Macdonald RL, Rogawski MA. Mechanisms of action of antiseizure drugs. Handb Clin Neurol 2012;108:663-681.
Rogawski MA, Loscher W. The neurobiology of antiepileptic drugs for the treatment of nonepileptic conditions. Nat Med 2004;10:685-692.
Meldrum BS, Rogawski MA. Molecular targets for antiepileptic drug development. Neurotherapeutics 2007;4:18-61.
Meisler MH, Kearney JA. Sodium channel mutations in epilepsy and other neurological disorders. J Clin Invest 2005;115:2010-2017.
Hugues M, Schmid H, Romey G, Duval D, Frelin C, Lazdunski M. The Ca2+-dependent slow K+ conductance in cultured rat muscle cells: characterization with apamin. EMBO J 1982;1:1039-1042.
Kohler M, Hirschberg B, Bond CT, et al. Small-conductance, calcium-activated potassium channels from mammalian brain. Science 1996;273:1709-1714.
Stocker M, Krause M, Pedarzani P. An apamin-sensitive Ca2+-activated K+ current in hippocampal pyramidal neurons. Proc Natl Acad Sci USA 1999;96:4662-4667.
Villalobos C, Shakkottai VG, Chandy KG, Michelhaugh SK, Andrade R. SKCa channels mediate the medium but not the slow calcium-activated afterhyperpolarization in cortical neurons. J Neurosci 2004;24:3537-3542.
Bond CT, Herson PS, Strassmaier T, et al. Small conductance Ca2+-activated K+ channel knock-out mice reveal the identity of calcium-dependent afterhyperpolarization currents. J Neurosci 2004;24:5301-5306.
Edgerton JR, Reinhart PH. Distinct contributions of small and large conductance Ca2+-activated K + channels to rat Purkinje neuron function. J Physiol 2003;548:53-69.
McCown TJ, Breese GR. Effects of apamin and nicotinic acetylcholine receptor antagonists on inferior collicular seizures. Eur J Pharmacol 1990;187:49-58.
Pedarzani P, Mosbacher J, Rivard A, et al. Control of electrical activity in central neurons by modulating the gating of small conductance Ca2+-activated K+ channels. J Biol Chem 2001;276:9762-9769.
Kobayashi K, Nishizawa Y, Sawada K, Ogura H, Miyabe M. K+-channel openers suppress epileptiform activities induced by 4-aminopyridine in cultured rat hippocampal neurons. J Pharmacol Sci 2008;108:517-528.
Walter JT, Alvina K, Womack MD, Chevez C, Khodakhah K. Decreases in the precision of Purkinje cell pacemaking cause cerebellar dysfunction and ataxia. Nat Neurosci 2006;9:389-397.
Alvina K, Khodakhah K. KCa channels as therapeutic targets in episodic ataxia type-2. J Neurosci 2010;30:7249-7257.
Shakkottai VG, Chou CH, Oddo S, et al. Enhanced neuronal excitability in the absence of neurodegeneration induces cerebellar ataxia. J Clin Invest 2004;113:582-590.
Shakkottai VG, do Carmo Costa M, Dell'Orco JM, et al. Early changes in cerebellar physiology accompany motor dysfunction in the polyglutamine disease spinocerebellar ataxia type 3. J Neurosci 2011;31:13002-13014.
Kasumu AW, Hougaard C, Rode F, et al. Selective positive modulator of calcium-activated potassium channels exerts beneficial effects in a mouse model of spinocerebellar ataxia type 2. Chem Biol 2012;19:1340-1353.
Verma-Ahuja S, Evans MS, Pencek TL. Evidence for decreased calcium dependent potassium conductance in hippocampal CA3 neurons of genetically epilepsy-prone rats. Epilepsy Res 1995;22:137-144.
N'Gouemo P, Yasuda RP, Faingold CL. Protein expression of small conductance calcium-activated potassium channels is altered in inferior colliculus neurons of the genetically epilepsy-prone rat. Brain Res 2009;1270:107-111.
Schulz R, Kirschstein T, Brehme H, Porath K, Mikkat U, Köhling R. Network excitability in a model of chronic temporal lobe epilepsy critically depends on SK channel-mediated AHP currents. Neurobiol Dis 2012;45:337-347.
Anderson NJ, Slough S, Watson WP. In vivo characterisation of the small-conductance KCa (SK) channel activator 1-ethyl-2-benzimidazolinone (1-EBIO) as a potential anticonvulsant. Eur J Pharmacol 2006;546:48-53.
Yang S, Xiao Y, Kang D, et al. Discovery of a selective NaV1.7 inhibitor from centipede venom with analgesic efficacy exceeding morphine in rodent pain models. Proc Natl Acad Sci USA 2013;110:17534-17539.
Ristori G, Romano S, Visconti A, et al. Riluzole in cerebellar ataxia: a randomized, double-blind, placebo-controlled pilot trial. Neurology 2010;74:839-845.
Acknowledgments
This work was supported by the CounterACT Program, National Institutes of Health Office of the Director (NIH OD), and the National Institute of Neurological Disorders and Stroke (NINDS), grant numbers U54NS079202 and R21NS072585. N.C. was supported by a National Heart, Lung & Blood Institute T32 Training Program in Basic and Translational Cardiovascular Science (T32HL086350). B.M.B. was supported by a National Institute of General Medical Sciences-funded Pharmacology Training Program (T32GM099608). We are further highly indebted to the NIH Anticonvulsant Screening program. This work would not have been possible without their help.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Author information
Authors and Affiliations
Corresponding author
Additional information
N.C. and H.M.N. contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 507 kb)
Rights and permissions
About this article
Cite this article
Coleman, N., Nguyen, H.M., Cao, Z. et al. The Riluzole Derivative 2-Amino-6-trifluoromethylthio-benzothiazole (SKA-19), a Mixed KCa2 Activator and NaV Blocker, is a Potent Novel Anticonvulsant. Neurotherapeutics 12, 234–249 (2015). https://doi.org/10.1007/s13311-014-0305-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13311-014-0305-y