Abstract
Introduction
A bidirectional relationship has been observed between type 2 diabetes mellitus and sarcopenia, especially among older adults. While previous studies have reported that imeglimin improves mitochondrial function, they have not assessed its effects on muscle strength in patients with type 2 diabetes. Therefore, we aimed to investigate the effects of imeglimin on muscle strength in patients with type 2 diabetes.
Methods
In this prospective cohort study, we recruited consenting patients with type 2 diabetes (20–75 years). Changes in lean body mass (LBM), fat mass, quadriceps muscle strength, and grip strength from baseline (week 0) to week 24 were evaluated and compared between patients treated with imeglimin therapy (group I) and those who did not take imeglimin (controls, group C).
Results
We recruited 27 patients treated with imeglimin (group I) and 29 controls (group C), and 50 of them completed the study (group I: n = 23; group C: n = 27). The change in LBM, total body fat mass, or skeletal muscle index from baseline to week 24 did not differ significantly between the two groups. However, group I exhibited a significantly higher percent change in quadriceps knee extension strength from baseline to week 24 than group C (13 ± 19% and 2.1 ± 14%, p = 0.022). Conversely, the difference in percent change in grip strength was not significant. Multivariable analysis showed that imeglimin use was significantly associated with a percent change in quadriceps knee extension strength, independent of age, sex, body mass index, and skeletal mass index (β = 0.325, p = 0.0014).
Conclusions
Imeglimin positively affected muscle strength in patients with type 2 diabetes without altering LBM. Therefore, imeglimin exerts a unique effect on skeletal muscles in humans. Further randomized controlled trials are needed to validate these findings.
Trial Registration
This research was registered in the University Hospital Medical Information Network (UMIN, UMIN000054715).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
No previous studies on imeglimin have assessed muscle strength in patients with type 2 diabetes mellitus. |
In this prospective cohort study, we aimed to investigate the effects of imeglimin on muscle strength and lean body mass (LBM) in patients with type 2 diabetes mellitus. |
What was learned from the study? |
The administration of imeglimin increased muscle strength in patients with type 2 diabetes mellitus without altering LBM. |
Imeglimin may positively affect skeletal muscle strength in patients with diabetes mellitus by improving mitochondrial function. |
Introduction
Sarcopenia, defined as the loss of skeletal muscle mass and strength with aging [1], has serious physiological and clinical consequences, such as falls, fractures, cognitive impairment, hospitalization, and mortality [2,3,4]. Patients with type 2 diabetes mellitus are at a high risk of developing sarcopenia [5]. Furthermore, a bidirectional relationship has been observed between type 2 diabetes and sarcopenia, especially among older adults. The skeletal muscles are the largest consumers of glucose, making sarcopenia a possible contributor to the development of type 2 diabetes. Moreover, the accumulation of advanced glycation end products and increased oxidative stress in patients with type 2 diabetes may adversely affect muscle mass and function [6].
Several medications are available for diabetes management. However, the effectiveness of pharmacotherapy in managing sarcopenia remains unclear. Glucagon-like peptide-1 receptor agonists (GLP-1RAs) and sodium-glucose co-transporter 2 inhibitors (SGLT2i), frequently prescribed based on the results of cardiovascular outcome trials, can reduce body weight by decreasing energy intake or increasing glucose output [7]. This may accelerate age-related muscle loss and lead to the development of sarcopenia in patients with type 2 diabetes.
Imeglimin is the first oral hypoglycemic agent that targets mitochondrial bioenergetics. It has a unique mechanism of action that involves dual effects: stimulation of insulin secretion in a glucose-dependent manner and improvement in insulin sensitivity [8]. Imeglimin reportedly improves the secretion of glucose-stimulated insulin and inhibits pancreatic beta (β)-cell apoptosis by maintaining or restoring the functional and structural integrity of mitochondria in β-cells, thereby preserving the β-cell mass [8, 9]. It also improves mitochondrial function, thereby improving insulin sensitivity in the liver.
Mitochondrial dysfunction contributes to the development of sarcopenia [10]. An age-related decline in skeletal muscle mitochondrial capacity has been associated with decreased walking speed and increased fatigability [11, 12]. Furthermore, the age-related loss of muscle mass has been attributed to the loss of proteostasis, inflammation, and oxidative stress due to impairments in mitochondrial function [12]. Hence, imeglimin may improve mitochondrial function, enhance skeletal muscle function, and reduce sarcopenia. However, no previous studies on imeglimin have focused on muscle strength and mass in patients with type 2 diabetes. Therefore, in the present study, we aimed to investigate how using imeglimin affects muscle mass and strength in patients with type 2 diabetes.
Methods
Study Design and Participants
This prospective cohort study was conducted at St. Marianna University Hospital (Kawasaki, Japan) following the Declaration of Helsinki and approved by the St. Marianna University Clinical Research Ethics Committee (No. 5603). The participants received a detailed explanation regarding the study’s aims and objectives at the time of recruitment, and written informed consent was obtained from all participants.
The study protocol was designed to evaluate the effects of oral hypoglycemic drugs, including imeglimin, on body composition and muscle strength in patients with type 2 diabetes aged 20–75 years attending our hospital between March 2022 and September 2023. Patients with new prescriptions for imeglimin were recruited for this protocol (group I). The choice of using imeglimin was left at the attending outpatient physician’s discretion. Patients who were not prescribed imeglimin were also invited to participate in this protocol, and those who consented were recruited and designated as the control group (group C). Patients who changed drug types during the protocol period were excluded. The date of consent was considered week 0, and we compared body composition and muscle strength findings between week 0 and week 24.
Measurements
The primary endpoints were the percent changes in the quadriceps muscle strength and grip strength from baseline (week 0) to week 24. Secondary endpoints included changes in lean body mass (LBM) and total body fat mass from week 0 to week 24. We also evaluated changes in each laboratory test parameter (plasma glucose; glycated hemoglobin [HbA1c]; glycoalbumin [GA]; insulin secretion: C-peptide immune immunoreactivity [CPR], lipid-related: triglycerides [TG], low-density lipoprotein [LDL], high-density lipoprotein [HDL]; and urine parameter: urinary albumin [assessed using spot urine]) from week 0 to week 24. Group I was followed up from the initiation of imeglimin treatment, and group C was followed up from when consent was obtained.
With the participant in the seated position and the knee joint in 90° flexion, muscle strength of the quadriceps was measured thrice on each side using a hand-held dynamometer (#Tas MF-01, Anima Corporation, Tokyo, Japan). The average value of three consecutive measurements was analyzed [13]. Hand grip strength was measured using a hand dynamometer (JAMAR® Hydraulic Hand Dynamometer, Sammons Preston Inc., Canada) with the participant in an upright position with the arms naturally lowered. The average value was used as the hand grip strength. Two trained raters (a physician and a nurse), other than the physicians involved in treatment-related decision-making, measured the muscle strength.
Body weight, LBM, and fat mass were measured using dual-energy X-ray absorptiometry (DEXA; Lunar PRODIGY Advance, GE Healthcare Japan Corp., Tokyo, Japan). Body mass index (BMI) (kg/m2) was calculated using the following formula:
The skeletal muscle index (SMI; kg/m2) was calculated using the following formula:
Statistical Analysis
Data are presented as mean ± standard deviation. A corresponding two-sample t test was used to assess the differences between groups. A paired t test was performed to evaluate changes in laboratory variables from baseline to the observation time point. The Shapiro–Wilk normality test was used to assess data normality, and all variables were normally distributed. Multiple regression analysis was performed to examine the independent associations between percent change in quadricep knee extension strength and clinically important variables, including age, sex, BMI, SMI, and imeglimin administration.
As this was an observational study, the effects of concomitant medications could not be ruled out. Therefore, a multivariate analysis was performed to determine whether concomitant medications affected the rate of change in muscle strength, with the presence or absence of the various types of medications as the explanatory variable and the rate of change in muscle strength as the dependent variable. We also examined differences in LBM and muscle strength in patients who were and were not receiving concomitant medications to determine whether concomitant medications influence LBM and muscle strength. Concomitant medications included insulin, SGLT2i, metformin, sulfonylureas and glinides (SUs), and incretin modulators (GLP-1RAs and dipeptidyl peptidase 4 inhibitors). All analysis were performed using JMP@Pro 16.2.0 (570,548), and statistical significance was set at p < 0.05.
Results
Baseline Characteristics
In the total, 56 patients with type 2 diabetes treated as outpatients were enrolled after they provided informed consent. Of them, 27 and 29 participants were assigned to groups I and C, respectively. Six participants withdrew from the study (group C = 2, group I = 4) due to the following reasons: lumbar hernia (n = 1), refusal to continue the program (n = 2), and drug-related side effects (n = 3). Thus, 50 patients were included in this prospective cohort study (group I, n = 23; group C, n = 27). The mean age of participants in groups C and I was 61 ± 10.9 and 58.7 ± 11.9 years, respectively, and the mean duration of diabetes was 13.7 ± 9.5 and 12.3 ± 9.5 years, respectively. The mean BMI of groups C and I was 25.0 ± 4.0 and 25.9 ± 5.2 kg/m2, respectively, and the mean HbA1c levels were 6.6 ± 0.63% (49 ± 6.9 mmol/mol) and 6.8 ± 0.93% (51 ± 10 mmol/mol) respectively, indicating relatively good glycemic control in both groups. The baseline variables for the remaining items were statistically similar in both groups (Table 1).
Changes in Muscle Strength and Body Composition
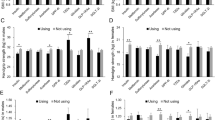
Table 2 presents the muscle strength and body composition at weeks 0 and 24 and the percent change from week 0 to week 24. Group I exhibited a significantly higher percent change in the quadricep knee extension force from week 0 to week 24 than group C (13 ± 19% and 2.1 ± 14%, p = 0.022). However, the difference in the percent change in grip strength from week 0 to week 24 was not statistically significant (− 0.19 ± 7.1% and − 3.3 ± 8.2%, p = 0.16) (Fig. 1A).
Change in the lean body mass (LBM) and skeletal mass index (SMI) from baseline and the percent changes in muscle strength from baseline at 24 weeks. Imeglimin(–) and Imeglimin(+) are the control (group C) and imeglimin (group I) groups, respectively. A Percent change in grip and knee extension strength in groups C and I at 24 weeks. B, C Changes in the lean body mass and SMI assessed using dual-energy X-ray absorptiometry (DEXA) in groups C and I at 24 weeks. Values are expressed as means ± standard deviations
Body composition, with regards to the amount of change in the LBM, total body fat mass, or SMI from week 0 to week 24, was not significantly different between the two groups (LBM: − 0.033 ± 1.7 kg and − 0.14 ± 0.97 kg, p = 0.78; total body fat mass: − 0.74 ± 2.2 kg and − 0.23 ± 1.1 kg, p = 0.28; skeletal mass index: 0.018 ± 0.34 kg/m2 and − 0.059 ± 0.29 kg/m2, p = 0.39) (Fig. 1B and C).
Furthermore, the quadriceps muscle strength increased significantly from 0 to 24 weeks in group I (p = 0.002), whereas no significant differences were observed in group C (p = 0.551). Regarding body composition, no significant change in total body fat mass and LBM was observed in both groups (total body fat mass: group I, p = 0.115; group C, p = 0.305; lean body mass: group I, p = 0.925; group C p = 0.455).
Changes in Other Parameters
The HbA1c levels did not change significantly from week 0 to week 24 in group C, whereas in group I, the HbA1c levels decreased but did not reach statistical significance. The GA level decreased by 1.0 ± 2.1% (p = 0.028) from week 0 to week 24 in group I (Table S1). Both groups had no significant changes in CPR levels and TG and cholesterol profiles.
Multiple Regression Analysis
We found a significant positive association of the percent change in quadriceps muscle strength with imeglimin administration independent of age, sex, BMI, and SMI (β = 0.325, p = 0.014; Table 3). Regarding the effect of concomitant medications other than imeglimin, we found that the significant positive effect of imeglimin on quadriceps muscle strength was independent of other medications (β = 0.306, p = 0.046; Table 3).
Differences due to Concomitant Medications
The effects of concomitant medications on LBM and muscle strength were examined separately (Table 4). The two groups did not differ significantly regarding changes in body composition and muscl4e strength. When groups I and C were analyzed separately, a significant difference was observed only for concomitant SUs use in group C (Table 5).
Discussion
The present study revealed two major findings: first, add-on treatment with imeglimin augmented knee extension strength; Second, group I showed no significant changes in body weight, LBM, or fat mass compared with group C after 24 weeks of treatment. Furthermore, the analysis of each concomitant medication revealed no significant differences in knee extension muscle strength or body composition in the entire study population.
We also conducted a separate analysis of each concomitant medication for groups I and C. In group I, no concomitant medications effect on the percent change in muscle strength was observed. However, in group C, a significant difference was observed for concomitant SUs. Previous studies have shown that SUs usage might negatively affect muscle strength [7]. However, considering that the rate of SUs usage was higher in group I than in group C and the multivariate analysis showed that imeglimin positively affected quadriceps muscle strength independent of concomitant SUs, we infer that this point does not affect the conclusions of this study.
In the multivariate analysis, imeglimin administration was a significant explanatory factor for the percent change in knee extension strength independent of age, sex, BMI, and SMI. These findings suggest that imeglimin exerts a positive effect on muscle strength irrespective of muscle mass. To our knowledge, this is the first study to demonstrate the effects of imeglimin on skeletal muscle strength in humans.
The knee extension muscle strength at the baseline was lower in group I than in group C, although no statistically significant difference was observed (p = 0.065). However, the cohort difference was negligible after 24 weeks of treatment. This was probably due to the slightly higher proportion of males in group C (15 males/12 females) than that in group I (11 males/12 females). The slight sex difference might affect the knee extension muscle strength at the baseline; however, administering imeglimin significantly increased the percent change in quadriceps knee extension muscle strength independent of sex, as shown in the multiple regression analysis in Table 3.
The primary action of imeglimin is to enhance glucose-stimulated insulin secretion in β-cells, which improves plasma glucose levels in patients with diabetes [14]. The contribution of the β-cell-protective effect and insulin secretion-stimulating action of imeglimin on muscle mass retention was expected; however, the present study demonstrated that imeglimin did not significantly impact LBM (Table 2). This finding is consistent with the results of a previous study that reported no changes in body composition in patients with type 2 diabetes receiving imeglimin [15].
In the present study, no changes in LBM were observed; however, muscle strength increased significantly in group I compared with that in group C. Imeglimin has two reported effects on skeletal muscle. First, it improves insulin signaling in muscle and has shown positive effects on insulin sensitivity. High-fat, high-sucrose diet (HFHSD) feeding altered insulin-stimulated phosphokinase B phosphorylation in the liver and skeletal muscle. Imeglimin improved insulin response in both tissues of HFHSD mice, indicating enhanced insulin sensitivity [16]. Second, imeglimin increases skeletal muscle glucose uptake and improves insulin sensitivity. In vitro, imeglimin increased glucose uptake dose-dependently in the H-2 Kb muscle cell line. In vivo, the decreased muscle glucose uptake by soleus and gastrocnemius in streptozotocin rats was significantly increased and restored to normal levels with imeglimin treatment [9, 17]. In addition, imeglimin has three reported effects on the liver. It improves insulin signaling, inhibits gluconeogenesis, and positively affects insulin sensitivity in the liver and skeletal muscle [9, 16, 17]. Imeglimin partially inhibits the mitochondrial respiratory chain complex I, restores complex III activity, and promotes fatty acid oxidation in the liver. It also decreases the production of reactive oxygen species (ROS) and increases the production of mitochondrial DNA, thereby contributing to enhanced mitochondrial function in the liver [16]. The effects of improving mitochondrial function observed in the liver may also be observed in the muscle. The relationship between the skeletal muscles and mitochondria has been widely reported. Coen et al. reported a decrease in mitochondrial capacity with aging, manifesting as decreased walking speed and increased fatigability. The authors also reported that mitochondria in aged muscles exhibited increased production of ROS compared with those in the muscles of young individuals [12, 18].
Exercise improves insulin sensitivity in the skeletal muscles and contributes to improved mitochondrial function [19]. Moreover, aerobic exercise improves mitochondrial content and function and increases mitochondrial turnover [20]. This process increases mitochondrial biosynthesis and mitophagy and enhances mitochondrial efficiency. Imeglimin decreases ROS production and increases mitochondrial DNA production [16]. ROS are potential therapeutic targets for mitochondrial dysfunction in skeletal muscles; therefore, improving ROS levels may benefit skeletal muscles. Despite differences in the mechanism of action, imeglimin and exercise therapy benefit the skeletal muscles, contributing to increased mitochondrial DNA production and decreased ROS production. Exercise improves mitochondrial function in skeletal muscle by activating peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1α) through 5' adenosine monophosphate-activated protein kinase (AMPK) phosphorylation.
Furthermore, it improves insulin sensitivity [20]. Aerobic exercise improves mitochondrial content and function and increases mitochondrial turnover [19]. This process increases mitochondrial biosynthesis and mitophagy in addition to improving mitochondrial efficiency. In contrast, imeglimin increases the production of mitochondrial DNA and decreases ROS levels without activating PGC1α [16]. Imeglimin and exercise therapy differ in the mechanism of action; however, they contribute to increased production of mitochondrial DNA and decreased ROS levels. Therefore, they both exert beneficial effects on skeletal muscle. These findings suggest that imeglimin-induced improvements in mitochondrial function may have contributed to the increased muscle strength observed in the present study. A significant improvement was observed in the quadricep muscle strength but not in grip strength. This discrepancy may be attributed to the difference in muscle size between the quadriceps and forearm muscle groups. Skeletal muscle distribution in the lower body is substantially greater than that in the upper body [21]. However, it remains unclear whether the effect of imeglimin on muscle strength can be attributed to improved mitochondrial function. Therefore, further research is required to confirm this hypothesis.
Furthermore, no significant differences were observed in the rates of change in body composition or LBM following the administration of concomitant medications. SGLT2i and GLP-1RAs result in robust body weight reduction in patients with type 2 diabetes. This reduction in body weight was observed during the first 4–16 weeks after treatment initiation, with no significant changes observed after that [22,23,24]. The present study included patients who had been receiving these drugs for an extended period; therefore, it is reasonable to assume that the effects of concomitant medications on body composition or muscle strength were not observed. The improved muscle strength observed in group I was mainly due to the effect of add-on treatment with imeglimin. The findings of the present study suggest that the administration of imeglimin may improve muscle strength without altering the body composition. This effect is unique to imeglimin and, to our knowledge, has not been reported for any other anti-diabetic drug [7].
This study has some limitations. First, this study could not be randomized or anonymized due to the limited sample size. The recruitment of participants was left to the discretion of outpatient physicians, and selection bias could not be excluded. Therefore, large-scale, multicenter trials should be undertaken to validate the present study’s findings. Second, the patients in the present study were receiving several concomitant anti-diabetic medications, such as SGLT2i and GLP-1RAs, which may affect muscle strength [7, 22,23,24]. Therefore, the possibility that concomitant medications may have exerted some effect on the results cannot be excluded. However, no significant differences were observed in muscle strength when each medication was examined separately, and the multivariate analysis showed that imeglimin increased quadriceps muscle strength independent of concomitant medications, suggesting that the effect was mainly due to the addition of imeglimin. Third, data on diet, exercise, or diabetic neuropathy were not obtained. These may have affected muscle function. Finally, although our study found no apparent effect of imeglimin on body composition, this is only because we could not detect small differences in our case series; therefore, an effect could be detected in an analysis involving a larger number of cases.
Conclusions
Our data suggest that administering imeglimin in patients with type 2 diabetes may positively affect muscle strength without affecting LBM. However, further randomized controlled trials must be conducted to validate these findings.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–23.
Visser M, Schaap LA. Consequences of sarcopenia. Clin Geriatr Med. 2011;27:387–99.
Vetrano DL, Landi F, Volpato S, et al. Association of sarcopenia with short- and long-term mortality in older adults admitted to acute care wards: results from the CRIME study. J Gerontol A Biol Sci Med Sci. 2014;69:1154–61.
Edwards MH, Dennison EM, Aihie Sayer A, Fielding R, Cooper C. Osteoporosis and sarcopenia in older age. Bone. 2015;80:126–30.
Kim TN, Park MS, Yang SJ, et al. Prevalence and determinant factors of sarcopenia in patients with type 2 diabetes: the Korean Sarcopenic Obesity Study (KSOS). Diabetes Care. 2010;33:1497–9.
Mesinovic J, Zengin A, De Courten B, Ebeling PR, Scott D. Sarcopenia and type 2 diabetes mellitus: a bidirectional relationship. Diabetes Metab Syndr Obes. 2019;12:1057–72.
Massimino E, Izzo A, Riccardi G, Della PG. The impact of glucose-lowering drugs on sarcopenia in type 2 diabetes: current evidence and underlying mechanisms. Cells. 2021;10:1958.
Hallakou-Bozec S, Vial G, Kergoat M, et al. Mechanism of action of imeglimin: a novel therapeutic agent for type 2 diabetes. Diabetes Obes Metab. 2021;23:664–73.
Yanai H, Adachi H, Hakoshima M, Katsuyama H. Glucose-lowering effects of imeglimin and its possible beneficial effects on diabetic complications. Biology (Basel). 2023;12:726.
Calvani R, Joseph AM, Adhihetty PJ, et al. Mitochondrial pathways in sarcopenia of aging and disuse muscle atrophy. Biol Chem. 2013;394:393–414.
Gonzalez-Freire M, Scalzo P, D’Agostino J, et al. Skeletal muscle ex vivo mitochondrial respiration parallels decline in vivo oxidative capacity, cardiorespiratory fitness, and muscle strength: the Baltimore longitudinal study of aging. Aging Cell. 2018;17: e12725.
Coen PM, Musci RV, Hinkley JM, Miller BF. Mitochondria as a target for mitigating sarcopenia. Front Physiol. 2018;9:1883.
Katoh M. Test–retest reliability of isometric ankle plantar flexion strength measurement performed by a hand-held dynamometer considering fixation: examination of healthy young participants. J Phys Ther Sci. 2022;34:463–6.
Hallakou-Bozec S, Kergoat M, Fouqueray P, Bolze S, Moller DE. Imeglimin amplifies glucose-stimulated insulin release from diabetic islets via a distinct mechanism of action. PLoS ONE. 2021;16: e0241651.
Uchida T, Ueno H, Konagata A, et al. Improving the effects of imeglimin on endothelial function: a prospective, single-center, observational study. Diabetes Ther. 2023;14:569–79.
Vial G, Chauvin MA, Bendridi N, et al. Imeglimin normalizes glucose tolerance and insulin sensitivity and improves mitochondrial function in liver of a high-fat, high-sucrose diet mice model. Diabetes. 2015;64:2254–64.
Fouqueray P, Leverve X, Fontaine E, Baquié M, Wollheim C. Imeglimin—a new oral anti-diabetic that targets the three key defects of type 2 diabetes. J Diabetes Metab. 2011;2:1–8.
Coen PM, Jubrias SA, Distefano G, et al. Skeletal muscle mitochondrial energetics are associated with maximal aerobic capacity and walking speed in older adults. J Gerontol A Biol Sci Med Sci. 2013;68:447–55.
van Tienen FH, Praet SF, de Feyter HM, et al. Physical activity is the key determinant of skeletal muscle mitochondrial function in type 2 diabetes. J Clin Endocrinol Metab. 2012;97:3261–9.
Kjøbsted R, Pedersen AJ, Hingst JR, et al. Intact regulation of the AMPK signaling network in response to exercise and insulin in skeletal muscle of male patients with type 2 diabetes: illumination of AMPK activation in recovery from exercise. Diabetes. 2016;65:1219–30.
Janssen I, Heymsfield SB, Wang ZM, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr. J Appl Physiol. 1985;2000(89):81–8.
Tobe K, Maegawa H, Nakamura I, Uno S. Safety and effectiveness of ipragliflozin in Japanese patients with type 2 diabetes mellitus and impaired renal function: subgroup analysis of a 3-year post-marketing surveillance study (Stella-Long TERM). Diabetol Int. 2021;12:181–96.
Terauchi Y, Fujiwara H, Kurihara Y, et al. Long-term safety and efficacy of the sodium-glucose cotransporter 2 inhibitor, tofogliflozin, added on glucagon-like peptide-1 receptor agonist in Japanese patients with type 2 diabetes mellitus: a 52-week open-label, multicenter, post-marketing clinical study. J Diabetes Investig. 2019;10:1518–26.
Sargeant JA, Henson J, King JA, et al. A review of the effects of glucagon-like peptide-1 receptor agonists and sodium-glucose cotransporter 2 inhibitors on lean body mass in humans. Endocrinol Metab (Seoul). 2019;34:247–62.
Acknowledgements
We are especially grateful to Yuko Yasuda of St. Marianna University School of Medicine, who measured patients' muscle strength and corrected the data.
Funding
No funding or sponsorship was received for this study or publication of this article. The Rapid Service Fee was funded by the authors.
Author information
Authors and Affiliations
Contributions
Takeshi Oyanagi, Shin Kawanabe, and Masakatsu Sone conceived the study idea and planned this study; Takeshi Oyanagi and Shin Kawanabe conducted data analyses; Takeshi Oyanagi, Shin Kawanabe, and Masakatsu Sone drafted the manuscript; Hidekazu Tsukiyama, Ami Nishine, Yuta Nakamura, Tomoko Nakagawa, Mayuko Kanou, Juri Kubota, Shingo Tsunemi, and Kenichi Yokota contributed to patient registration and critical review and editing of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors (Takeshi Oyanagi, Shin Kawanabe, Hidekazu Tsukiyama, Ami Nishine, Yuta Nakamura, Tomoko Nakagawa, Mayuko Kanou, Juri Kubota, Shingo Tsunemi, Kenichi Yokota and Masakatsu Sone) declare no conflict of interest.
Ethical Approval
Approval of the research protocol: The protocol for this research project has been approved by a suitably constituted Ethics Committee of the institution, and it conforms to the provisions of the Declaration of Helsinki: Clinical Research Ethics Committee of the St. Marianna University School of Medicine, Approval No. 5603. Approval date of Registry and the Registration No. of the study/trial: This study was registered with the UMIN Clinical Trials Registry (UMIN-CTR) on June 20, 2024, under the registration number UMIN000054715. The participants received a detailed explanation regarding the study’s aims and objectives, and written informed consent was obtained from all participants.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Oyanagi, T., Kawanabe, S., Tsukiyama, H. et al. The Effects of Imeglimin on Muscle Strength in Patients with Type 2 Diabetes: A Prospective Cohort Study. Diabetes Ther (2024). https://doi.org/10.1007/s13300-024-01639-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13300-024-01639-x