Abstract
Post-resective liver failure is a frequent complication of liver surgery and it is due to portal hyperperfusion of the remnant liver and to arterial vasoconstriction, as buffer response of the hepatic artery. In this context, splenectomy allows a reduction of portal flow and increases the survival chance in preclinical models. SerpinB3 is over-expressed in the liver in oxidative stress conditions, as a mechanism of cell defense to provide survival by apoptosis inhibition and cell proliferation. In this study, the expression of SerpinB3 was assessed as predictor of liver damage in in vivo models of major hepatic resection with or without splenectomy. Wistar male rats were divided into 4 groups: group A received 30% hepatic resection, group B > 60% resection, group C > 60% resection with splenectomy and group D sham-operated. Before and after surgery liver function tests, echo Doppler ultrasound and gene expression were assessed. Transaminase values and ammonium were significantly higher in groups that underwent major hepatic resection. Echo Doppler ultrasound showed the highest portal flow and resistance of the hepatic artery in the group with > 60% hepatectomy without splenectomy, while the association of splenectomy determined no increase in portal flow and hepatic artery resistance. Only the group of rats without splenectomy showed higher shear-stress conditions, reflected by higher levels of HO-1, Nox1 and of Serpinb3, the latter associated with an increase of IL-6. In conclusion, splenectomy controls inflammation and oxidative damage, preventing the expression of Serpinb3. Therefore, SerpinB3 can be considered as a marker of post-resective shear stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Major hepatic resection (MHR) determines not only parenchymal loss, but also a reduction of intrahepatic vascularization, resulting in an increase of the specific (for gram of tissue) portal flow and of the portal pressure in the remnant liver [1,2,3]. The shear-stress response favors hepatic regeneration [4]; however, an excessive portal pressure might damage sinusoidal endothelium and Kupffer cells, releasing inflammatory cytokines, with collapse of the microcirculation and hepatocellular injury [5]. In this scenario, the overproduction of reactive oxygen species can contribute to vascular dysfunction and activation of oxidant-sensitive transcription factors [6]. In addition, the hepatic artery buffer response (HABR) generates arterial hypoperfusion of the remnant liver, as consequence of portal hyperperfusion [7]. These features might also be responsible for the biliary ischemic damage and cholestasis [8]. At cellular level, hepatic regeneration is a complex process that involves both mitosis and apoptosis strictly and chronologically regulated in the initial steps. Multiple signaling pathways, induced by inflammation and oxidative stress, lead to the production of growth factors, cytokines, chemokines and molecules which act at the same time in the liver [9,10,11]. Immediately after hepatectomy, several early genes are activated by transcription factors that are latent in the quiescent liver [12, 13] resulting in increased DNA synthesis, cell size and replication, with preservation of essential metabolic functions [9]. Among cytokines, interleukin-6 (IL-6) is considered a key player, acting not only as mitogenic, but also as an anti-apoptotic factor for hepatocytes, being responsible for the activation of approximately 40% of the involved genes [14, 15].
SerpinB3 (also known as SCCA1, Squamous Cell Carcinoma Antigen) is a protease inhibitor, almost undetectable in normal hepatocytes and over-expressed in hepatocellular carcinoma [16, 17] and in several tumors of epithelial origin [18, 19]. SerpinB3 (SB3) expression has also been demonstrated to increase in response to Tumor Necrosis Factor-α (TNF-α) and Ras-driven inflammation [20, 21], leading to apoptosis resistance [22, 23], cell proliferation and increased IL-6 signaling [24, 25] by acting as an autocrine and/or paracrine mediator. In addition, in transgenic mice, SB3 is associated with a hyperdynamic circulatory syndrome-like pattern, where hepatic artery pulsatility index and portal vein blood flow were significantly increased compared to controls [26]. It has been demonstrated that portal flow diversion can improve the outcome in experimental models of MHR, since rats that underwent splenectomy in association with hepatectomy presented levels of transaminases significantly lower than those with hepatectomy alone [27]. Furthermore, splenectomy associated to extreme liver resection can balance the HABR mechanism, since a greater flow of the hepatic artery has been described in rats with major hepatectomy and splenectomy compared with those without splenectomy [28, 29].
In this study, we have assessed the possible role of SB3 as a marker of shear stress using an in vivo rat model of MHR in the presence or absence of splenectomy.
Methods
Experimental model
Twenty-one male Wistar rats, weighting 220 to 390 g, were housed for at least 7 days in a light- and temperature-controlled room with access to food and water. Rats, randomly divided into 4 groups, underwent the following procedures:
-
Group A: 30% liver resection (N. 5 rats),
-
Group B: > 60% liver resection (N. 7 rats),
-
Group C: > 60% liver resection and splenectomy (N. 6 rats),
-
Group D: sham operated, control group (N. 3 rats).
The animals were bred at the Animal Care Facility of the Experimental Surgery Division of the University of Padova. Surgical operations were performed under the Italian law (n° 116/92), in accordance with European Union regulations. All procedures, including echo Doppler ultrasound, blood and tissue sampling have been performed under general anesthesia induced by the inhalatory administration of Sevoflurane (4 ml/min for the induction and 1.5–3.5 ml/min for the maintenance, according to reduced hepatic metabolization due to different size liver resections) that was mixed to oxygen 0.5 L/min throughout the procedure.
Immediately before and 2 h after the surgical procedures, blood samples were obtained from the caudal vein of the rats from each group to assess standard liver function parameters including aspartate aminotransferase (AST), alanine aminotransferase (ALT), lactate dehydrogenase (LDH), Ammonium and Lactic Acid.
After midline laparotomy, hepatic and splenic intra-operatory echo Doppler ultrasound was carried out in each group. Liver lobules were subsequently mobilized by cutting the falciform and hepatogastric ligaments. The liver mass was reduced by resection of the left lateral lobe (Group A) and the left lateral, right lobe and caudate lobe (Group B and C). Liver resection was carried out with piercing sutures through the liver parenchyma with 5/0 silk. Rats from Group C received splenectomy together with liver resection and the splenic vessels were sectioned at the hilum after sutures with 5/0 silk. The control Group D did not receive any resection. The resected liver parenchyma was weighted and stored for further analysis, while the abdomen was closed by a suture in layers.
After an observation period of 2 h all the animals were subjected to blood sampling, as previously described, re-laparotomy and intra-operatory echo Doppler ultrasound. The remnant liver underwent en-bloc hepatectomy, was weighted and frozen in part, while the remaining liver specimens were fixed in 10% buffered formaldehyde and embedded in paraffin. Animals were killed by the inhalatory administration of CO2.
Echo Doppler ultrasound measurements
Echo Doppler ultrasound of splanchnic vessels was carried out in each group using a dedicated apparatus (Vevo2100 Visualsonics, probe MS-550D, 22–55 MHz) equipped with heated table that allowed monitoring of heart frequency, respiratory frequency and electro-cardiography as previously reported [30]. To obtain an optimal ultrasound interface, peritoneal cavity of each rat was filled with heated sterile saline solution, to permit adequate Doppler measurements without compressing examined parenchymas. Portal vein (PV) diameter, velocity and flow were assessed immediately upstream the emergency of the first portal branch. The Hepatic Artery (HA), Pulsatility Index (PI) and Resistance Index (RI) were evaluated at the hepatic hilum, while the Splenic Artery (SA), PI and RI were measured at the splenic hilum.
Histopathological analysis
Standard staining of the remnant liver with hematoxylin–eosin was used for light microscopy and the histopathological parameters analyzed were: vascular congestion, edema and glycogenic charge.
Molecular techniques
The frozen liver specimens were analyzed by real-time PCR to assess mRNA expression of Sb3, genes linked to inflammation as IL-6, TNF-α and oxidative stress genes as Heme Oxygenase 1 (HO-1), NADPH Oxidase 1 (Nox1) and NADPH Oxidase 2 (Nox2).
Total RNA was extracted using RNeasy Mini Kit (Qiagen GmbH, Hilden, Germany) according to the manufacturer’s instructions. After determination of the purity and the integrity of total RNA, 2 µg of each sample was reverse transcribed in cDNA using iScript cDNA Synthesis Kit (Bio-Rad, Milan, Italy). The reaction was performed at 25 °C for 5 min, then activation of the reverse transcriptase at 42 °C for 30 min and lastly at 85 °C for 5 min.
The real-time PCR was performed by the SYBR Green assay using a FastStart DNA MasterPLUS SYBR Green KitTM (Roche, Monza, Italy) in glass capillaries where the specimen was mixed with a blend containing a fluorescent molecule. After an initial denaturation step at 95 °C for 10 min, 45 cycles of amplification were carried out and included the following conditions: denaturation at 95 °C for 10 s, annealing at 60 °C for 30 s and extension at 72 °C for 10 s. Amplification of specific transcripts was confirmed by melting curve profiles at the end of each PCR cycle, using the specific routine built-up in the Light Cycler instrument. The housekeeping gene Hypoxanthine Phosphoribosyl Transferase (HPRT) was amplified in parallel in all amplification sets.mRNA expression of interest genes was calculated according to the threshold cycle of individual genes and the results were expressed as a relative ratio of the target to the housekeeping gene using the Light Cycler Relative Quantification software 4.05 (Roche Diagnostics, Monza, Italy). A negative control, samples containing all reagents but no cDNA template, were included in all runs.
Primers, designed from sequences of rat derived from the GenBank database using Primer 3 (Whitehead Institute, Massachusetts, USA) and Operon’s Oligo software (Operon, California, USA) and purchased from Eurofins MWG (Ebersberg, Germany) are reported in Table 1 (Supplementary material).
Statistical analysis
Each basal biochemical value, gene expression value and echo Doppler parameter has been compared to the basal values of the other groups and the same was carried out for the values obtained after the observation time. Moreover, a comparison was made between the basal and the final values in the context of each group. The following non-parametric tests have been chosen: Wilcoxon test for the comparison between two groups of paired data, Kruskal–Wallis test and Dunn post test for the comparison among more than two groups and Spearman test for the correlation in gene expression. Significance was established at p value < 0.05. The statistical significance of all tests used in the study was Employed software: GraphPad InStat (San Diego, CA, USA).
Results
Biochemical parameters
The group of rats that underwent > 60% hepatectomy showed a higher increase of biochemical parameters, including transaminases (AST, p = 0.017; ALT, p = 0.017) and ammonium (p = 0.048), compared to the groups of sham-operated controls and rats with > 60% hepatectomy and splenectomy, where the ammonium levels were even below the median values observed in controls. Among the groups with > 60% liver removal, in rats with associated splenectomy, transaminases showed a higher, although not significant trend increase, possibly due to the major surgical time of the procedure.
In addition, no significant differences between pre-operative and post-operative values were observed for LDH, tended to be more elevated in the group with major hepatectomy, and Lactic Acid in each group and neither among different groups (Fig. 1).
Hemodynamic effects
Portal vein flow velocity
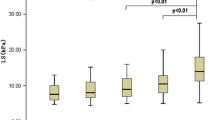
Overall mean portal velocity in each group did not show significant variations after surgical intervention (Fig. 2a), although the comparison between pre- and post-operative median velocities in group with splenectomy showed a trend in reduction even if it did not achieve statistical significance (Fig. 2b). These findings were not associated with changes of PV diameter. The median values of right portal flow showed a significant increase in post-operative period in the group of rats with > 60% hepatectomy (p = 0.047) (Fig. 3b). No statistical difference was evident among the other groups, even if in rats with > 60% hepatectomy with splenectomy a trend in reduction of portal flow was observed in post-operative period, likely due to removal of spleen contribution to portal flow (Fig. 3a and b).
Echo Doppler ultrasound results in PV, HA and SA. a Mean PV velocity pre and post > 60% hepatectomy and splenectomy, p = 0.047. b PV flow pre and post > 60% hepatectomy, p = 0.047. c HA Pulsatility Index (HA-PI) pre and post > 60% hepatectomy, p = 0.016. d HA Resistive Index (HA-RI) pre and post > 60% hepatectomy, p = 0.016. e SA Pulsatility Index (SA-PI) pre and post > 60% hepatectomy, p = 0.031. f SA Resistive Index (SA-RI) pre and post > 60% hepatectomy, p = 0.031
HA Doppler measurements
HA hemodynamic evaluation was carried out analyzing Pulsatility Index (HA-PI) and Resistive Index (HA-RI). Considering the median PI and RI of HA, we observed a significant increase between the pre-operative and post-operative period in the group of rats with > 60% hepatectomy (p = 0.016 for both HA-PI and HA-RI) (Fig. 3c and d), while pre- and post-operative values were similar in the other groups.
SA Doppler measurements
As for HA, even for SA Pulsatility Index (SA-PI) and Resistive Index (SA-RI) were considered. The median PI and RI of splenic artery could not be measured in one animal of group B due to technical reasons and in rats of group C in post-operative period due to splenectomy.
As shown in Fig. 3e and 3f, both PI and RI showed a significant increase in post-operative period in group with > 60% hepatectomy (p = 0.031 for both), while these parameters were unchanged in the group of rats with 30% hepatectomy and in sham-operated controls.
Liver tissue analysis
A rise in Sb3 levels, that is not physiologically detectable in normal liver, was observed after 2 h from surgery in the remnant liver only in rats that underwent > 60% hepatectomy (p = 0.042), while remained undetectable in the livers of all the other groups of rats, including those with > 60% hepatectomy with associated splenectomy (Fig. 4a).
mRNA expression in rat liver tissue detected by real-time polymerase chain reaction. a mRNA expression of Sb3 in rat liver after different degrees of hepatectomy. b Spearman rank correlation analysis of Sb3 mRNA and IL-6 mRNA levels. c mRNA expression of inflammatory cytokines and oxidative stress genes. Changes in mRNA gene expression are expressed as fold increase relative to resection at time 0 using the 2−ΔΔCT method. The results were normalized to the HPRT housekeeping gene. Columns represent median values and vertical bars represent standard error median
In parallel with these findings, only in the remnant liver of rats with > 60% hepatectomy a significantly higher upregulation of IL-6 (p = 0.004), of HO-1 (p = 0.036) and of Nox1 (p = 0.042) was observed, while these molecules were not upregulated in the group of rats with > 60% hepatectomy and splenectomy. It is worth to note that a significant overall direct correlation between IL-6 and Sb3 expression levels (Spearman test r = 0.6727, p = 0.039) was observed (Fig. 4b). TNF-α and Nox2 were not significantly affected by surgical procedures (Fig. 4c), at least at the chosen times of analysis.
Histopathologic analysis
In the remnant liver of the groups of rats with > 60% hepatectomy, either with or without splenectomy, a reduction of glycogen amount and mild edema increase was observed 2 h after resection, while vascular congestion was more increased in the group without splenectomy than in that with splenectomy (Fig. 5, Supplementary material), supporting the higher shear stress of the former group.
Discussion
The present study was carried out to evaluate the effect of different approaches of extreme hepatectomy on shear stress and to assess the possible usefulness of SB3 expression to monitor this phenomenon. Extreme hepatectomy (> 60%) with or without splenectomy determined a significant cytolysis with increased transaminase release, as previously described [29]. However, porto-arterial hemodynamics revealed a decreased relative portal flow and HABR response in rats that underwent splenectomy in association with extreme hepatectomy, supporting other preclinical studies, where different types of measurement were used [28].
At the same time, massive hepatectomy induced an increase in hepatic PI and RI, indicating arterial vasoconstriction that was not observed in minor hepatic resections or in case of associated splenectomy. Thus, the association between high-volume hepatectomy and splenectomy, showing mean portal flow and hepatic arterial resistance similar to minor hepatic resection and control groups, seems to prevent the hepatic hemodynamic alterations caused by excessive portal flow and associated HABR. The reduction of portal flow, determined by the splenectomy associated with MHR, has been indeed found to improve survival and to decrease hepatic cell damage [27, 28]. On the other hand, in rats with high-volume hepatectomy without splenectomy an increase in splenic arterial resistive and pulsatility index was registered, that can reflect the occurrence of some degree of portal hypertension determined in this case, as previously shown in humans [31].
As result of hemodynamic alterations following liver resection, a wide number of homeostasis regulators, as transcriptional factors, growth factors and cytokines are induced [32], leading to increased oxidative stress and hypoxia, likely accelerating liver regeneration [33]. In particular, oxidative stress conditions can induce the synthesis and release of SB3 [23], a molecule that we have previously observed to confer, after partial hepatectomy, resistance to apoptotic cell death and an additional stimulus for liver cell proliferation, leading to a final improvement of liver growth, at least in part determined by IL-6 production [24].
On basis of the scientific evidences about the activity of SB3 [19], this molecule might counteract acute liver damage by activation of pathways leading to hepatic regeneration, apoptosis inhibition and oxidative stress reduction. In fact, the levels of Sb3 raised only after MHR, where the shear stress was higher than in the other groups of animals, exposed to lower hepatectomy or protected by splenectomy. In agreement with these findings, the remnant livers of rats that underwent MHR showed not only higher values of the oxidative stress molecule HO-1, as shown by Solangi et al. [34], but also increased levels of IL-6, that were positively correlated with Sb3, likely acting as protective molecular response in this contest. IL-6 acts on hepatocytes via the IL-6 receptor, activating the signal transducer and activator of transcription 3 (STAT3) that in turn activates transcription of target genes at nuclear level [35,36,37] leading to the initiation of liver regeneration [38,39,40]. It is worth to note that STAT3 is also able to bind the promoter of SB3 activating its expression [41] and determining a positive loop between these two molecules.
In conclusion, in our experimental context of MHR, the resulting increased shear stress due to portal hyperperfusion, associated with increased arterial resistance, determines increased liver oxidative stress that up-regulates the expression of Sb3, likely as a protective mechanism. Therefore, SB3 in the remnant liver might be considered as a hepatic marker of post-resective shear stress. If these results will be confirmed in surgical practice, this molecule could become a prognostic parameter that could support clinical decisions.
Data Availability
The data presented in this study are available on request from the corresponding author.
References
Kubota K, Makuuchi M, Kusaka K, Kobayashi T, Miki K, Hasegawa K, Harihara Y, Takayama T (1997) Measurement of liver volume and hepatic functional reserve as a guide to decision-making in resectional surgery for hepatic tumors. Hepatology 26(5):1176–1181. https://doi.org/10.1053/jhep.1997.v26.pm0009362359
Lin XZ, Sun YN, Liu YH, Sheu BS, Cheng BN, Chen CY, Tsai HM, Shen CL (1998) Liver volume in patients with or without chronic liver diseases. Hepatogastroenterology 45(22):1069–1074
Kin Y, Nimura Y, Hayakawa N, Kamiya J, Kondo S, Nagino M, Miyachi M, Kanai M (1994) Doppler analysis of hepatic blood flow predicts liver dysfunction after major hepatectomy. World J Surg 18(1):143–149. https://doi.org/10.1007/BF00348207
Niiya T, Murakami M, Aoki T, Murai N, Shimizu Y, Kusano M (1999) Immediate increase of portal pressure, reflecting sinusoidal shear stress, induced liver regeneration after partial hepatectomy. J Hepatobiliary Pancreat Surg 6(3):275–280. https://doi.org/10.1007/s005340050118
Fukauchi T, Hirosi H, Onitsuka A, Hayashi M, Senga S, Imai N, Shibata M, Yamauchi K, Futamura N, Sumi Y (2000) Effects of portal-systemic shunt following 90% partial hepatectomy in rats. J Surg Res 89(2):126–131. https://doi.org/10.1006/jsre.1999.5810
Pibiri M, Leoni VP, Atzori L (2018) Heme oxygenase-1 inhibitor tin-protoporphyrin improves liver regeneration after partial hepatectomy. Life Sci 204:9–14. https://doi.org/10.1016/j.lfs.2018.05.011
Smyrniotis V, Kostopanagiotou G, Kondi A, Gamaletsos E, Theodoraki K, Kehagias D, Mystakidou K, Contis J (2002) Hemodynamic interaction between portal vein and hepatic artery flow in small-for-size split liver transplantation. Transpl Int 15(7):355–360. https://doi.org/10.1007/s00147-002-0425-x
Kelly DM, Zhu X, Shiba H, Irefin S, Trenti L, Cocieru A, Diago T, Wang LF, Quintini C, Chen Z, Alster J, Nakagawa S, Miller C, Demetris A, Fung JJ (2009) Adenosine restores the hepatic artery buffer response and improves survival in a porcine model of small-for-size syndrome. Liver Transpl 15(11):1448–1457. https://doi.org/10.1002/lt.21863
Michalopoulos GK (2007) Liver regeneration. J Cell Physiol 213(2):286–300
Mao SA, Glorioso JM, Nyberg SL (2014) Liver regeneration. Transl Res 163(4):352–362. https://doi.org/10.1016/j.trsl.2014.01.005
Zima T, Kalousova M (2005) Oxidative stress and signal transduction pathways in alcoholic liver disease. Alcohol Clin Exp Res 29(11 Suppl):110S-115S. https://doi.org/10.1097/01.alc.0000189288.30358.4b
Arai M, Yokosuka O, Chiba T, Imazeki F, Kato M, Hashida J, Ueda Y, Sugano S, Hashimoto K, Saisho H, Takiguchi M, Seki N (2003) Gene expression profiling reveals the mechanism and pathophysiology of mouse liver regeneration. J Biol Chem 278(32):29813–29818. https://doi.org/10.1074/jbc.M212648200
Haber BA, Mohn KL, Diamond RH, Taub R (1993) Induction patterns of 70 genes during nine days after hepatectomy define the temporal course of liver regeneration. J Clin Invest 91(4):1319–1326. https://doi.org/10.1172/JCI116332
Li W, Liang X, Leu JI, Kovalovich K, Ciliberto G, Taub R (2001) Global changes in interleukin-6–dependent gene expression patterns in mouse livers after partial hepatectomy. Hepatology 33(6):1377–1386. https://doi.org/10.1053/jhep.2001.24431
Jin X, Zimmers TA, Perez EA, Pierce RH, Zhang Z, Koniaris LG (2006) Paradoxical effects of short- and long-term interleukin-6 exposure on liver injury and repair. Hepatology 43(3):474–484. https://doi.org/10.1002/hep.21087
Pontisso P, Calabrese F, Benvegnù L, Lise M, Belluco C, Ruvoletto MG, Marino M, Valente M, Nitti D, Gatta A, Fassina G (2004) Overexpression of squamous cell carcinoma antigen variants in hepatocellular carcinoma. Br J Cancer 90(4):833–837. https://doi.org/10.1038/sj.bjc.6601543
Guido M, Roskams T, Pontisso P, Fassan M, Thung SN, Giacomelli L, Sergio A, Farinati F, Cillo U, Rugge M (2008) Squamous cell carcinoma antigen in human liver carcinogenesis. J Clin Pathol 61(4):445–447. https://doi.org/10.1136/jcp.2007.051383
Kato H (1996) Expression and function of squamous cell carcinoma antigen. Anticancer Res 16(4B):2149–2153
Sun Y, Sheshadri N, Zong WX (2017) SERPINB3 and B4: From Biochemistry to Biology. Semin Cell Dev Biol 62:170–177. https://doi.org/10.1016/j.semcdb.2016.09.005
Numa F, Takeda O, Nakata M, Nawata S, Tsunaga N, Hirabayashi K, Suminami Y, Kato H, Hamanaka S (1996) Tumor necrosis factor-alpha stimulates the production of squamous cell carcinoma antigen in normal squamous cells. Tumour Biol 17(2):97–101. https://doi.org/10.1159/000217972
Catanzaro JM, Sheshadri N, Zong WX (2014) SerpinB3/B4: mediators of Ras-driven inflammation and oncogenesis. Cell Cycle 13(20):3155–3156. https://doi.org/10.4161/15384101.2014.969991
Vidalino L, Doria A, Quarta S, Zen M, Gatta A, Pontisso P (2009) SERPINB3, apoptosis and autoimmunity. Autoimmun Rev 9(2):108–112. https://doi.org/10.1016/j.autrev.2009.03.011
Ciscato F, Sciacovelli M, Villano G, Turato C, Bernardi P, Rasola A, Pontisso P (2014) SERPINB3 protects from oxidative damage by chemotherapeutics through inhibition of mitochondrial respiratory complex I. Oncotarget 5(9):2418–2427. https://doi.org/10.18632/oncotarget.1411
Villano G, Quarta S, Ruvoletto MG, Turato C, Vidalino L, Biasiolo A, Tono N, Lunardi F, Calabrese F, Dall’olmo L, Dedja A, Fassina G, Gatta A, Pontisso P (2010) Role of squamous cell carcinoma antigen-1 on liver cells after partial hepatectomy in transgenic mice. Int J Mol Med 25(1):137–143
Sheshadri N, Catanzaro JM, Bott AJ, Sun Y, Ullman E, Chen EI, Pan JA, Wu S, Crawford HC, Zhang J, Zong WX (2014) SCCA1/SERPINB3 promotes oncogenesis and epithelial-mesenchymal transition via the unfolded protein response and IL6 signaling. Cancer Res 74(21):6318–6329. https://doi.org/10.1158/0008-5472.CAN-14-0798
Villano G, Verardo A, Martini A, Brocco S, Pesce P, Novo E, Parola M, Sacerdoti D, Di Pascoli M, Fedrigo M, Castellani C, Angelini A, Pontisso P, Bolognesi M (2020) Hyperdynamic circulatory syndrome in a mouse model transgenic for SerpinB3. Ann Hepatol 19(1):36–43. https://doi.org/10.1016/j.aohep.2019.06.021
Glanemann M, Eipel C, Nussler AK, Vollmar B, Neuhaus P (2005) Hyperperfusion syndrome in small-for-size livers. Eur Surg Res 37(6):335–341. https://doi.org/10.1159/000090333
Eipel C, Abshagen K, Ritter J, Cantré D, Menger MD, Vollmar B (2010) Splenectomy improves survival by increasing arterial blood supply in a rat model of reduced-size liver. Transpl Int 23(10):998–1007. https://doi.org/10.1111/j.1432-2277.2010.01079.x
Di Domenico S, Santori G, Traverso N, Balbis E, Furfaro A, Grillo F, Gentile R, Bocca B, Gelli M, Andorno E, Dahame A, Cottalasso D, Valente U (2011) Early effects of portal flow modulation after extended liver resection in rat. Dig Liver Dis 43(10):814–822. https://doi.org/10.1016/j.dld.2011.05.018
Di Pascoli M, Zampieri F, Verardo A, Pesce P, Turato C, Angeli P, Sacerdoti D, Bolognesi M (2016) Inhibition of epoxyeicosatrienoic acid production in rats with cirrhosis has beneficial effects on portal hypertension by reducing splanchnic vasodilation. Hepatology 64(3):923–930. https://doi.org/10.1002/hep.28686
Bolognesi M, Sacerdoti D, Merkel C, Gerunda G, Maffei-Faccioli A, Angeli P, Jemmolo RM, Bombonato G, Gatta A (1996) Splenic Doppler impedance indices: influence of different portal hemodynamic conditions. Hepatology 23(5):1035–1040. https://doi.org/10.1002/hep.510230515
Michalopoulos GK (2010) Liver regeneration after partial hepatectomy. Am J Path 176(1):2–13. https://doi.org/10.2353/ajpath.2010.090675
Schadde E, Tsatsaris C, Swiderska-Syn M, Breitenstein S, Urner M, Schimmer R, Booy C, Zgraggen BR, Wenger RH, Spahn DR, Hertl M, Knechtle S, Diehl AM, Schläpfer M, Beck-Schimmer B (2017) Hypoxia of the growing liver accelerates regeneration. Surgery 161(3):666–679. https://doi.org/10.1016/j.surg.2016.05.018
Solangi K, Sacerdoti D, Goodman AI, Schwartzman ML, Abraham NG, Levere RD (1988) Differential effects of partial hepatectomy on hepatic and renal heme and cytochrome P450 metabolism. Am J Med Sci 296(6):387–391. https://doi.org/10.1097/00000441-198812000-00004
Cressman DE, Diamond RH, Taub R (1995) Rapid activation of the Stat3 transcription complex in liver regeneration. Hepatology 21(5):1443–1449
Taub R (2004) Liver regeneration: from myth to mechanism. Nat Rev Mol Cell Biol 5(10):836–847. https://doi.org/10.1038/nrm1489
Heinrich PC, Behrmann I, Haan S, Hermanns HM, Müller-Newen G, Schaper F (2003) Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J 374(Pt 1):1–20. https://doi.org/10.1042/BJ20030407
Akerman P, Cote P, Yang SQ, McClain C, Nelson S, Bagby GJ, Diehl AM (1992) Antibodies to tumor necrosis factor-alpha inhibit liver regeneration after partial hepatectomy. Am J Physiol 263(4 Pt 1):G579–G585. https://doi.org/10.1152/ajpgi.1992.263.4.G579
Cressman DE, Greenbaum LE, DeAngelis RA, Ciliberto G, Furth EE, Poli V, Taub R (1996) Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science 274(5291):1379–1383. https://doi.org/10.1126/science.274.5291.1379
Li W, Liang X, Kellendonk C, Poli V, Taub R (2002) STAT3 contributes to the mitogenic response of hepatocytes during liver regeneration. J Biol Chem 277(32):28411–28417. https://doi.org/10.1074/jbc.M202807200
Ahmed ST, Darnell JE Jr (2009) Serpin B3/B4, activated by STAT3, promote survival of squamous carcinoma cells. Biochem Biophys Res Commun 378(4):821–825. https://doi.org/10.1016/j.bbrc.2008.11.147
Funding
Open access funding provided by Università degli Studi di Padova within the CRUI-CARE Agreement. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
EG: conceptualization, methodology, project administration, writing—original draft, writing—review and editing. GV: formal analysis, data curation, writing—original draft, visualization. SB: investigation, data curation, writing—original draft. MP: investigation, resources. FC: data curation, validation, supervision. DS: validation, supervision. UC: validation, supervision, project administration. PP: writing—original draft, writing—review and editing, funding acquisition. All the authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical standards, Research involving human participants and/or animals, and Informed consent
Animals experiments were conducted in accordance with the Principles of laboratory animal care (NIH publication no. 85–23, revised 1985; http://grants1.nih.gov/grants/olaw/references/phspol.htm).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gringeri, E., Villano, G., Brocco, S. et al. SerpinB3 as hepatic marker of post-resective shear stress. Updates Surg 75, 1541–1548 (2023). https://doi.org/10.1007/s13304-023-01531-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13304-023-01531-6