Abstract
Artificial Intelligence (AI) is playing an increasing role in several fields of medicine. AI is also used during laparoscopic cholecystectomy (LC) surgeries. In the literature, there is no review that groups together the various fields of application of AI applied to LC. The aim of this review is to describe the use of AI in these contexts. We performed a narrative literature review by searching PubMed, Web of Science, Scopus and Embase for all studies on AI applied to LC, published from January 01, 2010, to December 30, 2023. Our focus was on randomized controlled trials (RCTs), meta-analysis, systematic reviews, and observational studies, dealing with large cohorts of patients. We then gathered further relevant studies from the reference list of the selected publications. Based on the studies reviewed, it emerges that AI could strongly improve surgical efficiency and accuracy during LC. Future prospects include speeding up, implementing, and improving the automaticity with which AI recognizes, differentiates and classifies the phases of the surgical intervention and the anatomic structures that are safe and those at risk.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Artificial Intelligence (AI) is a discipline that studies models and the development of algorithms so that machines can acquire the ability to self-learn and achieve human-like performances in complex tasks [1]. These algorithms are based on the analysis of large amounts of data, in a limited time span, and the ability to achieve learning, problem-solving, and decision-making in complex scenarios. AI represents, therefore, a tool that with the potential to revolutionize the practice of medicine in many fields, including surgery [2, 3].
Today, medicine has reached a numerous and complex level of data production. Analyzing this complexity of data is not always feasible in a short time and with skills that are difficult to group into a single expert. Therefore, machines with AI capabilities can be a support tool for "observing" the data, learning from the data and supporting the doctors in everyday choices [1]. Currently, AI does not have the goal of replacing the doctor [4].
Initially, the AI was trained to recognize and classify radiologic images, then surgical actions such as sutures and knots [5]. Currently, AI is applied intraoperatively: it recognizes the phases of a surgical procedure and the anatomic regions of interest (ROIs). In this context, AI has found application during laparoscopic cholecystectomy (LC) [6].
Cholecystectomy is the most common operation performed worldwide by general surgeons with between 750,000 and 1,000,000 performed only in the U.S. annually [7]. LC was introduced into clinical practice approximately 30 years ago. It quickly became the gold standard operation for patients with symptomatic gallstones [8].
Despite the significant advantages, iatrogenic injuries can occur during LC [9]. Iatrogenic complications during LC require improved preventive strategies [10]. The difficulties of LC are linked to the recognition of the anatomic structures in case of inexperience of the surgeon or cholecystitis and to the recognition of the operating phase [11, 12]. One of the main complications is bile duct injury (BDI), caused by failure to recognize it by the surgeon [13, 14].
Historically, surgery has been one of the branches most inclined to innovation and adoption of new technologies able to offer advantages in terms of performance [13]. Despite its potential, the development of medical AI systems represents an untapped opportunity, especially in surgery [15, 16].
Some typologies of AI as Machine Learning (ML) and Deep Learning (DL) have been evaluated to overcome the surgical difficulties listed first. In the literature, several articles on this topic are present. To our knowledge, this is the first review that analyzes the application of AI during LC. Our review stems from the observation of the great importance that AI is assuming in recent years. The aim of this review is to evaluate the use of AI in recognizing the operative phases of LC, in identifying specific anatomic regions (at risk and not at risk) during the operation and its application during cholecystitis. Finally, we analyze the future prospects of this technology.
Methods
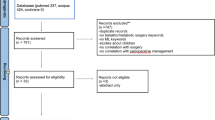
We performed a search on PubMed, Web of Science, Scopus and EMBASE using the following keywords: “artificial intelligence and cholecystectomy” AND “artificial intelligence and laparoscopic cholecystectomy” OR “application of artificial intelligence during a cholecystectomy” OR “AI and gallbladder anatomy” OR “AI and future perspectives in cholecystectomy” OR “Future of AI in automated laparoscopic cholecystectomy” OR “AI and surgery of cholecystectomy”. Articles in the literature on AI applied to laparoscopic cholecystectomy from January, 2010 to December, 2023 were analyzed (Table 1). Only English studies were included. Incomplete and non-peer reviewed in preprint articles were excluded. We focused our attention on randomized controlled trials (RCTs), meta-analysis, systematic reviews and observational studies on cohorts of patients. The articles included were either prospective or retrospective, monocentric or multicenter studies, and with a variable number of patients (hundreds to thousands) (Tables 2, 3).
Our main outcome is to evaluate the current various studies regarding the association between AI and LC. Our secondary outcome is to evaluate any future prospects for the application of AI in the same field of interest.
From a total of 32 articles analyzed, 26 items were selected and 6 excluded, as shown in Table 1.
Terminology of AI
AI has various fields of application and branches of interest (Table 4; Graphic 1–2) [17, 18]. The most developed branch is ML (Graphic 2) [19]. ML uses algorithms that derive answers from a large amount of data and statistical analyses [20]. ML can be supervised or unsupervised. Supervised ML uses a training dataset to teach models to produce the desired output. An algorithm measures the accuracy of the model and corrects it until the desired result: in this way, the AI model improves and learns as time goes by. Examples of ML are neural networks (Graphic 3), linear regression, logistic regression and random forest. Classifiers use algorithms to precisely assign test data into specific categories, while regression uses an algorithm to understand the relationship between dependent and independent variables and are useful for predicting specific numerical values. A practical application of ML is the recognition, classification and categorization of images and videos [18, 21].

Unsupervised ML does not use predetermined annotations, so it analyzes and clusters previously unlabeled datasets [22]. The main tasks of unsupervised ML are clustering, association and dimensionality reduction. Clustering is a data mining technique that groups unlabeled data based on their similarities or differences. This technique is useful for compressing images and videos. Association uses different rules to find relationships between variables in a given dataset. Dimensionality reduction is used when the number of dimensions or features in a given dataset is too high. It reduces the number of data inputs to a manageable size while preserving data integrity. This is often used during the processing phase of some preliminary data [17]. The fields of application of the Neuronal Networks and DL are shown in Graphs 4 and 5.
Results
The results of our review have been organized into paragraphs, divided into fields of application of AI during LC. The topics concern the phases of the surgical intervention and the anatomic structures that are safe and those at risk.
AI and the critical view of safety
Iatrogenic BDI is one of the intraoperative complications of LC [23, 24]. In 1995, Strasberg et al. indicated a surgical strategy to obtain a better intraoperative view of the gallbladder and reduce iatrogenic lesions during cholecystectomy [25]. This surgical approach is called Critical View of Safety (CVS) [26]. It is based on three principles: identification of Calot's triangle and the hepatoduodenal ligament, mobilization of the gallbladder fundus, identification, isolation and section of the cystic duct and cystic artery [27, 28]. The European Association of Endoscopic Surgery (EAES) recommends CVS as the best approach to cholecystectomy [29].
Despite the adoption of intraoperative CVS research, BDI rates remain higher than open cholecystectomy (0.1–0.2%), although decreasing over the years [30]. Some authors, such as Nijssen et al., have hypothesized incorrect awareness and standardized identification of CVS as the cause of this [31]. Nijssen's team analyzed videos of LC performed consecutively from 2009 to 2011 (1108 patients) in their hospital [31]. According to the surgeons' operating notes, CVS was detected in 80% of cases. However, according to the reviewers of these LCs, CVS was achieved in only 10.8% of cases and it was not achieved in any of the patients with BDI. The author concluded his article by highlighting the importance of video analysis of LCs to improve surgical technique and therefore reduce BDI. Rawlings et al. found similar findings in their study: according to the surgeons, CSV was detected in 100% of cases in 54 LCs analyzed, but from the reviewers’ analysis of videos of these LCs, CVS was identified in only 64% of cases [32].
These observations prompted Sanford and Strasberg to develop a method for the correct evaluation of CSV identified by surgeons from both photographic and video images of LCs [33]. The method consisted of a scale with 6 observation points, medial and lateral to the hepatocystic triangle. CVS was correctly identified in 96.5% of videos and 70% of LC images. Budding et al. assessed CVS through still images in only 27% of 63 LC procedures analyzed and reporting weak to minimal inter-rater agreement [34]. The result of these studies is the need to generate methods for the objective evaluation of LC frames or videos to improve both the surgical technique and the identification of CVS [35].
AI models were then created to try to achieve this [36]. Mascagni et al. performed a single-center study on this topic, analyzing the 60 s preceding clipping and cystic structures sectioning of 78 LC videos performed consecutively between March and June 2016 and attempting to document the achievement of CSV [37]. Video reviewers scored medial and lateral visualization of the hepatocystic triangle for each of the three CSV criteria. The authors' study is among the first to use LC videos instead of photographic images for CVS reporting, demonstrating a superiority in the consistency of CVS evaluation from videos compared to that from photographic images. According to the authors, analyzing videos of LCs before sectioning cystic structures could be a starting point for training AI models.
Mascagni et al. developed an AI model to reduce the subjectivity of CSV recognition [38]. According to the authors, the use of a DL model could automate CSV recognition. The ultimate goal was therefore to reduce the rate of iatrogenic injuries during LC. From 201 LC videos, 2854 images were extrapolated and used to train a deep neural network. The AI had to recognize the hepatocystic anatomy and classify the type of anatomic structure. The goal was to be able to predict the achievement of the CSV criteria. The AI was then trained with both a segmentation and classification model. This AI achieved a CSV criteria recognition accuracy level of 71.9% and a precision of 71.4%.
Also Madani et al. trained an AI model to recognize anatomic structures of interest and safe and unsafe areas to perform LC [39]. The authors used 290 videos from American and European hospitals and from each of them they extrapolated 10 frames previously clipping and sectioning the cystic structures. Three expert surgeons, for each frame, noted the possible presence of a specific anatomic structure (liver, gallbladder, hepatocystic triangle), a safe dissection area and an unsafe dissection area. A fourth hepatobiliary surgeon annotator evaluated the annotations applied on the pixels of the frames examined by the three surgeons previously. The safe dissection area (called Go) was located within the hepatocystic triangle near the inferior gallbladder border. The unsafe dissection area (called No-Go) was the area located most deeply in the hepatocystic triangle, together with the hepatoduodenal ligament and the hepatic hilum, and most likely to injure the cystic duct. The computer vision model obtained was that of semantic segmentation. Classic regions of interest (ROIs) are rectangles and include anatomic ROI. ROIs may have a limited role in the surgical field where structures have more complex geometric configurations and blend into their background. Semantic segmentation allows to delineate an anatomic structure along its exact boundaries pixel by pixel as an overlay on the original video. In fact, the authors superimposed the first model with Go areas on a second model with No-Go areas: these models were together named GoNoGoNet. A third model, called ChiletNet, simultaneously mapped the location of anatomic structures in each frame. In each of them, each pixel was classified as hepatocystic triangle, liver, gallbladder, or none of the above. To evaluate the performance of the models, the agreement between the expert surgeons' annotations and the AI's predictions of an anatomic structure was measured as semantic classification. For GoNoGoNet, the visual results were expressed either as a heat map (red region: highest probability of being in the Go region; blue region: lowest probability) or as a binary format (pixels in the Go region are highlighted and every pixel not in the Go region is not highlighted). Accuracy for pixel identification was greater than 90% for all structures.
The GoNoGoNet model was subsequently tested by another group of authors. Laplante et al. fed 47 frames of 25 LC videos to the aforementioned AI model [40]. The annotations applied by six expert surgeons were compared with those of the AI. The authors concluded the study by stating that the AI predictions had high specificity/PPV for Go zones and high sensitivity/NPV for No-Go zones and that the model's prediction was better for No-Go zones than for Go.
The GoNoGoNet model was also tested by Khalid et al. [6]. The authors tested the AI on 11 videos in which BDI was present. Another group with 11 videos without BDI was used as a control group. GoNoGoNet detected 33.6% more total tissue interactions in the No-Go zones in the BDI group compared to the control group. This meant that the BDI videos showed more dissections occurring outside the safe regions suggested by the AI.
For the first time, Endo et al. have developed an AI model capable of identifying the anatomic structures of interest, during LC: the extrahepatic bile duct (EHBD), the cystic duct (CD), the lower border of the liver S4 (S4) and the Rouviere sulcus (RS) [41]. The authors used images derived from 95 LC videos to train an AI DL model, annotating anatomic structures of interest. The authors excluded inflamed anatomic structures because they were difficult to annotate. An LC video of the 20 s preceding the detection of Calot's triangle was viewed by 4 experienced surgeons and 4 novice surgeons (with fewer than 50 LCs performed as first operator). The eight surgeons noted the anatomic landmarks mentioned above. Subsequently, the surgeons were shown the LC video with the annotations performed by the AI. In 26.9%, surgeons subsequently reformulated their notes, and 70% of these changes were considered safer changes, particularly for EHBD and CD. Subsequently, a questionnaire was submitted to the eight surgeons. In the questionnaire, it asked whether there was any change in each surgeon's perspective after watching the AI-driven video. Half of the interviewees considered the support given by AI in the safe recognition of anatomic structures to be significant.
Nakanuma et al. performed a similar experiment in their hospital center on ten cases of intraoperative LC, using an AI pre-trained to recognize anatomic structures [42]. Subsequently, a group of surgeons evaluated the accuracy of recognition of anatomic structures by the AI (CD, CBD, RS and S4). The structures of interest were delimited by ROIs in the shape of small tiles, of different color based on the anatomic structure. The small tiled automatically reposition and resized to form anatomic landmarks. In this case, the AI model (called YOLOv3) was directly connected to the integrated endoscopic operating room (EndoALPHA; Olympus Corp.). YOLOVv3 was installed on a monitor for landmark detection. In the operating room, there was also the main monitor that displays the endoscopic images. Then, the surgeons performed the 10 LCs while observing both monitors. The delay between the surgical video and the video showing the AI tracking information was 0.09 s. For the authors, the use of AI was considered useful for the recognition of anatomic structures of interest. Later, the same group of authors used two AIs simultaneously to identify anatomic landmarks during different phases of LC [43]. One AI had to recognize the surgical time during LC and the other AI had to recognize the anatomic structures of interest. Again, the goal was to evaluate an AI model's ability to prevent BDI. The authors used 1826 images from 92 LCs to train the AI to recognize CD, CBD, RS and S4. The annotations of the different surgical phases and anatomic structures were made by two surgeons with at least 10 years of experience. The two surgeons performed approximately 10 thousand notes. According to the authors, the dual AI system recognized anatomic structures in 92% of LC steps.
Recently, Kawamura et al. [36] have developed an AI capable of reporting CSV intraoperatively during LC. The authors used 72 videos, excluding cases of severe cholecystitis, as the identification of anatomic structures was more difficult and excluding LCs that ended in open cholecystectomies. From the videos, 71,379 images were obtained for annotation. Also in this study, two doctors (surgeon and gastroenterologist) made the annotations to train the AI to identify CSV. The AI was trained to automatically predict the anatomic ROI in which to find CSV. 20 of the 72 LC videos were used for data evaluation. The mean values of overall precision and accuracy were 0.971 and 0.834, respectively.
AI and recognition of cholecystectomy times
In addition to the application of AI in the recognition of anatomic structures during LC, some authors have trained AI in the recognition of surgical phases in LC videos. In particular, Golany et al. have developed an AI system (called Resnet50) capable of recognizing surgical phases during complex LC, such as in case of adverse events and complications [44]. The authors used 371 videos, of which 80% to train the AI and the remaining 20% to evaluate the AI. A group experienced surgeons (experience level more than 20 years) noted 8 steps for each LC video: trocar insertion, preparation, dissection of Calot's triangle, cropping and cutting, gallbladder dissection, gallbladder packing, cleansing and hemostasis, and gallbladder extraction. Beyond these eight phases, the authors defined the phase in which the instruments did not perform any surgical movement as "inactive" and the phase in which the camera was outside the patient's body as "out of body". LC videos considered complex were those with major bleeding, gallbladder perforation, major bile leak. Finally, a score was assigned for the difficulty of each surgical procedure, based on the Parkland Grading Scale [45, 46]. Finally, a final score was assigned for achieving the CSV. After the training, the AI had to be able to classify the surgical phase of the LC in which the given input (video frame) administered was located. To temporally classify the correct sequence of surgical stages, the authors integrated a variant of AI Cholec80, the so-called Multi-Stage Temporal Convolution Network (MS-TCN) [47]. Resnet50 achieved a classification accuracy of 78%. MS-TCN achieved a classification accuracy of 89%. The best results were obtained for the Calot's triangle dissection, clipping and cutting, and gallbladder dissection phases. The worst results were obtained for the preparation phase because it was confused with the dissection phase of Calot's triangle. Furthermore, as the complexity of the LC video increased, the accuracy of the model decreased, from 92% for lower complexity levels to 81% for higher complexity levels. According to the authors, adverse events during cholecystectomy influenced the accuracy of AI in recognizing surgical phases. In videos without adverse events, accuracy averaged 90%. In videos with cholecystitis, accuracy was 89%, in those with gallbladder perforation it was 87%, in those with major bile leak it was 77%. LC videos were extracted from 5 different hospitals to evaluate any difference in AI accuracy in identifying surgical phases during cholecystectomy. The AI was trained on videos from four hospitals and evaluated on the fifth. Differences in precision were observed due to the different devices and different surgical techniques used. The recognition precision of the surgical phases varied from 79 to 90.6%.
Similarly, Cheng et al. conducted a multicenter study with the aim of developing an AI capable of recognizing the surgical phases of LC [48]. The authors used 90 videos (from which they extracted 156,584 frames) of LC to train the AI and another 60 (from which they extracted 15,395 frames) to evaluate it. The videos came from 4 Chinese hospitals. Both young and experienced surgeons performed LCs, to analyze the difference in surgical stages. LC was divided into six surgical phases: establishing access (EA); lysis of adhesion (AL); mobilize the hepatocystic triangle (MHT); dissect the gallbladder from the liver bed (DGB); clear the operational region (COR); extract the gallbladder (EG). The authors included cases of acute cholecystitis. In cases of acute cholecystitis, the surgical phases lasted longer. The authors used one AI to identify surgical phases and another AI to classify surgical phases from the perspective of temporal sequences. The AI achieved an accuracy of 91.05%.
Limits of AI applied to LC
Most of the studies analyzed presented common limits.
Recruiting expert surgeons capable of annotating an AI system was the main challenge. In fact, to annotate frames from LC videos it is necessary to train the surgeons themselves in this task through specific training. The number of annotating surgeons for each study was often small (less than 10). This blurred the intersection regions within the expert-annotated heatmaps and potentially reduced the performance of the AI models. Furthermore, expert surgeons presented differences in their annotations during the AI training phase on the LC images proposed to them, caused by different personal experience.
Furthermore, in some cases, the AIs showed a variation in the accuracy of recognition of both safe and unsafe zones and surgical phases when given LC inputs derived from structures other than the one in which the AI had been trained. This could be caused both by the different surgical technique with which the different LCs are performed and by the different medical devices used during the LCs. Another explanation could be the small number of LC videos with which the AIs were trained (often only a hundred LPs). Furthermore, the classic rectangular shape of the ROI could affect the visual field of the operating surgeon during LC: in fact, some authors have reported a flickering in the dynamic images of the surgical field when the ROI was applied. In only one study analyzed, the authors replaced the rectangular ROI with tile-shaped safe and unsafe anatomic structure visualization technology (YOLOv3), capable of automatic repositioning and scaling to form anatomic landmarks. Furthermore, using this form of visualization technology, the authors observed a reduction in flickering of the anatomic structure of interest.
In addition, most studies were performed on LCs without complex situations such as acute and chronic cholecystitis, not allowing an adequate evaluation of AI in these contexts and in most studies, the dataset for training the AI came from a single center, preventing adequate generalization of the results.
Another limitation of the studies concerned the angle of the laparoscopic scope. In fact, the GoNoGoNet system identified the safe and unsafe areas only when Calot's triangle was framed by the laparoscopic optics together with the gallbladder and the liver. When vision magnification occurred during LC, the AI model misinterpreted the actual safe and unsafe zones. Furthermore, the presence of fumes and fluid loss (such as bile in case of gallbladder perforation) worsened the performance of AIs in terms of accuracy in recognizing safe and unsafe zones and surgical phases during LC. In other cases, AI models had difficulty recognizing structures of anatomic interest covered or hidden by adipose or fibrous tissue. The cause of this was both the absence of a sufficient number of annotations during the training phase of the AI by the annotating surgeons and the presence of anatomic boundaries which were consequently not well defined.
Another limitation common to most studies is that the evaluation of the technique for detecting CVS and of safety and danger zones during LC surgery using a heat map and comparing them with previous surgical images was limited to simulation using videos surgical, which had not reached operating room specifications. In only one study, the AI system was connected to the integrated endoscopic operating room (EndoALPHA; Olympus Corp.).
Finally, an investigation method for the prevention of BDI by AI has not currently been created, probably due to the still small number of studies conducted in this field of research. To conclude, AI can suggest safer dissection areas, but this does not imply that operating surgeons will actually incorporate such advice into their mental model, behaviors, and actions. This is because there are important design and implementation barriers to bringing AI into the operating room that have yet to be explored, such as usability, workflow, interface, data pipeline, and infrastructure. According to some authors, the majority of surgeons believed that AI would be beneficial for patient care but only a minority believed that it could improve decisions and intraoperative performance by requiring its on-demand use, with the possibility of deactivating it if necessary to reduce distractions due for example to ROI boxes. Although it is certainly advantageous to limit distractions in the surgical field, this functionality would probably reduce the usefulness of AI applied to LCs.
Future perspectives
All the studies examined agree that AI has wide possibilities for improvement in future but that new studies are needed to confirm the advantages in the fields of application of this review. In many studies, AI has been helpful in recognizing the location of CD and EHBD, which may be helpful in avoiding BDI. Indiocyanin green (ICG) is currently used for this purpose during many LCs. However, the ICG has some disadvantages: the rare presence of patients with allergic reactions to its administration, the ICG-emission range wider than the real one, the limited emission time and the inability to show RS and S4. Unlike ICG, AI could present advantages: it has no impact on the patient and has no time limits in its intraoperative use. We can state that the simultaneous use of ICG and AI could potentially support the recognition of more accurate anatomic information during LC.
Another important application of these findings is in surgical training and performance evaluation and in the emerging field of automated coaching. Studies agree that the information detected by AI is objectively useful because it adds clinical meaning to the operating surgeon's assessment during LC. Therefore, in future, AI could be used both as a training model and as an intraoperative support for novice surgeons to recognize anatomic structures that are more difficult to interpret and thus reduce the risk of BDI and other complications of LCs. Indeed, several studies have evaluated the ability of AI models to recognize CSV in LC. This ability has been evaluated in several studies. Currently, AI models have been created that detect CVS with high accuracy in real time during LC. We can state that surgical safety could be improved by using image classification technology to identify the achievement of CVS during LC.
In future, it could improve the system for detecting anatomic structures of surgical interest. Currently, only in one study does the ROI have a shape that is not rectangular but rather dynamic tiles. A future perspective could be the implementation of the adoption of the ROI model with dynamic tiles to recognize anatomic structures that are difficult to recognize (such as when hidden by fat or fibrosis) or to reduce peripheral flicker of images during LC.
In only one of the studies examined, the AI system was integrated with the laparoscopic system of the operating room. This gave the operating surgeon the possibility of simultaneously observing the laparoscopic images on a monitor and the suggestions given by the AI on another monitor near the first. It is hoped that in future the number of AI models integrated into the laparoscopic systems of operating rooms can be increased, to allow the surgeon to simultaneously evaluate the indications of the AI and the laparoscopic images in real time.
Conclusions
Our review suggests that during LC, AI can be used to identify safe and unsafe dissection zones and other anatomic structures. The possibility of contributing to the prevention of BDI and recognition of anatomic structures and surgical steps during LC was also evident, thanks to semantic and temporal segmentation. Furthermore, the AI provided important awareness to both novices and experts, prompting them to recognize anatomic landmarks related to BDI reduction. As more evidence emerges over time about the safety and effectiveness of using AI in the operating room, these automated computer vision tasks have the potential to increase performance and possibly be used in future for real-time decision support and other quality improvement initiatives.
However, large-scale evaluation is needed and the development of guidelines for both the annotation and clinical application of AI surgical support devices is also important.
Data availability
No additional data are available.
References
Hamet P, Tremblay J (2017) Artificial intelligence in medicine. Metabolism 69S:S36–S40. https://doi.org/10.1016/j.metabol.2017.01.011
Howard J (2019) Artificial intelligence: implications for the future of work. Am J Ind Med 62:917–926
Palomba G, Fernicola A, Della Corte M, Capuano M, De Palma GD, Aprea G (2024) Artificial intelligence in screening and diagnosis of surgical diseases: a narrative review. AIMS Public Health 11(2):557–576. https://doi.org/10.3934/publichealth.2024028
Akinrinmade AO, Adebile TM, Ezuma-Ebong C et al (2023) Artificial intelligence in healthcare: perception and reality. Cureus 15:e45594
Kaul V, Enslin S, Gross SA (2020) History of artificial intelligence in medicine. Gastrointest Endosc 92:807–812
Khalid MU, Laplante S, Masino C et al (2023) Use of artificial intelligence for decision-support to avoid high-risk behaviors during laparoscopic cholecystectomy. Surg Endosc 37:9467
Keiler A, Pernegger C, Hornof R (1992) Laparoscopic cholecystectomy–current status. Wien Klin Wochenschr 104:29–38
Fielding GA (1992) Laparoscopic cholecystectomy. Aust N Z J Surg 62:181–187
Shea JA, Healey MJ, Berlin JA et al (1996) Mortality and complications associated with laparoscopic cholecystectomy: a meta-analysis. Ann Surg 224:609–620
Strasberg SM (2002) Avoidance of biliary injury during laparoscopic cholecystectomy. J Hepatobiliary Pancreat Surg 9:543–547
Davidoff AM, Pappas TN, Murray EA et al (1992) Mechanisms of major biliary injury during laparoscopic cholecystectomy. Ann Surg 215:196–202
Soper NJ, Stockmann PT, Dunnegan DL et al (1992) Laparoscopic cholecystectomy the new ‘gold standard’? Arch Surg 127:917–921
Hugh TB (2002) New strategies to prevent laparoscopic bile duct injury—surgeons can learn from pilots. Surgery 132:826–835
Way LW, Stewart L, Gantert W et al (2003) Causes and prevention of laparoscopic bile duct injuries: analysis of 252 cases from a human factors and cognitive psychology perspective. Ann Surg 237:460–469
Johnson-Mann CN, Loftus TJ, Bihorac A (2021) Equity and artificial intelligence in surgical care. JAMA Surg 156:509–510
Loftus TJ, Upchurch GR, Bihorac A (2021) Building an artificial intelligence-competent surgical workforce. JAMA Surg 156:511–512
Choi RY, Coyner AS, Kalpathy-Cramer J et al (2020) Introduction to machine learning, neural networks, and deep learning. Transl Vis Sci Technol 9:14
Chen M, Decary M (2020) Artificial intelligence in healthcare: an essential guide for health leaders. Healthc Manag Forum 33:10–18
Deo RC (2015) Machine learning in medicine. Circulation 132:1920–1930
Lo Vercio L, Amador K, Bannister JJ et al (2020) Supervised machine learning tools: a tutorial for clinicians. J Neural Eng 17:062001
Ramesh AN, Kambhampati C, Monson JRT et al (2004) Artificial intelligence in medicine. Ann R Coll Surg Engl 86:334–338
Wiljer D, Hakim Z (2019) Developing an artificial intelligence-enabled health care practice: rewiring health care professions for better care. J Med Imaging Radiat Sci 50:S8–S14
de’Angelis N, Catena F, Memeo R et al (2021) WSES guidelines for the detection and management of bile duct injury during cholecystectomy. World J Emerg Surg. https://doi.org/10.1186/s13017-021-00369-w
Waage A, Nilsson M (2006) Iatrogenic bile duct injury: a population-based study of 152 776 cholecystectomies in the Swedish inpatient registry. Arch Surg 141:1207–1213
Strasberg SM, Hertl M, Soper NJ (1995) An analysis of the problem of biliary injury during laparoscopic cholecystectomy. J Am Coll Surg 180:101
Manatakis DK, Antonopoulou MI, Tasis N et al (2023) Critical view of safety in laparoscopic cholecystectomy: a systematic review of current evidence and future perspectives. World J Surg 47:640–648
Stefanidis D, Chintalapudi N, Anderson-Montoya B et al (2017) How often do surgeons obtain the critical view of safety during laparoscopic cholecystectomy? Surg Endosc 31:142–146
Strasberg SM (2019) A three-step conceptual roadmap for avoiding bile duct injury in laparoscopic cholecystectomy: an invited perspective review. J Hepatobiliary Pancreat Sci 26:123–127
Eikermann M, Siegel R, Broeders I et al (2012) Prevention and treatment of bile duct injuries during laparoscopic cholecystectomy: the clinical practice guidelines of the European Association for Endoscopic Surgery (EAES). Surg Endosc 26:3003–3039
Pucher PH, Brunt LM, Davies N et al (2018) Outcome trends and safety measures after 30 years of laparoscopic cholecystectomy: a systematic review and pooled data analysis. Surg Endosc 32:2175–2183
Nijssen MAJ, Schreinemakers JMJ, Meyer Z et al (2015) Complications after laparoscopic cholecystectomy: a video evaluation study of whether the critical view of safety was reached. World J Surg 39:1798–1803
Rawlings A, Hodgett SE, Matthews BD et al (2010) Single-incision laparoscopic cholecystectomy: initial experience with critical view of safety dissection and routine intraoperative cholangiography. J Am Coll Surg 211:1–7
Sanford DE, Strasberg SM (2014) A simple effective method for generation of a permanent record of the Critical View of Safety during laparoscopic cholecystectomy by intraoperative ‘doublet’ photography. J Am Coll Surg 218:170–178
Buddingh KT, Morks AN, Ten Cate Hoedemaker HO et al (2012) Documenting correct assessment of biliary anatomy during laparoscopic cholecystectomy. Surg Endosc 26:79–85
Emous M, Westerterp M, Wind J et al (2010) Registering the critical view of safety: photo or video? Surg Endosc 24:2527–2530
Kawamura M, Endo Y, Fujinaga A et al (2023) Development of an artificial intelligence system for real-time intraoperative assessment of the critical view of safety in laparoscopic cholecystectomy. Surg Endosc 37:8755–8763
Mascagni P, Fiorillo C, Urade T et al (2020) Formalizing video documentation of the critical view of safety in laparoscopic cholecystectomy: a step towards artificial intelligence assistance to improve surgical safety. Surg Endosc 34:2709–2714
Mascagni P, Vardazaryan A, Alapatt D et al (2022) Artificial intelligence for surgical safety: automatic assessment of the critical view of safety in laparoscopic cholecystectomy using deep learning. Ann Surg 275:955
Madani A, Namazi B, Altieri MS et al (2022) Artificial intelligence for intraoperative guidance: using semantic segmentation to identify surgical anatomy during laparoscopic cholecystectomy. Ann Surg 276:363–369
Laplante S, Namazi B, Kiani P et al (2023) Validation of an artificial intelligence platform for the guidance of safe laparoscopic cholecystectomy. Surg Endosc 37:2260–2268
Endo Y, Tokuyasu T, Mori Y et al (2023) Impact of AI system on recognition for anatomical landmarks related to reducing bile duct injury during laparoscopic cholecystectomy. Surg Endosc 37:5752–5759
Nakanuma H, Endo Y, Fujinaga A et al (2023) An intraoperative artificial intelligence system identifying anatomical landmarks for laparoscopic cholecystectomy: a prospective clinical feasibility trial (J-SUMMIT-C-01). Surg Endosc 37:1933–1942
Fujinaga A, Endo Y, Etoh T et al (2023) Development of a cross-artificial intelligence system for identifying intraoperative anatomical landmarks and surgical phases during laparoscopic cholecystectomy. Surg Endosc 37:6118–6128
Golany T, Aides A, Freedman D et al (2022) Artificial intelligence for phase recognition in complex laparoscopic cholecystectomy. Surg Endosc 36:9215–9223
Madni TD, Nakonezny PA, Barrios E et al (2019) Prospective validation of the Parkland Grading Scale for Cholecystitis. Am J Surg 217:90–97
Madni TD, Leshikar DE, Minshall CT et al (2018) The Parkland grading scale for cholecystitis. Am J Surg 215:625–630
Czempiel T, Paschali M, Keicher M, et al (2020) TeCNO: Surgical Phase Recognition with Multi-Stage Temporal Convolutional Networks. Lecture Notes in Computer Science (including subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics) 12263 LNCS, pp 343–352
Cheng K, You J, Wu S et al (2022) Artificial intelligence-based automated laparoscopic cholecystectomy surgical phase recognition and analysis. Surg Endosc 36:3160–3168
Funding
Open access funding provided by Università degli Studi di Napoli Federico II within the CRUI-CARE Agreement. This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
AF: study conception, data acquisition, writing and final approval of the draft; GP: writing, editing and final approval of the draft; MC: writing and editing; GDDP: final approval and editing, GA: study conception, writing-review, editing and final approval. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Conflict of interest
All authors meet the criteria for authorship and have participated in writing this manuscript. There was no conflict of interest for any of the author. Moreover, none of the authors of the study have received subsidies from Public Bodies or from any other sources for the execution of this study.
Provenance
Not commissioned.
Human and animal rights statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study formal consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fernicola, A., Palomba, G., Capuano, M. et al. Artificial intelligence applied to laparoscopic cholecystectomy: what is the next step? A narrative review. Updates Surg (2024). https://doi.org/10.1007/s13304-024-01892-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13304-024-01892-6