Abstract
Common complications of coronavirus disease 2019 (COVID-19) related ARDS and ventilation are barotrauma-induced pneumothorax, pneumatocele and/or empyema. We analysed indications and results of video-assisted thoracoscopic surgery (VATS) in complicated COVID-19 patients. This is a retrospective single-institution study analysing a case series of patients treated by VATS for secondary spontaneous pneumothorax (SSP), pneumatocele and empyema complicating COVID-19, not responding to drainage in Lodi Maggiore Hospital between February 2020 and May 2021. Out of 2076 patients hospitalized in Lodi Maggiore Hospital with COVID-19, nine Males (0,43%; mean age 58,1–33–81) were treated by VATS for complications of pneumonia (6 SSP and 3 empyema; 1 case complicated by haemothorax). 7 patients (77%) had CPAP before surgery for 21.3 days mean (4–38). Mean Operative time was 80.9 min (38–154). Conversion rate was 0%. 3 (33%) patients were admitted to ICU before VATS. Treatments were: bullectomy in six patients (66%), drainage of the pleural space in all patients, pleural decortication and fluid aspiration in five cases (55%). two patients (22%) needed surgery interruption and bilateral ventilation to restore adequate oxygenation. Mortality was 1/9 (11%) due to respiratory failure for persistent pneumonia. In one patient (11%) redo surgery was performed for bleeding. Mean postop Length of Stay (LOS) was 37.9 days (10–77). Our report shows that VATS can be considered an extreme, but effective treatment for COVID-19 patients with SSP, pneumatocele or empyema, for patients who can tolerate general anaesthesia. Attention must be paid to the aerosol-generation of infected droplets.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The clinical and epidemiological characteristics of the new Coronavirus infection (SARS-CoV-2) led the World Health Organization to declare on March 11th, 2020 a public health emergency of international concern and a worldwide pandemic. As of May 5th, 2022 a total of 512,690,034 confirmed cases and 6,252,316 deaths had been reported across 587 countries all over the world [1]. Coronavirus disease 2019 (COVID-19) causes an interstitial pneumonia with different degrees of severity. In most cases the infection leads to a mild respiratory, flu-like syndrome with fever, fatigue and dry cough, but in 10–20% of cases it may progress to a severe clinical syndrome with serious respiratory distress, usually appearing 8–14 days after the onset of the illness [2,3,4]. Acute respiratory distress syndrome (ARDS) is the main cause of respiratory failure, and it is associated with high morbidity and mortality rates [5]. The affected patients need increasing respiratory support, including nasal catheters and masks, high-flow nasal cannula, non-invasive ventilation (NIV) and mechanical ventilation. A common complication of ARDS, and the consequent need of ventilation (NIV or mechanical), is barotrauma that may lead to pneumothorax and pneumomediastinum [6, 7].
The Maggiore Hospital of Lodi is the first line hospital in the epicentre of the Italian pandemic. Thoracic complications of COVID-19 are usually treated by drainage. In selected cases, when drainage treatments fails, surgical treatment with video-assisted thoracoscopic surgery (VATS) might be the last management option. We hereby report our series of cases surgically treated in a single-institution, analysing indications, treatments and outcomes.
Methods
Lodi Maggiore hospital was the very first epicentre of the first wave of COVID-19 pandemic in Western countries. This is a retrospective single-institution study analysing our case series of patients treated by VATS for secondary spontaneous pneumothorax (SSP), pneumatocele, and/or empyema complicating coronavirus disease 2019 (COVID-19) not responding to drainage and/or antibiotic treatment, between February 2020 and May 2021. All the patients in this series had confirmed COVID-19 diagnosis by reverse transcriptase polymerase chain reaction (rtPCR).
All the patients with complications of COVID-19 had thoracic CT scan. VATS was defined as a procedure performed under general anaesthesia in operatory room with possible monopulmonary ventilation using 3–4 thoracoport to access in the thoracic cavity. Indications for surgery were complicated SSP, pneumatocele or empyema not responding to treatment with worsening respiratory failure and/or sepsis or eventually worsening pneumothorax despite drainage positioning. All surgery were performed by 1 surgical team fully protected by PPE; no member of the team was contaminated during the observational time. Data collection was performed after December 2021 to allow at least a 3-month follow-up (mean follow-up 13.1 months, range 3–23).
The primary end point of this study was to evaluate indications for surgery in complicated COVID-19 patients, the number and incidence of complications requiring surgery, and finally the early outcomes of these procedures.
Results
In Lodi Maggiore Hospital, 3984 patients were treated for suspected or confirmed COVID-19 between Feb 2020 and May 2021. 2076 patients were hospitalized due to respiratory failure needing oxygen supply and/or CPAP, and/or intubation and ICU. 9 cases (0,43%) underwent VATS. All patients were males (100%) mean age was 58.1 (33–81). Complications of pneumonia leading to VATS were 6 SSP (66%) and 3 Empyema (33%); in 1 case complicated by haemothorax (11%). All the complications did not improve after first line treatments such as drainage and or antibiotic. Complicated patients had a mean BMI of 25.4 (19.1–31.1). 7 patients (77%) had CPAP before surgery for a mean of 21.3 days (4–38). Mean Positive End Expiratory Pressure (PEEP) during CPAP before surgery was 21.3 (4–38). Mean Operative time for VATS was 80.9 min (38–154). 3 patients (33%) were in ICU before VATS. No procedure was converted to open thoracotomy (conversion rate 0%). Treatments were bullectomy with pneumatocele resection in six patients (66%), drainage in the pleural space in all the patients, in 5 cases (55%) pleural decortications, fluid aspiration and collection of samples for cultural purposes to assess the correct antimicrobial management. In two patients (22%) respiratory failure during surgery needed bilateral ventilation to restore adequate value of Hb saturation and/or paO2. After restoring of adequate paO2 the surgical procedure restarted. In 1 case (11%—patients 8) haemorrhagic complication—due to bleeding in the chest wall causing massive haemothorax—was managed by redo surgery on postop day 6 with a mini thoracotomy, control of the source of bleeding by ligation of intercostal vessels and drainage with no subsequent recurrence of haemothorax. Mortality rate was 1/9 (11%) due to respiratory failure for persistent pneumonia and sepsis, 5 days after surgery (patients 2—CT scan on postop day 4 showing absence of residual pneumothorax but persistent ground glass bilateral pneumonia). Mean postoperative length of stay was 37.9 days (10–77).
Cfr. Table 1 (Pts, Comorbidities, Outcomes).
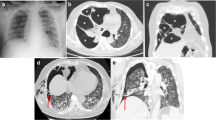
(Figs. 1, 2, 3, 4 and 5 diagnostic and intraoperative imaging).
Discussion
The virus that causes COVID-19 leads to a severe acute respiratory syndrome. The major morbidity and mortality is largely due to an interstitial pneumonia that evolves to ARDS in 10–20% of patients [5]. A series of complications tend to occur, especially in critically ill patients admitted to ICU, including shock, sepsis and multi-organ dysfunction [8]. One of the main causes of sepsis is the parapneumonic superinfection: most common micro-organisms isolated in COVID-19 patients include multi-drug resistant Acinetobacter baumannii, Klebsiella pneumoniae, Pseudomonas aeruginosa, Candida albicans and Candida glabrata [4]. In our case, the second patient resulted positive for Candida glabrata and MRSA, worsening his already compromised general conditions. Moreover, the progressive inflammation of interstitial pneumonia results in fibrin accumulation and pulmonary fibrosis. This structural remodelling leads to a higher risk of alveolar rupture [9] and subsequent secondary spontaneous pneumothorax (SSP) and/or pneumatocele.
In COVID-19 patients, oxygen support is the mainstay of treatment: nasal catheters and masks for mild and moderate patients, HFNC, non-invasive, and mechanical ventilation for severe critical patients.
Considering the increased stiffness of the lung, these patients require higher levels of PEEP to maximize and maintain alveolar recruitment and, thereby, to improve blood oxygenation. In fact, an Italian case series showed that COVID-19 patients in Lombardy required mechanical ventilation with a median PEEP level of 14 cm H2O [10].
Since one of the well-known complications of ventilatory support is barotrauma (4–15%) [11], the association between the rigidity of the alveolar wall and the need of high-pressure ventilation places COVID-19 patients at increased risk of SSP. During SARS-CoV-1 pandemic of 2003 Yam [et al.] similarly reported an incidence of barotrauma of 6,6–15% associated to NIV and 34% related to mechanical ventilation [6]. Most of the patients in the present case series developed SSP when a severe lung injury had already progressed after receiving non-invasive or mechanical ventilation for several days.
The first line treatment of pneumothorax is the insertion of an intercostal chest tube. However, SSP has to be considered different from primary pneumothorax, since it is related to life-threatening symptoms, various localizations of bullae, high rates of morbidity and mortality and a higher recurrence after chest drain. On these bases the British Thoracic Society Guidelines [12] advises a more aggressive and emergent treatment in SSP, proposing a timely surgical approach. The surgical treatment consists of a bullectomy under thoracoscopic vision, aiming to treat only the bullae associated with air-leakage by mechanical stapling. In the reported cases, the chest tube was not sufficient to avoid the lung shrinkage, so it was decided to promptly perform VATS under general anaesthesia, to repair the leakage. The main contraindications to surgical approach are strictly related to the patient anaesthetic risk and the requirement of single-lung ventilation. All these observations may lead to ask whether COVID-19 patients who develop SSP are suitable for surgery.
There is an analogue series published by Chang and colleagues [14] of 13 patients managed by thoracic surgery in New York. The incidence of complications requiring surgery is similar in the two groups (0.7% in Chang series vs 0.43% in our series), as well as the performed procedures (empyema drainage, resections of pneumatocele, drainage of persistent pneumothorax). There are some small differences in mortality rate, and redo surgery rate but the small amount of patients in both groups does not allow to draw any conclusion about those topics. The difference in days between the onset of complications and surgery might be an interesting point of discussion with a bigger number because it might be related to better outcome.
In one case report, Dr. Castiglioni and colleagues [15] chose to treat their patient with double pneumatoceles following COVID-19 directly by a thoracotomy due to the impossibility of a single lung prolonged ventilation. No patient in the present series presented the same problem, but it was necessary to interrupt the thoracoscopy in two cases, a double lung ventilation was performed for some minutes to restore adequate ventilation and then the surgical procedure was restarted. In those two cases, a very strict cooperation with the anaesthesia team was mandatory.
On one hand, the risk of infection for healthcare workers must be considered: the presence of air-leakage in a ventilated lung generates a massive aerosolization of contaminated droplets, increasing the risk of transmission. For this reason, it is mandatory to use high-level PPE (personal protective equipment) and to clearly designate and maintain clean and dirty areas within the operating theatre [13]. On the other hand, in literature there are no reported data on COVID-19 patients’ anaesthetic and perioperative risk following thoracoscopic bullectomy or pneumatocele resection. Based on our limited experience, this procedure has proved to be effective in the treatment of secondary spontaneous pneumothorax in COVID-19, without anaesthesiologic or surgical periprocedural complications, achieving an improvement of respiratory function.
Conclusions
In conclusion, if patients with secondary spontaneous pneumothorax, pneumatocele and or empyema complicating COVID-19 are tolerant for surgery under general anaesthesia, the surgical treatment can be considered as the most effective treatment. Therefore, thoracoscopic bullectomy is safe and feasible in COVID-19 patients, although the operative and perioperative management are more complex and particular attention should be paid to preventive measures to avoid transmission of the novel coronavirus.
Data availability
All data generated or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ARDS:
-
Acute respiratory distress syndrome
- COVID-19:
-
Coronavirus disease 2019
- CPAP:
-
Continuous positive airway pressure
- FiO2 :
-
Fraction of inspired O2
- PEEP:
-
Positive end-expiratory pressure
- SSP:
-
Secondary spontaneous pneumothorax
- VATS:
-
Video-assisted thoracoscopic surgery
- LOS:
-
Length of stay
- ICU:
-
Intensive care unit
- PPE:
-
Personal protective equipment
- rtPCR:
-
Reverse transcriptase polymerase chain reaction
- CT:
-
Computed tomography
- MRSA:
-
Methicillin-resistant Staphylococcus aureus
References
https://www.ecdc.europa.eu/en/geographical-distribution-2019-ncov-cases. Accessed 5 May 2022
Chen N, Zhou M, Dong X et al (2020) Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 395(10223):507–513
Xu XW, Wu XX, Jiang XG et al (2020) Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ 368:m606
Huang C, Wang Y, Li X et al (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395:497–506
Zhou M, Zhang X, Jieming Qu (2020) Coronavirus disease 2019 (COVID-19): a clinical update. Front Med. https://doi.org/10.1007/s11684-020-0767-8
Yam LYC, Chen RC, Zhong NS (2003) SARS: ventilator and intensive care. Respirology 8:S31–S35
Gomersall CD, Joynt GM, Lam P et al (2004) Short-term outcome of critically ill patients with severe acute respiratory syndrome. Intensive Care Med 30:381–387. https://doi.org/10.1007/s00134-003-2143-y
Guan WJ, Ni ZY, Hu Y et al (2020) For China medical treatment expert group for COVID-19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. [Epub ahead of print]. https://doi.org/10.1056/NEJMoa2002032
Sun R, Liu H, Wang X (2020) Mediastinal emphysema, giant bulla, and pneumothorax developed during the course of COVID-19 pneumonia. Korean J Radiol. https://doi.org/10.3348/kjr.2020.0180
Grasselli G, Zangrillo A, Zanella A et al (2020) For the COVID-19 Lombardy ICU Network. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. https://doi.org/10.1001/jama.2020.5394
Hsu C-W, Sun S-F et al (2014) Iatrogenic pneumothorax related to mechanical ventilation. World J Crit Care Med 3(1):8–14. https://doi.org/10.5492/wjccm.v3.i1.8
MacDuff A, Arnold A, Harvey J (2010) Management of spontaneous pneumothorax: British Thoracic Society pleural disease guideline. Thorax 65:ii18–ii31
Dexter F, Parra MC, Brown JR et al (2020) Perioperative COVID-19 defense: an evidence-based approach for optimization of infection control and operating room management. Anesth Analg. https://doi.org/10.1213/ANE.0000000000004829
Chang SH, Chen D, Paone D et al (2021) Thoracic surgery outcome for patients with coronavirus disease 2019. J Thor Cardiovasc Surg. https://doi.org/10.1016/j.jtcvs.2021.01.069
Castiglioni M, Pelosi G, Meroni A et al (2021) Surgical resections of superinfected pneumatoceles in a COVID-19 patient. Ann Thorac Surg 111:e 23–5. https://doi.org/10.1016/j.athoracsur.2020.06.008
Charlson ME, Pompei P et al (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40:373–383
Funding
The authors state no funds grants or any other support.
Author information
Authors and Affiliations
Contributions
All the authors contributed equally to the clinical and operative management of the patients. BP, AFT and LM served as principal authors of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study was performed in accordance with the ethical standards laid down in the 1964 Helsinki Declaration; ethical committee approval is not required in our Institution for retrospective analyses with anonymous data.
Informed consent
All patients signed the institution informed consent for thoracic surgery. No specific consent for this type of retrospective analyses is required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bisagni, P., Armao, F.T., Longhi, M. et al. VATS in complicated COVID-19 patients: case series. Updates Surg 75, 717–722 (2023). https://doi.org/10.1007/s13304-022-01420-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13304-022-01420-4