Abstract
Obesity increases surgical morbidity and mortality in open pancreaticoduodenectomy (OPD). Its influence on robotic pancreaticoduodenectomy (RPD) remains uncertain. This study aimed to investigate the impact of body mass index (BMI) on the early experience of RPD. Between June 2015 and April 2020, 68 consecutive RPDs were performed at the National Cheng Kung University Hospital. The patients were categorized as normal-weight (BMI < 23 kg/m2), overweight (BMI = 23–27.5 kg/m2), and obese (BMI > 27.5 kg/m2) according to the definition of obesity in Asian people from the World Health Organization expert consultation. Preoperative characteristics, operative details, and postoperative outcomes were prospectively collected. The cumulative sum was used to assess the learning curves. The average age of the patients was 64.8 ± 11.7 years with an average BMI of 24.6 ± 3.7 kg/m2 (23 normal-weight, 29 overweight, and 16 obese patients). Eighteen patients were required to overcome the learning curve. The overall complication rate was 51.5%, and the major complication rate (Clavien grade ≥ III) was 19.1%. The normal-weight group showed the most favorable outcomes. The blood loss, major complication rate, peripancreatic fluid collection rate, and conversion rate were higher in the obese group than in the non-obese group. There were no differences in the operative time, clinically relevant postoperative pancreatic fistula, postoperative hemorrhage, delayed gastric emptying, bile leak, wound infection, reoperation, hospital stay, and readmission rate between the obese and non-obese groups. Multivariate analysis showed obesity as the only independent factor for major complications (OR: 5.983, CI: 1.394–25.682, p = 0.001), indicating that obesity should be considered as a surgical risk factor during the implementation of RPD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity is one of the most critical public health problems in the world and the prevalence of obesity has a rapidly increased among the Taiwanese and Chinese populations in recent decades [1, 2]. Obesity is not only associated with an increased risk of developing metabolic syndrome and cardiovascular diseases but has been considered as a surgical risk factor for complications in various kinds of abdominal surgeries. Increased fat deposition in the visceral organs and a heavy omentum would impair surgical exposure and obscure the operative field, resulting in longer operative time and more blood loss. Hence, obesity is considered an independent factor for conversion to open surgery, which might result in a longer operation time, greater blood loss, and higher postoperative complications [3,4,5].
Pancreaticoduodenectomy (PD) is one of the most complicated operations in abdominal surgeries, requiring extensive dissection and three delicate digestive reconstructions. The mortality rate substantially decreases to 2–5%, and the postoperative morbidity remains as high as 40–60% [6,7,8,9]. Previous studies have reported increased morbidity and mortality in obese patients undergoing open pancreaticoduodenectomy (OPD) when compared with non-obese patients [10,11,12,13]. The robotic platform provides surgeons with ergonomic conditions and dexterity and has an increasing application in general, urological, and gynecological procedures; however, the penetration of robotic pancreaticoduodenectomy (RPD) is still slow and uncommon owing to the complex high-risk procedure and steep learning curve. RPD has been shown to provide better postoperative outcomes including lesser blood loss, lesser surgical site infection, fewer pulmonary complications, and a shorter hospital stay than OPD [14,15,16]. Case selection is an important issue in RPDs, especially during the learning curve phase. Although obesity is not a contraindication of minimally invasive pancreatic resection (MIPR) [17], the body mass index (BMI) should be considered when assessing surgical risks. However, the impact of BMI on postoperative morbidities of RPD has seldom been discussed. The purpose of this study was to investigate the impact of BMI on surgical outcomes during the early development period of RPD at a high-volume tertiary hospital.
Materials and methods
Between June 2015 and April 2020, 68 consecutive RPDs were performed at the National Cheng Kung University Hospital, a tertiary referral center in Tainan, Taiwan. The first RPD was completed in June 2015 after the installation of the da Vinci Si surgical system (Intuitive Surgical Inc., Sunnyvale, CA) and all operations were performed using the da Vinci Si surgical system. The indications for RPD were periampullary tumors and the patients who were generally suitable for laparoscopic surgery; the specific contraindications for RPD included large tumors (> 5 cm), tumors with major vessel invasion, locally advanced tumors, tumors with bulky lymphadenopathy, previous severe pancreatitis, and previous major abdominal surgery. For pancreatic adenocarcinoma, the tumor with a size larger than 2 cm was considered a contraindication in our study due to a high risk of vessel invasion and the concern of tumor dissemination during dissection., BMI > 35 mg/m2 was considered as a relative contraindication for RPD. All operations were performed by a single experienced laparoscopic surgeon (YJ Chao) and assisted by senior experienced surgeons with experience in advanced laparoscopic techniques. Before the first RPD, the surgeon had performed 210 OPDs, 72 laparoscopic distal pancreatectomies, 10 robotic distal pancreatectomies, and 8 robotic distal gastrectomies. The patients were divided into three groups: normal weight, BMI < 23 kg/m2; overweight; BMI = 23–27.5 kg/m2; and obese, BMI > 27.5 kg/m2, according to the definition of overweight and obesity in Asian individuals from the World Health Organization expert consultation [18]. The dilated pancreatic duct was defined as a pancreatic duct diameter > 3 mm. All events recorded within 90 days of surgery were reported as postoperative complications. Postoperative complications were stratified according to the Clavien–Dindo classification [19]. Delayed gastric emptying (DGE), postoperative pancreatic fistula (POPF), and postoperative pancreatectomy hemorrhage (PPH) were determined based on the International Study Group of Pancreatic Surgery [20,21,22]. The conversion was defined as a change from robotic surgery to open surgery. Reoperation was considered a secondary operation because of severe complications after RPD. Re-admission was defined as any surgery-related admission within 30 days after discharge. Mortality was defined as any death occurring within 90 days or during the index hospitalization. Data were collected prospectively from June 2015 to August 2020, including clinicopathological characteristics and postoperative outcomes. This study was approved by the Institutional Review Board of the National Cheng Kung University Hospital and written informed consent was obtained from all patients.
Surgical procedure
After the induction of general anesthesia, the patient is placed in 30-degree reverse Trendelenburg position with legs split and the table is rotated 10 degrees to the left. Pneumoperitoneum is created from the sub-umbilical port and the pneumoperitoneum pressure is set a routine pressure of 12 mmHg. Laparoscopic exploration is performed to assess unexpected peritoneal seeding or liver metastasis. The remaining 5 trocars are shown in the Supplementary Fig. 1. The robotic system is docked over the patient’s head. The assistant surgeon stands between the patient’s legs. The left lobe of the liver is retracted using the W-shaped liver retraction technique to expose the porta hepatis [23]. The transverse colon is lifted upward to expose the fourth part of the duodenum and the proximal jejunum is subsequently divided at 20 cm distal to the ligament of Treitz. An extensive Kocher maneuver is performed to mobilize the transverse colon and duodenum. The right gastrocolic and right gastric vessels are clipped using Hem-o-lok and divided. The proximal duodenum is divided using a linear stapler. The pylorus and first portion of the duodenum are usually preserved for pylorus-preserving PD if no tumor invasion or obvious duodenal ulcer is observed. The lymph node stations 8a and 12a are harvested and the gastroduodenal artery is clipped using Hem-o-lok and divided. Cholecystectomy is performed and the common bile duct was dissected away from the hepatic artery and portal vein. The retropancreatic tunnel is dissected and the pancreatic neck is transected using ultrasonic shears. The uncinate process is dissected from the right side margin of the superior mesenteric artery, and the common bile duct is subsequently transected. Pancreatic anastomosis is performed using a modified Blumgart anastomosis. An internal stent is routinely placed if the diameter of the pancreatic duct is less than 5 mm. The hepaticojejunostomy is created using a one-layer continuous suture with 4-0 or 5-0 prolene. For pylorus-preserving PD, duodenojejunostomy is performed through mini-laparotomy after specimen retrieval. For classical PD, a linear stapled gastrojejunostomy is created intracorporeally. Before the closure of the abdominal wound, two Jackson Pratt drains are routinely inserted at the Morrison pouch and behind the pancreaticojejunostomy, respectively.
Statistics
The results were expressed as the mean ± standard variation or median with interquartile range for quantitative data. The Chi-square test, Fisher exact test, one-way analysis of variance among groups, and Kruskal–Wallis test followed by all pairwise comparisons were used for statistical analysis. The learning curve was measured using the cumulative sum analysis (CUSUM) method to assess the technical competence of certain procedures [24]. The CUSUM of the operative time (CUSUMOT) was measured from the first to the last patient. The CUSUMOT was calculated as CUSUMOT = ∑in = 1(xi − μ), where xi is the mean value of the overall operation time and μ is the operation time of each case. Statistical significance was set at p < 0.05. All data analyses were performed using SPSS version 19.0 (IBM SPSS, Chicago, IL, USA).
Results
The mean age of the patients was 64.8 ± 11.7 years including 30 men and 38 women. The average BMI was 24.6 ± 3.7 kg/m2. There were 23 normal-weight, 29 overweight, and 16 obese patients. A total of 18 patients had dilated pancreatic ducts. The mean operative time was 317 ± 67 min and the estimated blood loss was 155 ± 217 mL. Pylorus-preserving PD was performed in 57 patients (83.8%) and traditional PD in 11 patients (16.2%). Pathologic examinations showed ampullary adenocarcinoma (33.8%), pancreatic adenocarcinoma (19.1%), cholangiocarcinoma (13.2%), intraductal papillary mucinous neoplasm (16.2%), ampullary adenoma (5.9%), chronic pancreatitis (2.9%), neuroendocrine tumor (1.5%), and other benign diseases (7.4%). Two patients (2.9%) were converted to open surgery in the obese group due to severe inflammation at the pancreatic head and tumor adhesive to the superior mesenteric vein and none in the normal-weight and overweight groups. Thirty-three patients experienced complications with an overall complication rate of 51.5%, and the major complication rate (Clavien grade ≥ III) was 19.1%. There were 17.6% CR-POPF (16.1% grade B POPF, 1.5% grade C POPF), 8.9% grade B/C PPH, 11.8% grade B/C DGE, and 5.9% bile leakage. Twelve patients (17.6%) had peripancreatic fluid collections, and eight of them required drainage. One patient required reoperation due to failed embolization of the pseudoaneurysm from the gastroduodenal artery due to POPF. Eventually, the patient had multiple organ failure and died on a postoperative day 114. The median hospital stay was 15 days (interquartile range: 11–22 days), with an 11.8% readmission rate (Supplemental Table 1).
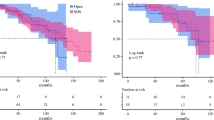
Learning curve analysis
The learning curve assessment was performed using the CUSUM method for the operative time. Additionally, the peak indicated the cutoff point of the learning curve, which was observed in the 18th case (Fig. 1). The weight status of the patients (normal-weight, overweight, and obese) was not a contra-indicator for RPD during the pre-learning curve phase and showed a similar distribution between the pre-learning curve and after-learning curve phase (obese/overweight/normal-weight: 4/10/4 in the pre-learning curve phase; 12/19/19 in the post-learning curve phase, p = 0.378)(Fig. 2).
Demographic among the groups of normal weight, overweight, and obese patients
The BMI was significantly different between the three groups (p < 0.0001). The operative time was shorter in the normal-weight group than in the overweight (282 vs. 301 min, p = 0.049) and obese group (282 vs. 343 min, p = 0.043). Meanwhile, the normal-weight group had lower intraoperative blood loss than the normal-weight group (0 vs. 100 mL, p = 0.053) and obese groups (0 vs 175 mL, p = 0.005). There were no differences in age, sex, ASA score, dilated pancreatic duct, pancreatic texture, operative procedure, pathological diagnosis, distribution of the learning curve, retrieval of lymph nodes, and hospital stay between the groups (Table 1).
Morbidity and mortality among groups of normal weight, overweight, and obese patients
The obese group had the highest overall complication rate followed by the overweight and the normal-weight groups (75% vs. 48% vs. 30%), which was significantly higher in the obese group than in the normal weight group (p = 0.004) (Table 2). The major complication rate in the obese group was 50%, which was significantly higher than that in the overweight (p = 0.037) and normal-weight groups (p = 0.001). There was no CR-POPF in the normal-weight group, which was significantly lower than that in the obese group (p = 0.033). The obesity group had a higher risk of postoperative peripancreatic fluid collection than the normal-weight group (p = 0.019) and overweight group (p = 0.021). The PPH, DGE, bile leakage, wound infection, reoperation, and readmission were comparable between the groups.
We further divided the patients into two groups: non-obese (BMI ≦ 27.5 kg/m2) and obese (BMI > 27.5 kg/m2). The obese group had more patients with ampullary adenocarcinomas (p = 0.011). However, when considering ampullary tumors (adenocarcinomas and adenomas), there was no difference between groups (obese: 44% vs non-obese: 37.5%, p = 0.779). Furthermore, the obese group had a smaller tumor size (p = 0.031), higher blood loss (p = 0.027), and higher conversion rate (11.1% vs. 0%, p = 0.053) than those in the non-obese group. There were no significant differences in the age, sex, ASA, dilated pancreatic duct, pancreatic texture, operative procedure, learning curve phase, operative time, retrieval lymph node, and hospital stay (Supplemental Table 2). The obese group had higher overall postoperative complications (75% vs. 40.4%, p = 0.005) and major complication rates (50% vs. 9.6%, p = 0.001) than the non-obese group (Supplemental Table 3). Finally, we analyzed the risk factors correlated with morbidity during the development period of RPD. In the univariate analysis, obesity and conversion were critical factors leading to major complications. In the multivariate analysis, we found that the only risk factor was obesity (OR [95%CI): 5.983 [1.394–25.682], p = 0.016) (Table 3).
Discussion
Overweight and obesity have become important health problems in Asian countries as well as in Western countries. Only a few studies from Western countries have focused on the surgical outcomes of PD patients with high BMI, and data with respect to Asians are still limited. This is the first Asian study to investigate the impact of BMI during the implementation of RPD. We stratified the body weight status according to the definition of obesity for the Asian population by the World Health Organization (WHO). We found the obesity group (BMI > 27.5 kg/m2) had the highest overall and major complication rate. The major complication rate was significantly higher in the overweight than in the normal-weight and overweight groups. In contrast, the normal-weight group (BMI < 23 kg/m2) had the most favorable outcomes, with the lowest overall complication rate and hospital stay. Meanwhile, obesity was the only independent factor for major complications in multivariate analysis. The definition of obesity in Asian population from the World Health Organization expert consultation is feasible to stratify the surgical risks in RPD during the implementation phase. Although obesity is not considered as a contraindication in MIPR according to the Miami International Evidence-based Guideline [17], obesity should be considered as increasing surgical risks and the selection criteria of RPD should be more restricted for obese patients during the implementation phase. Currently, the ideal approach of PD for obese patients remains unclear and only one retrospective study showed that RPD had less wound infection and fewer CR-POPF than OPD [25].
Postoperative peripancreatic fluid collection (43.8%) was the most common complication in obese patients, which might be ascribed to a wider dissection area, larger dead space, more tissue damage, delayed mobilization, and more frequent dysfunction of drains than non-obese patients. The WHO defines overweight as a BMI ≥ 25 kg/m2 and obesity as a BMI ≥ 30 kg/m2, while the definition of obesity for the Asian is different from that in other countries. Chinese and South Asian individuals usually have a thinner bone and lower muscle mass than Caucasians [26]. Furthermore, Asians have higher body fat and risks of cardiovascular events than Whites for the same body weight and BMI [27]. In particular, a higher body fat contributed to increased abdominal adipose tissue and visceral adipose tissue in Chinese and South Asians [28, 29]. This would increase the surgical challenges and surgical morbidities in digestive surgeries. Previous studies from Western countries have shown that overweight patients receiving OPD may have increased operation time, blood loss [30], and surgical morbidities [10, 12, 13] compared to normal-weight patients. Chang et al. presented data from the National Clinical Database in the United States, which showed that obesity increased the risk of wound infection, reoperation, failure of extubation in 48 h, infection, pulmonary embolism, and renal insufficiency compared to the control group [11]. In an early report from Asia, the Japanese group analyzed 97 patients undergoing OPD and divided them into overweight (BMI > 25 kg/m2) and non-overweight groups (BMI ≤ 25 kg/m2). They found that the overweight group experienced an increase in the rate of postoperative peripancreatic fluid collection (14.3% vs. 2.9%, p < 0.05). Although the categorization of body constitution was not inappropriate for the Asian population, a higher BMI was associated with an increased risk of peripancreatic fluid collection, which is consistent with our results.
Although the first case series of RPD was published in 2003 [31], the application of a robotic system for PD is still slow and uncommon owing to the steep learning curve. Operative time was one of the most commonly used parameters to evaluate the learning curve. A learning curve exists for every procedure and 20–100 cases would be required to overcome the learning curve of RPD from different reports [32,33,34,35,36]. The duration of a learning curve depends on several factors, including the selection of patients, the surgeon’s volume/knowledge/experience for PD, the experience of advanced minimally invasive surgery, and the maturation of the teamwork. In 2015, Boone et al. presented the largest study in the United States, including 200 cases with a mean BMI of 28 ± 5 kg/m2 and the learning curve was passed after 80 cases [35] (Table 4). In 2016, Napoli and Boggi et al. analyzed 70 consecutive cases in Italy with a mean BMI of 23.6 kg/m2 and found the operative time dropped after 33 cases [32]. Subsequently, the Taiwanese group from Shyr et al. reported that 20 cases were needed to overcome the learning curve [34]. In our series, only 18 cases were required to overcome the learning curve, and the learning curve appeared to decrease with the dissemination of RPD. We had a short learning curve for RPD, which may be due to (1) the previous experience in OPDs and minimally invasive surgery, (2) a matured team of minimally invasive surgery, (3) the well-established procedure that was followed, (4) a relatively lower BMI (24.6 ± 3.7 kg/m2) [35]. The appropriate selection of patients during the learning curve phase is the key to decreasing the operative time and blood loss, resulting in the shortening of the learning curve and reducing postoperative morbidities. The learning endpoint by operative time did not reflect the proficiency and the true learning curve might be longer than the CUSUM analysis. In the largest study of RPD from China, 100 patients were required to overcome the learning curve by operative time and the oncological and surgical outcomes were much improved after 250 cases of RPD [36]. They concluded that more than 200 cases were required to measure the learning curve in RPD appropriately. Although we learned how to master the robotic system and passed the learning curve in 18 patients, the proficiency still needs more cases to stabilize the operative time and postoperative morbidity. Hence, the results of this study showed the states during the implementation phase of RPD, and the postoperative outcomes of obesity at the proficiency stage need further investigation with a large patient number.
Our study had several limitations. First, this was a retrospective study with a selection bias and low number of patients. To avoid early recurrence after RPD, in this study, the number of pancreatic cancers was relatively low, although there was no statistical difference among the disease types. Second, the patients for RPD were well-selected and we avoided difficult cases of RPD even after the learning curve; therefore, we had a low conversion rate (2.9%). We did not use high BMI as an exclusion criterion for RPD initially; the highest BMI was 35.6 kg/m2 in our patients. Although BMI > 35 kg/m2 was a relative contraindication for RPD in our institute, only three patients (3/311, 1.0%) receiving PD had BMI higher than 35 kg/m2 during this period. In Park's study, BMI might not correlate with visceral fat, and high visceral fat was considered a risk factor for developing CR-POPF [37]. However, calculating visceral fat requires additional software, which is inconvenient and unpopular. The application of visceral fat for risk stratification may be limited. Third, our results could not represent other countries in Asia, and some modifications for the definition of obesity should be considered according to different populations.
Conclusions
During the implementation phase of RPD, obesity is an acceptable risk factor in a well-prepared team and obese patients have a higher major complication rate with more postoperative peripancreatic fluid collection than non-obese patients. Obesity is the only independent factor for major complications in RPD and should be considered when assessing surgical risks during the early development period.
References
Chang HC, Yang HC, Chang HY et al (2017) Morbid obesity in Taiwan: prevalence, trends, associated social demographics, and lifestyle factors. PLoS One 12:e0169577
Gordon-Larsen P, Wang H, Popkin BM (2014) Overweight dynamics in Chinese children and adults. Obes Rev 15(Suppl 1):37–48
Hotouras A, Ribas Y, Zakeri SA et al (2016) The influence of obesity and body mass index on the outcome of laparoscopic colorectal surgery: a systematic literature review. Colorectal Dis 18:O337–O366
Paajanen H, Kakela P, Suuronen S et al (2012) Impact of obesity and associated diseases on outcome after laparoscopic cholecystectomy. Surg Laparosc Endosc Percutan Tech 22:509–513
Yu X, Yu H, Fang X (2016) The impact of body mass index on short-term surgical outcomes after laparoscopic hepatectomy, a retrospective study. BMC Anesthesiol 16:29
Gordon TA, Bowman HM, Tielsch JM et al (1998) Statewide regionalization of pancreaticoduodenectomy and its effect on in-hospital mortality. Ann Surg 228:71–78
Gouma DJ, van Geenen RC, van Gulik TM et al (2000) Rates of complications and death after pancreaticoduodenectomy: risk factors and the impact of hospital volume. Ann Surg 232:786–795
Cameron JL, He J (2015) Two thousand consecutive pancreaticoduodenectomies. J Am Coll Surg 220:530–536
Gleeson EM, Shaikh MF, Shewokis PA et al (2016) WHipple-ABACUS, a simple, validated risk score for 30-day mortality after pancreaticoduodenectomy developed using the ACS-NSQIP database. Surgery 160:1279–1287
Noun R, Riachy E, Ghorra C et al (2008) The impact of obesity on surgical outcome after pancreaticoduodenectomy. JOP 9:468–476
Chang EH, Sugiyama G, Smith MC et al (2019) Obesity and surgical complications of pancreaticoduodenectomy: an observation study utilizing ACS NSQIP. Am J Surg 220(1):135–139
Ekstrom E, Ansari D, Williamsson C et al (2017) Impact of body constitution on complications following pancreaticoduodenectomy: a retrospective cohort study. Int J Surg (Lond Engl) 48:116–121
Del Chiaro M, Rangelova E, Ansorge C et al (2013) Impact of body mass index for patients undergoing pancreaticoduodenectomy. World J Gastrointest Pathophysiol 4:37–42
Kamarajah SK, Bundred JR, Marc OS et al (2020) A systematic review and network meta-analysis of different surgical approaches for pancreaticoduodenectomy. HPB 22:329–339
Nassour I, Wang SC, Christie A et al (2018) Minimally invasive versus open pancreaticoduodenectomy: a propensity-matched study from a national cohort of patients. Ann Surg 268:151–157
Zureikat AH, Postlewait LM, Liu Y et al (2016) A multi-institutional comparison of perioperative outcomes of robotic and open pancreaticoduodenectomy. Ann Surg 264:640–649
Asbun HJ, Moekotte AL, Vissers FL et al (2020) The Miami international evidence-based guidelines on minimally invasive pancreas resection. Ann Surg 271:1–14
WHO Expert Consultation (2004) Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet (Lond Engl) 363:157–163
Clavien PA, Barkun J, de Oliveira ML et al (2009) The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 250:187–196
Wente MN, Bassi C, Dervenis C et al (2007) Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 142:761–768
Bassi C, Marchegiani G, Dervenis C et al (2017) The 2016 update of the international study group (isgps) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery 161:584–591
Wente MN, Veit JA, Bassi C et al (2007) Postpancreatectomy hemorrhage (PPH): an international study group of pancreatic surgery (ISGPS) definition. Surgery 142:20–25
Wang CJ, Chao YJ, Sy ED et al (2019) A simple method of intracorporeal “W-shaped” liver retraction technique for minimally invasive gastric cancer surgery. Surg Laparosc Endosc Percutan Tech 29:e24–e28
Bokhari MB, Patel CB, Ramos-Valadez DI et al (2011) Learning curve for robotic-assisted laparoscopic colorectal surgery. Surg Endosc 25:855–860
Girgis MD, Zenati MS, Steve J et al (2017) Robotic approach mitigates perioperative morbidity in obese patients following pancreaticoduodenectomy. HPB 19:93–98
Kepley AL, Nishiyama KK, Zhou B et al (2017) Differences in bone quality and strength between Asian and Caucasian young men. Osteoporos Int 28:549–558
Deurenberg P, Deurenberg-Yap M, Guricci S (2002) Asians are different from Caucasians and from each other in their body mass index/body fat per cent relationship. Obes Rev 3:141–146
Lear SA, Humphries KH, Kohli S et al (2007) The use of BMI and waist circumference as surrogates of body fat differs by ethnicity. Obesity 15:2817–2824
Lear SA, Humphries KH, Kohli S et al (2007) Visceral adipose tissue accumulation differs according to ethnic background: results of the Multicultural Community Health Assessment Trial (M-CHAT). Am J Clin Nutr 86:353–359
Williams TK, Rosato EL, Kennedy EP et al (2009) Impact of obesity on perioperative morbidity and mortality after pancreaticoduodenectomy. J Am Coll Surg 208:210–217
Giulianotti PC, Coratti A, Angelini M et al (2003) Robotics in general surgery: personal experience in a large community hospital. Arch Surg 138:777–784
Napoli N, Kauffmann EF, Palmeri M et al (2016) The learning curve in robotic pancreaticoduodenectomy. Dig Surg 33:299–307
Zhang T, Zhao ZM, Gao YX et al (2019) The learning curve for a surgeon in robot-assisted laparoscopic pancreaticoduodenectomy: a retrospective study in a high-volume pancreatic center. Surg Endosc 33:2927–2933
Shyr BU, Chen SC, Shyr YM et al (2018) Learning curves for robotic pancreatic surgery-from distal pancreatectomy to pancreaticoduodenectomy. Medicine 97:e13000
Boone BA, Zenati M, Hogg ME et al (2015) Assessment of quality outcomes for robotic pancreaticoduodenectomy: identification of the learning curve. JAMA Surg 150:416–422
Shi Y, Wang W, Qiu W et al (2019) Learning curve from 450 cases of robot-assisted pancreaticoduocectomy in a high-volume pancreatic center: optimization of operative procedure and a retrospective study. Ann Surg. https://doi.org/10.1097/SLA.0000000000003664
Park CM, Park JS, Cho ES et al (2012) The effect of visceral fat mass on pancreatic fistula after pancreaticoduodenectomy. J Invest Surg 25:169–173
Acknowledgement
We sincerely thank Dr. Hao-Chen Wang (Clinical Medicine Research Center, National Cheng Kung University Hospital, Tainan, Taiwan) for providing the consulting services for manuscript editing and writing.
Funding
No financial support was available for this work.
Author information
Authors and Affiliations
Contributions
YJC and YSS contributed to the conception and design of the study. CJW, WHL, PJS, and TKL assisted in the operation. YJC and YSS performed the data analyses and wrote the manuscript. YSS helped perform the analysis through constructive discussions.
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest or financial ties to disclose among authors.
Ethical approval
All procedures performed in our study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This article does not contain any actions performed on animals.
Informed consent
Informed consent was obtained from all individuals included in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chao, YJ., Liao, TK., Su, PJ. et al. Impact of body mass index on the early experience of robotic pancreaticoduodenectomy. Updates Surg 73, 929–937 (2021). https://doi.org/10.1007/s13304-021-01065-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13304-021-01065-9