Abstract
This retrospective analysis of the prospective IGOMIPS registry reports on 1191 minimally invasive pancreatic resections (MIPR) performed in Italy between 2019 and 2022, including 668 distal pancreatectomies (DP) (55.7%), 435 pancreatoduodenectomies (PD) (36.3%), 44 total pancreatectomies (3.7%), 36 tumor enucleations (3.0%), and 8 central pancreatectomies (0.7%). Spleen-preserving DP was performed in 109 patients (16.3%). Overall incidence of severe complications (Clavien–Dindo ≥ 3) was 17.6% with a 90-day mortality of 1.9%. This registry analysis provided some important information. First, robotic assistance was preferred for all MIPR but DP with splenectomy. Second, robotic assistance reduced conversion to open surgery and blood loss in comparison to laparoscopy. Robotic PD was also associated with lower incidence of severe postoperative complications and a trend toward lower mortality. Fourth, the annual cut-off of ≥ 20 MIPR and ≥ 20 MIPD improved selected outcome measures. Fifth, most MIPR were performed by a single surgeon. Sixth, only two-thirds of the centers performed spleen-preserving DP. Seventh, DP with splenectomy was associated with higher conversion rate when compared to spleen-preserving DP. Eighth, the use of pancreatojejunostomy was the prevalent reconstruction in PD. Ninth, final histology was similar for MIPR performed at high- and low-volume centers, but neoadjuvant chemotherapy was used more frequently at high-volume centers. Finally, this registry analysis raises important concerns about the reliability of R1 assessment underscoring the importance of standardized pathology of pancreatic specimens. In conclusion, MIPR can be safely implemented on a national scale. Further analyses are required to understand nuances of implementation of MIPR in Italy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Minimally invasive (MI) surgery was probably the greatest innovation in general surgery in the last century, and was immediately rewarding in operations requiring large incisions to perform low complexity surgeries.
Feasibility of MI pancreatic resections (MIPR) was shown over 25 years ago [1, 2]. However, MIPR had a slow implementation due to the intrinsic complexity of pancreatic surgery, often including multiple digestive reconstructions, and the lack of an obvious advantage in terms of improved outcomes [3]. Oncologic adequacy was an additional concern, especially for pancreatic cancer [4]. Thanks also to the advent of robotic technology, recent evidence shows that in selected patients, MIPR can offer clear advantages over the conventional open surgery [5,6,7]. These newer data come from centers of excellence that have surpassed a quite steep learning curve. In MI pancreatoduodenectomy (PD; MIPD), true proficiency is acquired after 250 procedures [8], making generalizability of results achieved by champion surgeons questionable.
The most effective tool to depict daily practice of MIPR is probably a prospective registry. Participation in national and international registries was also recommended by the International Evidence-Based Guidelines on Minimally Invasive Pancreatic Resections with the purpose of ensuring safe and wide expansion of MIPR [9]. At the moment, there are some generalist registries for pancreatic surgery [10], but only two registries for MI pancreatic surgery: the European Registry (http://www.e-mips.com/registry) and the Italian Registry (IGOMPIS: https://www.yoursuite.it/IGOMIPS/).
IGOMIPS is a prospective registry, established in 2019, that includes the majority of MIPR performed in Italy [11]. We herein report the first comprehensive analysis of the IGOMIPS registry with the aim of providing a snapshot of daily practice of MIPR in Italy. Detailed data are provided for distal pancreatectomy (DP) and PD, as these procedures account for over 90% of all MIPR.
Materials and methods
IGOMIPS
The Independent Ethics Committee of the Humanitas Institute (authorization number 2167) established the IGOMIPS registry in 2019 following approval. IGOMIPS was recorded in the Registry of Patient Registries (RoPR) of the Agency for Healthcare and Research and Quality, US Department of Health (Registry of Patient Registries. Content last reviewed April 2019 https://www.ahrq.gov/ropr/index.html).
A detailed description of IGOMIPS was previously reported [11]. Briefly, IGOMIPS is a prospective registry for MI pancreatic surgery capturing operative and outcome data up to 90-days after surgery. All Italian centers performing MI pancreatic surgery can apply to participate to IGOMIPS, following protocol approval by the local Ethical Committee. IGOMIPS has some unique features permitting several analyses that are not feasible in other registries. First, every procedure must be declared the day before surgery, so that concordance between planned and performed procedures can be defined. Second, IGOMIPS includes progressive case numbers for centers and individual surgeons, thus permitting to analyze performance based on experience. Third, participating centers are periodically audited to verify the quality of data and adherence to registry protocol.
Study design
This study provides a retrospective review of MIPR performed at 34 IGOMPIS centers between September 2019 and June 2022. Comparisons and statistical analysis aim to provide insights on current practice of MIPR on a national basis.
Statistical computations were performed using the software STATA 17.0 (StataCorp. 2017. Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC.). Descriptive and inferential statistics were carried out with the analytical models adequate for the type of variable studied (e.g., Mann–Whitney test, chi- square). Two-sided P values lower than 0.05 were considered statistically significant. All continuous variables were reported as the median and interquartile range (IQR).
Data analysis
A per protocol analysis of MIPR enrolled in IGOMIPS up to June 2022 was performed. Other MI pancreatic surgeries (e.g., diagnostic laparoscopy, and biliary or gastric by-pass) were not included in the analysis.
Operations converted to open surgery were still analyzed as MI procedures (intention-to-treat analysis). We also reported rates of conversion to open surgery and agreement between planned and performed procedures.
Center volume
The cut-off of ≥ 20 MIPR proposed by the International Evidence-Based Guidelines on Minimally Invasive Pancreatic Resections [9] was used to identify high-volume centers. Outcomes of centers above and below this cut-off were compared.
Postoperative complications
Pancreas-specific complications were defined and graded as proposed by the International Study Group of Pancreatic Surgery [12,13,14,15]. Severity of all complications was assessed according to the Clavien and Dindo scale [16]. Severe complications were those with a score ≥ 3.
Results
During the study period, a total of 1293 MI pancreatic procedures were reported to IGOMIPS from 34 Italian centers from 11 different regions accounting for 49,791,952 out of 59,030,133 inhabitants as of January 1st, 2022 (84.3%) (http://dati.istat.it/Index.aspx?DataSetCode=DCIS_POPRES, accessed January 15, 2023).
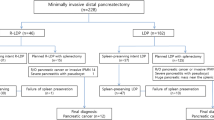
Figure 1 shows case enrollment based on number of active centers and trimester. Figure 2 reports the study flowchart. Overall 1191 MIPR were analyzed following exclusion of 102 procedures, because an MIPR was not performed (n = 60; 4.0%) or due to missing data (n = 42; 4.2%). Number of procedures per center ranged from 1 to 161 with a median of 12 [49]. Most procedures (854/1191; 71.7%) were performed at nine centers performing ≥ 20 MIPR per year. Overall, the top five most active centers enrolled 618 MIPR (51.9%). DPWS was performed at all centers. PD was performed at 19 centers (55.9%) and SPDP at 21 centers (61.7%). All but one of the top five centers performed MIPD. Nineteen centers (55.9%) located in Northern Italy reported 743 MIPR (62.4%), 8 centers (23.5%) located in Central Italy reported 342 MIPR (28.7%), and 7 centers (20.6%) located in Southern Italy reported 106 MIPR (8.9%). Four of the five top recruiting centers were located in Northern Italy and reported a total of 461 MIPR. A single center, located in Central Italy, was the top recruiter with 155 MIPR (13.0%).
The MIPR most commonly reported to IGOMIPS was MIDP (n = 668; 55.7%), followed by MIPD (n = 435; 36.3%), total pancreatectomy (n = 44; 3.7%) tumor enucleation (n = 36; 3.0%), and central pancreatectomy (n = 8; 0.7%). MIDP was reported either with (DPWS) (n = 559; 46.7%) or without splenectomy (SPDP) (n = 109; 9.1%). Laparoscopy and robotic assistance were used in 635 (53.3%) and 510 (42.8%) MIPR, respectively. A hybrid laparoscopic (n = 42; 3.6%) or robotic (n = 4; 0.3%) was used in a minority of patients.
Overall, the use of laparoscopy was prevalent over robotic assistance in DPWS (69.9 vs 29.5%; p < 0.0001), but the opposite was true for PD (34.9% vs 55.6%; p < 0.0001). In SPDP (48.6% vs 50.5%), tumor enucleation (47.2% vs 52.8%), and TP (47.7% vs 50.0%), the use of laparoscopy and robotic assistance was similar. When the analysis was restricted to the 24 centers (70.6%) where a robotic platform was available and accessible for MIPR, the use of robotic assistance was prevalent for PD (36.6% vs 59.0%; p < 0.0001), SPDP (44.4% vs 55.6%; p = 0.230), TP (45.0% vs 55.0%; p = 0.527), and tumor enucleation (20.8% vs 79.2%; p = 0.004), while the prevalence of laparoscopy for DPWS was no longer evident (51.5% vs 48.5%). Central pancreatectomy was performed only in centers with robotic availability and all cases were performed under robotic assistance (Table 1).
Despite two high-volume centers did not perform this operation, the proportion of MIPD over all MIPR was higher at high-volume centers (303/516–58.7% vs 132/675–19.6%; p < 0.0001). Also, the proportion of SPDP was higher at high-volume centers (91/455–20.0% vs 18/213–8.4%; p < 0.001) (Fig. 3). PD and SPDP were mostly performed by the same surgeon, but the fraction of procedures performed by the same surgeon was higher at low-volume centers (MIPD: 93.7% vs 83.6%; p = 0.017) (MIDP: 96.1% vs 68.8%; p = 0.038) (Table 2).
Distal pancreatectomy
DPWS was performed at all the 34 IGOMIPS centers, while SPDP was performed at 21 centers (61.7%). Severe postoperative complications occurred in 69 patients (10.3%) and 2 patients died (0.3%). Repeat surgery and hospital readmission were required in 15 (2.4%) and 64 (9.5%) patients, respectively. Median length of hospital stay was 7 days [6,7,8,9,10].
Table 3 provides a summary of baseline characteristics, intraoperative data, and postoperative outcome of patients undergoing either DPWS or SPDP.
Radical Anterograde Modular Pancreatosplenectomy (RAMPS) was performed in 101 of 232 DPWS for pancreatic cancer (45.9%). Ninety-five patients had an anterior RAMPS (94.1%) and 6 had a posterior RAMPS (5.9%). Fourteen patients had a vascular resection (9.3%). Excluding posterior RAMPS, 18 patients required resection of an extrapancreatic organ (7.8%). Patients operated at high-volume center were more likely to have received neoadjuvant chemotherapy (21.6% versus 8.9%, p = 0.015) and showed a trend for having undergone more frequently RAMPS (51.0% versus 36.0%, p = 0.034).
Data on modality of pancreatic transection were available for 534 patients (80.0%). A stapler was used to divide and seal the pancreatic stump in most MIDP (n = 427, 80.0%). In the remaining patients, the pancreas was either divided by harmonic shears alone (n = 55; 10.3%) or by an energy device plus fish-mouth sutures (n = 52; 9.7%).
Overall, 114 of 668 patients developed a clinically relevant postoperative pancreatic fistula (17.0%) (grade B: 113; 16.9%) (grade C: 1; 0.1%). Closure of pancreatic remnant by harmonic shears alone was associated with higher incidence of clinically relevant postoperative pancreatic fistula (harmonic shears: 36.4%; stapler: 18.4%; energy device plus sutures: 14.0%; p = 0.004), as well as of severe postoperative complications (harmonic shears: 27.3%; energy device plus sutures: 13.5%; stapler: 8.7% p < 0.0001). Neither staple line reinforcement (n = 188; 46.7%) nor the application of sealants on the pancreatic stump (n = 134; 20.7%) affected incidence of clinically relevant postoperative pancreatic fistula as well as of severe complications.
Out of 548 planned DPWS, 526 (96.0%) were actually performed. The 22 (3.9%) unplanned procedures were 6 SPDP, 9 tumor enucleations, 1 PD, and 6 total pancreatectomies. Conversion to open surgery was required in 45 of these patients (8.2%). On the other hand, 33 patients had an unplanned DPWS instead of SPDP (n = 25), tumor enucleation (n = 4), PD (n = 1), total pancreatectomy (n = 2), and central pancreatectomy (n = 1).
Out of 127 planned SPDP, 96 (75.6%) were actually performed. The 31 (24.4%) unplanned procedures were 25 DPWS, 4 tumor enucleations, and 2 central pancreatectomies. Conversion to open surgery was required in two of these patients (1.6%). On the other hand, 13 patients had an unplanned SPDP instead of DPWS (n = 6), enucleation (n = 4), and central pancreatectomy (n = 3).
Most MIDP were performed laparoscopically (n = 444; 66.4%), but robotic assistance was prevalent for SPDP (50.5% in SPDP versus 29.5% in DPWS; p < 0.001). Splenic vessels were preserved along with the spleen in 78 patients (84.7%).
Conversion to open surgery was required in 48 MIDP (6.6%), and occurred more frequently in DPWS (n = 43; 7.7%) than in SPDS (n = 1; 0.9%) (p = 0.009). However, 25 patients planned for SPDP underwent DPWS. In an intention-to-treat analysis, conversion rate for planned DPWS was 8.2% (n = 45) and for planned for SPDP 1.6% (n = 2) (p = 0.008).
Histology was available for 639 MIDP (95.6%). Pancreatic cancer was the leading diagnosis (n = 237; 37.0%), followed by neuroendocrine tumor (n = 198; 31.2%), mucinous cystoadenoma (n = 52; 8.2), intraductal papillary mucinous neoplasm (IPMN) without malignant degeneration (n = 44; 6.9%), solid pseudopapillary tumor (n = 38; 5.9%), serous cystadenoma (n = 20; 3.1%), and metastasis from renal carcinoma (n = 11; 1.7%). The remaining 39 patients had less frequent tumor types (6.1%). Frequency of individual tumor types was similar between high- and low-volume centers (Fig. 4).
Laparoscopic versus robotic DPWS
As shown in Table 4, compared to laparoscopy, robotic DPWS was associated with longer median operative time (280 [120] versus 240 [110] minutes; p < 0.0001) and longer median duration of hospital stay (8 [5] versus 7 [4] days; p = 0.002), but lower rates of conversion to open surgery (10.1% vs 2.5%; p = 0.002) and lower estimated blood loss (100 [150] versus 150 [200] mL; p < 0.0001). Laparoscopic DPWS also showed a trend to statistical significance for lower incidence of intraabdominal fluid collections (16.1% vs 23.2%; p = 0.053).
In patients with pancreatic cancer, despite similar T stage, nodal status, and number of examined lymph nodes, robotic DPWS was associated with lower rates of R1 resection (7.3% vs 28.0%; p = 0.002).
Laparoscopic versus robotic SPDP
As shown in Table 5, laparoscopic and robotic SPDP were similar in all respects, including need for unplanned splenectomy (laparoscopic: 22.7% vs robotic: 18.5%; p = 0.572).
Pancreatoduodenectomy
Table 6 provides a summary of baseline characteristics, intraoperative data, and postoperative outcome for patients undergoing MIPD.
The pylorus was preserved in 227 MIPD (55.2%), vein resection and reconstruction were performed in 20 procedures (4.6%), and resection of adjacent organs in 5 operations (1.2%). Details of digestive reconstruction were available for 413 patients. A single jejunal loop was employed in 379 MIPD (91.7%) and the pancreatic anastomosis was a pancreatico-jejunostomy in 400 patients (96.8%) and a pancreatogastrostomy in 13 (3.1%). Pancreatico-jejunostomy was performed end-to-side in 379 of 400 MIPD (94.7%), mostly using either a Blumgart (n = 60; 15.0%) or a modified Blumgart technique (n = 108; 27.0%). A double layer of sutures was used in 342 pancreatico-jejunostomy (85.5%) and a duct stent was used in 226 patients (55.1%).
Conversion to open surgery was required in 45 MIPD (10.3%). Median length of hospital stay was 13 days [8,9,10,11,12,13,14,15,16,17,18,19,20,21,22]. Severe postoperative complications occurred in 121 patients (27.8%) and 21 patients died (4.8%). Clinically relevant postoperative pancreatic fistula was diagnosed in 73 patients (17.1%) (grade B: 61; 14.3%) (grade C: 12; 2.8%). Repeat surgery and hospital readmission were required in 48 (12.6%) and 49 (13.0%) patients, respectively. Two patients (0.4%) required more than one reintervention.
Out of 437 planned MIPD, 422 (96.6%) were performed. The 15 (3.4%) unplanned procedures were one DPWS, one tumor enucleation, and 13 total pancreatectomies. Conversion to open surgery was required in 44 of these patients (10.1%). On the other hand, 13 patients had an unplanned MIPD instead of DPWS (n = 4), tumor enucleation (n = 3), total pancreatectomy (n = 4), central pancreatectomy (n = 1), and ampullectomy (n = 1).
Most PD were performed at high-volume centers (n = 303; 69.3%) using robotic assistance (n = 242; 55.6%). Laparoscopy was used in 152 PD (34.9%). When the analysis was restricted to the 16 centers that have a robot, implementation of PD increased to 44.6% and the use of robotic assistance to 59.0%. Adoption of robotic assistance increased over time. It was 7.1% in 2019, 47.6% in 2020, 57.7% in 2021, and 71.1% in 2022 (p < 0.0001). A hybrid technique, mostly using a mini-incision for some open anastomosis, was used in 37 additional laparoscopic (8.5%) and 4 robotic PD (0.9%), respectively.
Histology was available for 399 PD (91.7%). Pancreatic cancer was the leading diagnosis (n = 193; 48.4%), followed by adenocarcinoma of the ampulla of Vater (n = 77; 19.4%), distal common bile duct cancer (n = 42; 10.4%), and duodenal cancer (n = 14; 3.5%). There were also 25 neuroendocrine tumors (6.4%) and 17 benign cystic tumors (3.8%). The remaining 31 patients had less frequent tumor types (8.1%). Frequency of individual tumor types was similar between high- and low-volume centers.
Laparoscopic versus robotic PD
As shown in Table 7, comparing laparoscopic PD and robotic PD showed that the latter was performed in younger patients (67 years versus 69 years; p = 0.017) and in persons with a lower prevalence of previous abdominal surgery (31.9% versus 42.1%; p = 0.041). However, the prevalence of patients with ASA score ≥ 3 (47.1% versus 36.8%; p = 0.045) was higher in robotic PD. Robotic PD was associated with lower estimated blood loss (200 [200] ml versus 300 [350] ml; p = 0.0001), fewer conversions to open surgery (4.8% versus 17.8%; p < 0.0001), and reduced rate of severe postoperative complications (23.1% versus 33.0%; p = 0.024). Postoperative mortality was also reduced after robotic PD (3.3% versus 7.2%; p = 0.076), despite the difference did not reach statistical significance. Laparoscopic PD was associated with fewer intraabdominal fluid collections (21.4% versus 34.6%; p = 0.008).
In patients operated for a malignant tumor, with an identical prevalence of T3/4 tumors and a higher prevalence of node positive patients in robotic PD (68.7% versus 57.3%; p = 0.049), robotic PD showed a higher median number of examined lymph nodes (30 [20] versus 21.5 [14]; p = 0.0001) but a higher rate of R1 resection (23.7% versus 14.0%; p = 0.050).
Results of MIPR based on center volume
Results of MIPR based on center volume are presented in Table 8. Center volume had an effect on selected outcome measures.
MIDP at high-volume centers was associated to lower postoperative mortality (0 vs 0.9%; 0.038), despite similar incidence of severe postoperative complications (11.6 vs 7.5%; p = 0.102) and longer operative time (256 [210–333] vs 240 [195–300]; p = 0.006). MIDP at high-volume centers was associated also to higher number of examined lymph nodes (26 [18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38] vs 15 [11,12,13,14,15,16,17,18,19,20,21,22,23]; p = 0.0001) but higher R1 rate (26.7 vs 8.3%; p = 0.004).
MIPD at high-volume centers was associated with lower intraoperative blood loss (200 [100–300] ml vs 300 [150–500] ml; p = 0.0001) and lower conversion to open surgery (4.9% vs 22.7%; p = 0.0001), despite older patient age, higher BMI, and higher proportion of ASA score ≥ 3.
Discussion
This report of 1,191 contemporary MIPR from the prospective IGOMIPS registry provides some important information. First, robotic assistance was preferred for all types of MIPR but DPWS. Second, robotic assistance was associated with reduced rates of conversion to open surgery and lower amount of estimated blood loss. Third, robotic PD had trend toward lower mortality when compared to laparoscopic PD. Fourth, despite the use of robotic assistance was prevalent for SPDP, it did not increase the rate of spleen preservation. Fifth, DPWS was associated with higher conversion rate when compared to SPDP. Sixth, pancreatico-jejunostomy was prevalent in PD, but the technique of pancreatic anastomosis showed considerable variation. Seventh, performing ≥ 20 MIPR per year was associated with lower postoperative mortality and higher number of examined lymph nodes in DP and lower conversion to open surgery in SPDP. Performing ≥ 20 MIPD was associated with lower intraoperative blood loss and lower conversion to open surgery despite older patient age, higher BMI, and higher ASA score. Complex MIPR resections (i.e., PD and SPDP) were mostly performed at high-volume centers. Eight, most MIPR were performed by a single surgeon irrespective of center volume, but at high-volume centers, one-third of DP were performed by multiple surgeons as compared to < 20% for DPWS and < 5% for SPDP at low-volume centers. The importance of these findings is emphasized by the fact that figures refer to contemporary daily practice of MIPR on a national basis.
This registry analysis raises also important questions about the reliability of R1 assessment (i.e., the importance of standardized pathology of specimens) and the consequences of unplanned MIPR especially when this means an increase in technical complexity (e.g., from MIDP to MIPD).
Despite the lack of clear evidence of superiority of robotic assistance over the conventional laparoscopy in MIPR, the use of robotic assistance was prevalent for all types of MIPR but DPWS and increased over time. There has been a tenfold increase in robotic MIPR between 2019 and 2022, at hospitals where a robot is available. Recent evidence shows that robotic assistance outperforms laparoscopy in MIDP, for some outcome measures [17,18,19]. Considering the high costs of robotic assistance and the need to select the procedures in which this new technology may be conveniently employed, it is not surprising that the use of conventional laparoscopy was prevalent for DPWS. For MIPD, advantages of robotic assistance are more evident [20,21,22] as shown also by the high implementation of robotic PD in the IGOMIPS registry. One of the most striking pieces of evidence favoring robotic PD is provided by the Dutch trial on laparoscopic versus open PD that was terminated due to excess mortality in the laparoscopic arm [23]. Since then, Dutch surgeons have embraced robotic PD and have achieved excellent outcomes [24].
Little doubt exists that the use of robotic assistance reduces the rate of conversion to open surgery and the amount of blood loss [17,18,19,20,21,22]. The IGOMIPS registry confirms these results in daily practice, showing that advantages of robotic assistance in MIPR are not reserved to the few centers that have pioneered robotic surgery.
One striking result from this registry analysis is that robotic assistance, when compared to laparoscopy, reduced the incidence of severe postoperative complications, and could reduce postoperative mortality of MIPD. Despite higher prevalence of patients at increased operative risk (ASA score ≥ 3: 47.1% versus 36.8%; p = 0.045), incidence of severe postoperative complications was lower in robotic PD. Difference in mortality showed only a trend toward statistical significance. However, it may still be important to note that robotic PD was associated with a mortality rate of 3% at a national level. This mortality rate is equivalent to the value reported in the benchmark study for open PD when patients have an ASA score ≥ 3[25]. In robotic PD, approximately 50% of the patients had an ASA score ≥ 3.
Not surprisingly, MIPD performed at low-volume centers was associated with worse outcomes. Only 5 of 19 centers (26.3%) performing MIPD met the threshold of ≥ 20 procedures per year defined by the Miami guidelines [9]. Considering that approximately 30–40% of all PD can be MIPD, meeting this cut-off means that at least 50 PD are performed annually. Although just few centers met this annual volume at a national level, the importance of annual volume for the outcome of PD is well established [26]. A Dutch study showed that at least 40 PD per year are required to improve postoperative mortality [27]. A more recent study from Norway showed that ≥ 40 PD may not be enough to reduce postoperative mortality [28], and a study Korea demonstrated that mortality improves if the annual volume of PD is ≥ 54 [29]. Therefore, the annual volume ≥ 50 PD, permitting ≥ 20 MIPD, seems appropriate to offer good clinical outcomes.
This analysis also shows that most MIPR are performed by a single surgeon at most centers. This was especially true for MIPD. If, on the one hand, convincing evidence demonstrates that 250 robotic PD are required to achieve truly optimal outcomes [8], thus reinforcing the need for centralization, on the other hand, this high number of procedures raises the difficult question about how MIPD can safely diffuse on a large scale [30]. This study raises also important questions on how to train the next generation of pancreatic surgeons, and how to retrain the current generation of pancreatic surgeons that is mostly composed by open surgeons.
SPDP was mostly performed at high-volume centers, further underscoring the importance of volume in MIPR. Preserving the spleen is believed to be important in the rare patients with a benign but symptomatic tumor or a premalignant pancreatic tumor that require an MIDP. Spleen preservation prevents overwhelming sepsis and thrombocytosis and preserves overall immune function [31,32,33]. In addition, it could reduce blood loss and operative time, while limiting the rate of postoperative pancreatic fistula and delayed gastric emptying [31, 34,35,36]. However, SPDP, especially when the splenic vessels are also spared (Kimura procedure), is technically demanding and requires greater technical skills when compared to DPWS. This is mostly why robotic assistance is believed to improve the ability to preserve the spleen during MIDP [17, 18]. The fact that SPDP was mostly performed at high-volume centers is a possible explanation for the lack of an increased spleen preservation rate in the robotic group in this registry analysis.
In the IGOMIPS registry, DPWS was associated with higher rates of conversion to open surgery when compared to SPDP. In general, SPDP is technically more complex than DPWS. However, SPDP can be converted to DPWS when spleen preservation is not feasible, while primary DPWS is more frequently associated with difficulty factors, such as malignant histology, tumor proximity to the superior mesenteric-portal vein, sinistral portal hypertension, and splenomegaly making conversion to open surgery more likely to occur in these patients [37].
This registry analysis also showed that in MIPD, the pancreatic anastomosis is nearly always an end-to-side pancreatojejunostomy, but the surgical technique varied considerably among centers. A Blumgart or a modified Blumgart pancreatico-jejunostomy was used in 168 patients (38.6%), being the technique used more frequently. Practice in MIPD is probably influenced by experience in open PD. However, the minimally invasive approach may put additional difficulties, sometimes forcing surgeons to oversimplify the technique. This is probably why some surgeons prefer single layer running pancreatojejunostomy [38], despite this technique was associated with increased rates of postoperative pancreatic fistula in a large multicenter study [39]. The Blumgart technique is quite easy to perform during MIPD and combines the principle of duct-to-mucosa anastomosis to jejunal wrapping over the pancreatic stump. A recent study showed that modified Blumgart pancreatojejunostomy is associated with low rates of grade C postoperative pancreatic fistula in either open or robotic PD [40]. A modified Blumgart anastomosis is included in the standardized training pathway developed by the Dutch Pancreatic Cancer Group [24].
One key information from this study is that implementation of MIPR was not associated with high rates of resection for benign tumors, such as serous cystadenoma. A recent study showed that approximately 2% of the patients undergoing surgery for an incidentally discovered pancreatic cystic lesion have a final histology of serous cystadenoma [41]. Despite the different denominator in this study and in the IGOMIPS registry, the 3% rate of resection for serous cystadenoma reported herein is quite reassuring that availability of MI techniques does not result in unnecessary surgery [42].
This registry analysis showed conflicting data about R1 rates. DP at high-volume centers was associated with higher rates of R1 resection, which appears counterintuitive. Furthermore, robotic PD was associated with higher rates of R1 resection, while robotic DP showed lower R1 resection, versus laparoscopy. Margins status is an important quality metric in pancreatic surgery [43], but objective assessment relies on standardized and high quality of pathology. The higher number of examined lymph nodes suggests more accurate histology at high-volume centers, supporting the hypothesis of underestimation of R1 at low-volume centers. In addition, administration on neoadjuvant treatments decreases R1 rates [44], and a study from Esposito et al. showed that most resections for pancreatic cancer is R1 [45]. It is therefore difficult to believe that at high-volume centers, where patients receive neoadjuvant chemotherapy more frequently, R1 rates are truly higher when compared to low-volume centers. Clearly, quality of pathology makes most of the difference. Pathology of pancreatic specimens should become truly standardized to permit meaningful comparison on margin status.
This study has several limitations. First, despite prospective enrollment in the IGOMIPS registry, accuracy of information depends on individual centers. However, prospective data acquisition is the best possible method to ensure high quality of information. Second, some results may be influenced by local practice and/or quality of some hospital services (e.g., pathology). The large number of cases reported to the registry is expected to dilute the effect of these confounders. Third, relatively few centers provided most cases. Therefore, even in a national registry, quality of care mostly refers to specialized centers.
In conclusion, this registry analysis shows that MIPR can be safely implemented on a national scale. A few high-volume pancreatic centers perform most procedures, but results achieved at low-volume centers appears acceptable. Further analysis, on a larger sample, is required to understand nuances of implementation of MIPR in Italy.
Availability of data and materials
The datasets generated during the current study are available from the corresponding author on reasonable request. All materials are available upon request.
Code availability
Not applicable.
Change history
02 December 2023
A Correction to this paper has been published: https://doi.org/10.1007/s13304-023-01709-y
References
Cuschieri A (1994) Laparoscopic surgery of the pancreas. J R Coll Surg Edinb 39:178–184
Gagner M, Pomp A (1994) Laparoscopic pylorus-preserving pancreatoduodenectomy. Surg Endosc 8:408–410. https://doi.org/10.1007/BF00642443
Dokmak S, Ftériche FS, Aussilhou B, Bensafta Y, Lévy P, Ruszniewski P, Belghiti J, Sauvanet A (2015) Laparoscopic pancreaticoduodenectomy should not be routine for resection of periampullary tumors. J Am Coll Surg 220:831–838. https://doi.org/10.1016/j.jamcollsurg.2014.12.052
van Hilst J, de Rooij T, Abu Hilal M, Asbun HJ, Barkun J, Boggi U, Busch OR, Conlon KC, Dijkgraaf MG, Han HS, Hansen PD, Kendrick ML, Montagnini AL, Palanivelu C, Røsok BI, Shrikhande SV, Wakabayashi G, Zeh HJ, Vollmer CM, Kooby DA, Besselink MG (2017) Worldwide survey on opinions and use of minimally invasive pancreatic resection. HPB (Oxford) 19:190–204. https://doi.org/10.1016/j.hpb.2017.01.011
Khachfe HH, Nassour I, Hammad AY, Hodges JC, AlMasri S, Liu H, deSilva A, Kraftician J, Lee KK, Pitt HA, Zureikat AH, Paniccia A (2022) Robotic pancreaticoduodenectomy: increased adoption and improved outcomes - Is laparoscopy still justified? Ann Surg. https://doi.org/10.1097/SLA.0000000000005687
Rosemurgy AS, Ross SB, Espeut A, Nguyen D, Crespo K, Syblis C, Vasanthakumar P, Sucandy I (2022) Survival and robotic approach for pancreaticoduodenectomy: a propensity score-match study. J Am Coll Surg 234:677–684. https://doi.org/10.1097/XCS.0000000000000137
Napoli N, Kauffmann EF, Vistoli F, Amorese G, Boggi U (2021) State of the art of robotic pancreatoduodenectomy. Updates Surg 73:873–880. https://doi.org/10.1007/s13304-021-01058-8
Shi Y, Jin J, Qiu W, Weng Y, Wang J, Zhao S, Huo Z, Qin K, Wang Y, Chen H, Deng X, Peng C, Shen B (2020) Short-term outcomes after robot-assisted vs open pancreaticoduodenectomy after the learning curve. JAMA Surg 155:389–394. https://doi.org/10.1001/jamasurg.2020.0021
Asbun HJ, Moekotte AL, Vissers FL, Kunzler F, Cipriani F, Alseidi A, D’Angelica MI, Balduzzi A, Bassi C, Björnsson B, Boggi U, Callery MP, Del Chiaro M, Coimbra FJ, Conrad C, Cook A, Coppola A, Dervenis C, Dokmak S, Edil BH, Edwin B, Giulianotti PC, Han HS, Hansen PD, van der Heijde N, van Hilst J, Hester CA, Hogg ME, Jarufe N, Jeyarajah DR, Keck T, Kim SC, Khatkov IE, Kokudo N, Kooby DA, Korrel M, de Leon FJ, Lluis N, Lof S, Machado MA, Demartines N, Martinie JB, Merchant NB, Molenaar IQ, Moravek C, Mou YP, Nakamura M, Nealon WH, Palanivelu C, Pessaux P, Pitt HA, Polanco PM, Primrose JN, Rawashdeh A, Sanford DE, Senthilnathan P, Shrikhande SV, Stauffer JA, Takaori K, Talamonti MS, Tang CN, Vollmer CM, Wakabayashi G, Walsh RM, Wang SE, Zinner MJ, Wolfgang CL, Zureikat AH, Zwart MJ, Conlon KC, Kendrick ML, Zeh HJ, Hilal MA, Besselink MG, International Study Group on Minimally Invasive Pancreas Surgery (I-MIPS) (2020) The Miami international evidence-based guidelines on minimally invasive pancreas resection. Ann Surg 271:1–14. https://doi.org/10.1097/SLA.0000000000003590
Mackay TM, Gleeson EM, Wellner UF, Williamsson C, Busch OR, Groot Koerkamp B, Keck T, van Santvoort HC, Tingstedt B, Pitt HA, Besselink MG; Global Audits on Pancreatic Surgery Group (GAPASURG). Transatlantic registries of pancreatic surgery in the United States of America, Germany, The Netherlands, and Sweden: Comparing design, variables, patients, treatment strategies, and outcomes. Surgery 169:396-402. https://doi.org/10.1016/j.surg.2020.07.012
Zerbi A, Capretti G, Napoli N, Belli G, Coppola R, Falconi M, Salvia R, Valeri A, Alfieri S, Berti S, Butturini G, Conzo G, Coratti A, Dalla Valle R, Garulli G, Ettorre GM, Ferrari G, Ferrero A, Jovine E, Maida P, Minni F, Molino C, Nardo B, De Paolis P, Testini M, Boggi U (2020) The Italian National Registry for minimally invasive pancreatic surgery: an initiative of the Italian Group of Minimally Invasive Pancreas Surgery (IGoMIPS). Updates Surg 72:379–385. https://doi.org/10.1007/s13304-020-00808-4
Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham M, Allen P, Andersson R, Asbun HJ, Besselink MG, Conlon K, Del Chiaro M, Falconi M, Fernandez-Cruz L, Fernandez-Del Castillo C, Fingerhut A, Friess H, Gouma DJ, Hackert T, Izbicki J, Lillemoe KD, Neoptolemos JP, Olah A, Schulick R, Shrikhande SV, Takada T, Takaori K, Traverso W, Vollmer CR, Wolfgang CL, Yeo CJ, Salvia R, Buchler M, International Study Group on Pancreatic Surgery (ISGPS) (2016) update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery 161:584–591. https://doi.org/10.1016/j.surg.2016.11.014
Wente MN, Veit JA, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR, Neoptolemos JP, Padbury RT, Sarr MG, Yeo CJ, Büchler MW (2007) Postpancreatectomy hemorrhage (PPH): an International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery 142:20–25. https://doi.org/10.1016/j.surg.2007.02.001
Wente MN, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR, Neoptolemos JP, Padbury RT, Sarr MG, Traverso LW, Yeo CJ, Büchler MW (2007) Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 142:761–768. https://doi.org/10.1016/j.surg.2007.05.005
Besselink MG, van Rijssen LB, Bassi C, Dervenis C, Montorsi M, Adham M, Asbun HJ, Bockhorn M, Strobel O, Büchler MW, Busch OR, Charnley RM, Conlon KC, Fernández-Cruz L, Fingerhut A, Friess H, Izbicki JR, Lillemoe KD, Neoptolemos JP, Sarr MG, Shrikhande SV, Sitarz R, Vollmer CM, Yeo CJ, Hartwig W, Wolfgang CL, Gouma DJ, International Study Group on Pancreatic Surgery (2017) Definition and classification of chyle leak after pancreatic operation: A consensus statement by the International Study Group on Pancreatic Surgery. Surgery 161:365–372. https://doi.org/10.1016/j.surg.2016.06.058
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213
Li P, Zhang H, Chen L, Liu T, Dai M (2022) Robotic versus laparoscopic distal pancreatectomy on perioperative outcomes: a systematic review and meta-analysis. Updates Surg. https://doi.org/10.1007/s13304-022-01413-3
Lof S, van der Heijde N, Abuawwad M, Al-Sarireh B, Boggi U, Butturini G, Capretti G, Coratti A, Casadei R, D’Hondt M, Esposito A, Ferrari G, Fusai G, Giardino A, Groot Koerkamp B, Hackert T, Kamarajah S, Kauffmann EF, Keck T, Marudanayagam R, Nickel F, Manzoni A, Pessaux P, Pietrabissa A, Rosso E, Salvia R, Soonawalla Z, White S, Zerbi A, Besselink MG, Abu Hilal M, European Consortium on Minimally Invasive Pancreatic Surgery (E-MIPS) (2021) Robotic versus laparoscopic distal pancreatectomy: multicentre analysis. Br J Surg 108:188–195. https://doi.org/10.1093/bjs/znaa039
Zhang X, Chen W, Jiang J, Ye Y, Hu W, Zhai Z, Bai X, Liang T (2022) A comparison of robotic versus laparoscopic distal pancreatectomy: a single surgeon’s robotic experience in a high-volume center. Surg Endosc 36:9186–9193. https://doi.org/10.1007/s00464-022-09402-8
Ouyang L, Zhang J, Feng Q, Zhang Z, Ma H, Zhang G (2022) Robotic versus laparoscopic pancreaticoduodenectomy: an up-to-date system review and meta-analysis. Front Oncol 12:834382. https://doi.org/10.3389/fonc.2022.834382
Lof S, Vissers FL, Klompmaker S, Berti S, Boggi U, Coratti A, Dokmak S, Fara R, Festen S, D’Hondt M, Khatkov I, Lips D, Luyer M, Manzoni A, Rosso E, Saint-Marc O, Besselink MG, Abu Hilal M, European consortium on Minimally Invasive Pancreatic Surgery (E-MIPS) (2021) Risk of conversion to open surgery during robotic and laparoscopic pancreatoduodenectomy and effect on outcomes: international propensity score-matched comparison study. Br J Surg 108(1):80–87. https://doi.org/10.1093/bjs/znaa026
Kamarajah SK, Bundred J, Marc OS, Jiao LR, Manas D, Abu Hilal M, White SA (2020) Robotic versus conventional laparoscopic pancreaticoduodenectomy a systematic review and meta-analysis. Eur J Surg Oncol 46:6–14. https://doi.org/10.1016/j.ejso.2019.08.007
van Hilst J, de Rooij T, Bosscha K, Brinkman DJ, van Dieren S, Dijkgraaf MG, Gerhards MF, de Hingh IH, Karsten TM, Lips DJ, Luyer MD, Busch OR, Festen S, Besselink MG, Dutch Pancreatic Cancer Group (2019) Laparoscopic versus open pancreatoduodenectomy for pancreatic or periampullary tumours (LEOPARD-2): a multicentre, patient-blinded, randomised controlled phase 2/3 trial. Lancet Gastroenterol Hepatol 4:199–207. https://doi.org/10.1016/S2468-1253(19)30004-4
Zwart MJW, Nota CLM, de Rooij T, van Hilst J, Te Riele WW, van Santvoort HC, Hagendoorn J, Borei Rinkes IHM, van Dam JL, Latenstein AEJ, Takagi K, Tran KTC, Schreinemakers J, van der Schelling GP, Wijsman JH, Festen S, Daams F, Luyer MD, de Hingh IHJT, Mieog JSD, Bonsing BA, Lips DJ, Hilal MA, Busch OR, Saint-Marc O, Zehl HJ 2nd, Zureikat AH, Hogg ME, Molenaar IQ, Besselink MG, Koerkamp BG, Dutch Pancreatic Cancer Group (2022) Outcomes of a multicenter training program in robotic pancreatoduodenectomy (LAELAPS-3). Ann Surg 276:e886–e895. https://doi.org/10.1097/SLA.0000000000004783
Sánchez-Velázquez P, Muller X, Malleo G, Park JS, Hwang HK, Napoli N, Javed AA, Inoue Y, Beghdadi N, Kalisvaart M, Vigia E, Walsh CD, Lovasik B, Busquets J, Scandavini C, Robin F, Yoshitomi H, Mackay TM, Busch OR, Hartog H, Heinrich S, Gleisner A, Perinel J, Passeri M, Lluis N, Raptis DA, Tschuor C, Oberkofler CE, DeOliveira ML, Petrowsky H, Martinie J, Asbun H, Adham M, Schulick R, Lang H, Koerkamp BG, Besselink MG, Han HS, Miyazaki M, Ferrone CR, Fernández-Del Castillo C, Lillemoe KD, Sulpice L, Boudjema K, Del Chiaro M, Fabregat J, Kooby DA, Allen P, Lavu H, Yeo CJ, Barroso E, Roberts K, Muiesan P, Sauvanet A, Saiura A, Wolfgang CL, Cameron JL, Boggi U, Yoon DS, Bassi C, Puhan MA, Clavien PA (2019) Benchmarks in pancreatic surgery: a novel tool for unbiased outcome comparisons. Ann Surg 270:211–218. https://doi.org/10.1097/SLA.0000000000003223
Prades J, Arnold D, Brunner T, Cardone A, Carrato A, Coll-Ortega C, De Luze S, Garel P, Goossens ME, Grilli R, Harris M, Louagie M, Malats N, Minicozzi P, Partelli S, Pastorekova S, Petrulionis M, Price R, Sclafani F, Smolkova B, Borras JM (2020) Bratislava Statement: consensus recommendations for improving pancreatic cancer care. ESMO Open 5:e001051. https://doi.org/10.1136/esmoopen-2020-001051
van der Geest LG, van Rijssen LB, Molenaar IQ, de Hingh IH, Groot Koerkamp B, Busch OR, Lemmens VE, Besselink MG, Dutch Pancreatic Cancer Group (2016) Volume-outcome relationships in pancreatoduodenectomy for cancer. HPB (Oxford) 18:317–324. https://doi.org/10.1016/j.hpb.2016.01.515
Nymo LS, Kleive D, Waardal K, Bringeland EA, Søreide JA, Labori KJ, Mortensen KE, Søreide K, Lassen K (2020) Centralizing a national pancreatoduodenectomy service: striking the right balance. BJS Open 4:904–913. https://doi.org/10.1002/bjs5.50342
Kim CG, Jo S, Kim JS (2012) Impact of surgical volume on nationwide hospital mortality after pancreaticoduodenectomy. World J Gastroenterol 18:4175–4181. https://doi.org/10.3748/wjg.v18.i31.4175
Levi Sandri GB, Abu Hilal M, Dokmak S, Edwin B, Hackert T, Keck T, Khatkov I, Besselink MG, Boggi U, Innovation E-AHPBA, Committee D (2022) Figures do matter: a literature review of 4587 robotic pancreatic resections and their implications on training. J Hepatobiliary Pancreat Sci. https://doi.org/10.1002/jhbp.1209
Nakata K, Shikata S, Ohtsuka T, Ukai T, Miyasaka Y, Mori Y, Velasquez VVDM, Gotoh Y, Ban D, Nakamura Y, Nagakawa Y, Tanabe M, Sahara Y, Takaori K, Honda G, Misawa T, Kawai M, Yamaue H, Morikawa T, Kuroki T, Mou Y, Lee WJ, Shrikhande SV, Tang CN, Conrad C, Han HS, Chinnusamy P, Asbun HJ, Kooby DA, Wakabayashi G, Takada T, Yamamoto M, Nakamura M (2018) Minimally invasive preservation versus splenectomy during distal pancreatectomy: a systematic review and meta-analysis. J Hepatobiliary Pancreat Sci 25:476–488. https://doi.org/10.1002/jhbp.569
Moekotte AL, Lof S, White SA, Marudanayagam R, Al-Sarireh B, Rahman S, Soonawalla Z, Deakin M, Aroori S, Ammori B, Gomez D, Marangoni G, Abu Hilal M, Minimally Invasive liver and Pancreatic Surgery Study Group-UK (MI-LAPS UK) (2020) Splenic preservation versus splenectomy in laparoscopic distal pancreatectomy: a propensity score-matched study. Surg Endosc 34:1301–1309. https://doi.org/10.1007/s00464-019-06901-z
Lee W, Hwang DW, Han HS, Han IW, Heo JS, Unno M, Ishida M, Tajima H, Nishizawa N, Nakata K, Seyama Y, Isikawa Y, Hwang HK, Jang JY, Hong T, Park JS, Kim HJ, Jeong CY, Matsumoto I, Yamaue H, Kawai M, Ohtsuka M, Mizuno S, Asakuma M, Soejima Y, Hirashita T, Sho M, Takeda Y, Park JI, Kim YH, Kim HJ, Yamaue H, Yamamoto M, Endo I, Nakamura M, Yoon YS (2022) Comparison of infectious complications after spleen preservation versus splenectomy during laparoscopic distal pancreatectomy for benign or low-grade malignant pancreatic tumors: A multicenter, propensity score-matched analysis. J Hepatobiliary Pancreat Sci. https://doi.org/10.1002/jhbp.1213
Choi SH, Seo MA, Hwang HK, Kang CM, Lee WJ (2012) Is it worthwhile to preserve adult spleen in laparoscopic distal pancreatectomy? Perioperative and patient-reported outcome analysis. Surg Endosc 26:3149–3156. https://doi.org/10.1007/s00464-012-2306-4
Dai MH, Shi N, Xing C, Liao Q, Zhang TP, Chen G, Wu WM, Guo JC, Liu ZW, Zhao YP (2017) Splenic preservation in laparoscopic distal pancreatectomy. Br J Surg 104:452–462. https://doi.org/10.1002/bjs.10434
Shi N, Liu SL, Li YT, You L, Dai MH, Zhao YP (2016) Splenic preservation versus splenectomy during distal pancreatectomy: a systematic review and meta-analysis. Ann Surg Oncol 23:365–374. https://doi.org/10.1245/s10434-015-4870-z
Ohtsuka T, Ban D, Nakamura Y, Nagakawa Y, Tanabe M, Gotoh Y, Velasquez VVDM, Nakata K, Sahara Y, Takaori K, Honda G, Misawa T, Kawai M, Yamaue H, Morikawa T, Kuroki T, Mou Y, Lee WJ, Shrikhande SV, Tang CN, Conrad C, Han HS, Palanivelu C, Asbun HJ, Kooby DA, Wakabayashi G, Takada T, Yamamoto M, Nakamura M (2018) Difficulty scoring system in laparoscopic distal pancreatectomy. J Hepatobiliary Pancreat Sci 25:489–497. https://doi.org/10.1002/jhbp.578
Liu Q, Zhao Z, Gao Y, Zhao G, Tan X, Wang C, Liu R (2020) Novel single-layer continuous suture of pancreaticojejunostomy for robotic pancreaticoduodenectomy. J Hepatobiliary Pancreat Sci 27:56–63. https://doi.org/10.1002/jhbp.682
Klompmaker S, van Hilst J, Wellner UF, Busch OR, Coratti A, D’Hondt M, Dokmak S, Festen S, Kerem M, Khatkov I, Lips DJ, Lombardo C, Luyer M, Manzoni A, Molenaar IQ, Rosso E, Saint-Marc O, Vansteenkiste F, Wittel UA, Bonsing B, Groot Koerkamp B, Abu Hilal M, Fuks D, Poves I, Keck T, Boggi U, Besselink MG, European consortium on Minimally Invasive Pancreatic Surgery (E-MIPS), (2020) Outcomes after minimally-invasive versus open pancreatoduodenectomy: a pan-european propensity score matched study. Ann Surg 271:356–363. https://doi.org/10.1097/SLA.0000000000002850
Menonna F, Napoli N, Kauffmann EF, Iacopi S, Gianfaldoni C, Martinelli C, Amorese G, Vistoli F, Boggi U (2021) Additional modifications to the Blumgart pancreaticojejunostomy: Results of a propensity score-matched analysis versus Cattel-Warren pancreaticojejunostomy. Surgery 169:954–962. https://doi.org/10.1016/j.surg.2020.08.013
Lombardo C, Iacopi S, Menonna F, Napoli N, Kauffmann E, Bernardini J, Cacciato Insilla A, Boraschi P, Donati F, Cappelli C, Campani D, Caramella D, Boggi U (2018) Incidence and reasons of pancreatic resection in patients with asymptomatic serous cystadenoma. Pancreatology 18:577–584. https://doi.org/10.1016/j.pan.2018.06.001
Boggi U (2022) Laparoscopic duodenum-preserving total pancreatic head resection for pancreatic tumors: the difficult balance among overtreatment, ideal treatment, and undertreatment. Langenbecks Arch Surg 407:3859–3861. https://doi.org/10.1007/s00423-022-02512-w
Hank T, Hinz U, Tarantino I, Kaiser J, Niesen W, Bergmann F, Hackert T, Büchler MW, Strobel O (2018) Validation of at least 1 mm as cut-off for resection margins for pancreatic adenocarcinoma of the body and tail. Br J Surg 105:1171–1181. https://doi.org/10.1002/bjs.10842
Jung HS, Kim HS, Kang JS, Kang YH, Sohn HJ, Byun Y, Han Y, Yun WG, Cho YJ, Lee M, Kwon W, Jang JY (2022) Oncologic benefits of neoadjuvant treatment versus upfront surgery in borderline resectable pancreatic cancer: a systematic review and meta-analysis. Cancers (Basel) 14:4360. https://doi.org/10.3390/cancers14184360
Esposito I, Kleeff J, Bergmann F, Reiser C, Herpel E, Friess H, Schirmacher P, Büchler MW (2008) Most pancreatic cancer resections are R1 resections. Ann Surg Oncol 15:1651–1660. https://doi.org/10.1245/s10434-008-9839-8
Acknowledgements
IGOMIPS was founded by the Associazione Italiana Studio Pancreas (AISP), the Società Italiana di Chirurgia Endoscopica (SICE), and the Italian Chapter of the International Hepato-Pancreato-Biliary Association (AICEP) under the auspices of the Italian Society of Surgery (SIC) and the Italian Association of Hospital Surgeons (ACOI). The authors gratefully thank AISP, SICE, and AICEP for their support to this national registry. Moreover, the authors would also like to thank the RicerChiAmo Onlus for its support.
Funding
Open access funding provided by Università di Pisa within the CRUI-CARE Agreement. The IGOMIPS registry is founded by several private bodies through AISP, including RicerChiAmo Onlus. This article did not receive any specific grant from funding agencies in public, commercial, or non-profit sectors.
Author information
Authors and Affiliations
Consortia
Contributions
All authors contributed to the conception of this work or to the acquisition and interpretation of data. All authors were involved in drafting and critical revision of the manuscript; they also approved the final version for publication. All authors agreed to be accountable for all aspects of the work. Surgeons contributing patient cases to this registry, but not qualifying for authorship, are listed in the Appendix. This study is based on the analysis of a national, prospective registry, on minimally invasive pancreatic surgery. All authors provided data of patients operated at their respective Institutions. Substantial contributions were made to the conception or design of the work (UB and AZ), the acquisition, analysis (GF, GB, LM, MAH, MV, FDB, RT, MV, EJ, AF, UB, SA, RC, GE, LM, CM, RDV, GE, RM, GZ, AB, SG, AB, AC, GG, RR, MM, and FB), interpretation of data for the work (GD, NN, SP, and AE), drafting of the work (UB and AZ), or revising it critically for important intellectual content (GB, RC, MF, and RS). All authors finally approved the version to be published. Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved (all authors).
Corresponding author
Ethics declarations
Conflict of interest
There are no conflict of interest or competing interests to declare. The authors declare they have no conflict of interest. The IGOMPIS registry was preregistered in the Registry of Patient Registries (RoPR) of the Agency for Healthcare and Research and Quality, US Department of Health (Registry of Patient Registries. Content last reviewed April 2019 https://www.ahrq.gov/ropr/index.html).
Ethics approval
This registry was conducted in compliance with applicable laws, regulations, and guidance. We obtained an independent ethics committee review as well as written and dated approval/favorable opinion from the independent ethics committee (IEC) for the registry protocol/amendment(s). The study was performed in accordance with the tenets of the Declaration of Helsinki.
Consent to participate
All patients provided written informed consent to participate in the study.
Research involving human participants and/or animals
The IGOMIPS registry was approved by the Independent Ethics Committee of the Humanitas Institute (authorization number 2167) and subsequently by local Ethics Committee of all participating centers.
Informed consent and ethical approval
All patients signed an informed consent to be included in the IGOMIPS registry and to use their anonymized data for research purposes.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The following surgeons contributed patients to the IGOMIPS registry, but do not qualify for full authorships. They should be listed as Collaborators are listed in the Supplementary Appendix Section.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Boggi, U., Donisi, G., Napoli, N. et al. Prospective minimally invasive pancreatic resections from the IGOMIPS registry: a snapshot of daily practice in Italy on 1191 between 2019 and 2022. Updates Surg 75, 1439–1456 (2023). https://doi.org/10.1007/s13304-023-01592-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13304-023-01592-7