Abstract
Introduction
Type 2 diabetes (T2D) represents a remarkable disease burden in Japan, and the cost-effectiveness of pharmacotherapy is an important consideration. In this study, we compared the long-term effects of the type of initial medication, as well as the initial frequency of clinic visits, on the occurrence of T2D-related complications. Additionally, we compared the medical costs associated with each treatment pattern.
Methods
We analyzed electronic health record data collected from multiple primary care clinics in Japan. Patients were selected based on being primarily prescribed either biguanides (BG) or DPP-4 inhibitors (DPP-4i) during a 3-month baseline period, both of which are commonly used as first-choice medications in Japan. We then followed the onset of T2D-related complications and conducted survival analyses. Additionally, we calculated the accumulated medical costs up to the onset of an event or loss to follow-up, and summarized the annual costs per patient for each treatment pattern.
Results
A total of 416 Japanese patients with T2D who initiated treatment between January 2015 and September 2021 were included. The median follow-up period was 2.69 years. The survival analysis showed that the use of DPP-4is and frequent visits from the beginning of treatment did not offer a benefit in suppressing the onset of complications later on. On the other hand, it was found that the annual medical costs for the group using DPP-4i with frequent visits were about 1.9 times higher than for the group using BGs with less frequent visits.
Conclusions
The results suggest that for Japanese patients with T2D, the use of BGs along with relatively long follow-up intervals in the beginning of treatment can remarkably reduce medical costs while providing a level of complication suppression equivalent to that of the use of DPP-4is or frequent visits.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Why carry out this study? |
Patients with type 2 diabetes (T2D) in Japan and Western countries exhibit pathophysiological differences, leading to variations in preferred first-line medications. |
There is still limited evidence comparing the impact of initial medication choice and frequency of visits on treatment outcomes and medical costs. |
This study compared the long-term effects of the type of initial medication and the initial frequency of visits, on the occurrence of T2D-related complications and medical costs. |
What was learned from the study? |
No clear benefits on the occurrence of long-term complications were observed for either the choice of initial medication or the frequency of visits. On the other hand, the initial treatment policy could double the medical costs up to the occurrence of event. |
The results suggest that for Japanese patients with T2D, especially for those with mild obesity, starting treatment with BG medication and a relatively long observation interval, followed by modifications in treatment policy based on the physician's comprehensive judgment, can achieve equivalent treatment outcomes while reducing medical costs. |
Introduction
Type 2 diabetes (T2D) is a complex metabolic disorder characterized by high blood glucose, insulin resistance, and lack of insulin. T2D is closely associated with obesity and sedentary lifestyles, and its prevalence is increasing worldwide [1]. Uncontrolled T2D can lead to a range of complications affecting nearly every organ in the body, potentially resulting in multi-organ dysfunction. The primary concerns include both microvascular (such as nephropathy, neuropathy, and retinopathy) and macrovascular complications (such as heart disease, stroke, and peripheral vascular disease). In managing T2D, it is important not only to focus on glycemic control but also to prevent associated complications [2, 3]. As of 2021, approximately 537 million adults globally are living with diabetes, and this number is projected to rise to 643 million by 2030. The majority, over 90%, of these cases are T2D [4]. Diabetes represents an enormous economic burden, with global costs reaching USD 1.3 trillion as of 2015, and it is predicted to increase to USD 2.1 trillion by 2030 [5]. In the pharmacological treatment of diabetes, cost-effectiveness is an important consideration. Biguanides (BGs) have been used as the first-line treatment in Western countries for a long time given its effectiveness in reducing cardiovascular complications, relative safety, and low cost [6,7,8].
The economic burden of diabetes in Japan is remarkable and increasing, with medical costs rising by USD 1 million annually, reaching USD 11 million in 2009 [9]. While cost-effective diabetes control remains a critical issue in Japan, a study using the National Database of Health Insurance Claims data, which covers nearly the entire Japanese population, revealed a notable difference between Western countries and Japan. In Japan, dipeptidyl peptidase-4 inhibitors (DPP-4is), which are more expensive than biguanides, are most frequently used as the first-line treatment [10]. One of the reasons for this difference in prescription patterns is attributed to the difference in the pathophysiology of T2D between Japanese and White patient populations: White patients typically experience a marked increase in insulin resistance as they transition from normal glucose tolerance to T2D; Japanese patients show a trend towards lower insulin secretion rather than a significant increase in insulin resistance [11, 12]. These pathophysiological differences manifest as the relatively low body mass index (BMI) characteristic of Japanese patients. A previous meta-analysis reported that the glycemic control capability of DPP-4is decreases in populations with higher BMI [13], which could explain why DPP-4is are preferred as the first-line medication in Japan. In any case, it is important to prescribe medication considering the characteristics of both the patient and the drug.
While there are prior studies using medical claims data to investigate the effects of T2D pharmacotherapies on glycated hemoglobin (HbA1c) reduction in a Japanese population [14], the real-world evidence regarding the prevention of diabetes complications in Japanese population remains scarce. In this study, we aim to explore cost-effective T2D pharmacotherapies based on real-world evidence using actual clinical practice-based electronic health record (EHR) data from primary care clinics. Reflecting the reality of prescriptions in Japan, we investigate the effects of the type of initial medication (BGs or DPP-4is) and prescription interval on the prevention of complications and their impact on costs.
Methods
Study Design and Data Source
For this study, pseudonymized real-world dataset was provided by Allied Medical, K.K. The dataset is based on medical practice data, comprising 58,682 patients from 34 primary care clinics across Japan. The dataset is owned by Allied Medical, K.K., and the research team conducted the analysis with permission granted by the company.
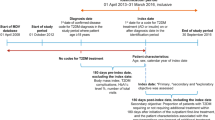
In this study, the initiation of treatment is defined as the first visit accompanied by a prescription for diabetes medication, and we included patients who initiated treatment after January 2015. The first 90 days from the first visit were considered the baseline period, with the time afterward defined as the observation period. Patients were included based on the following criteria: (1) having at least one measurement of HbA1c and estimated glomerular filtration rate (eGFR) during the baseline period, (2) having a prescription proportion of days covered (PDC) [15] of 0.75 or higher for the medication of interest (BGs or DPP-4is) during the baseline period. Patients who met the following criteria were excluded: (1) having PDC of 0.75 or higher for both BGs and DPP-4is during the baseline period, (2) experiencing endpoint during the baseline period or prior to the first clinic visit at other clinics (self-report). Patients were divided into the BG arm and the DPP-4i group based on their prescription patterns during the baseline period, and analyses were conducted regardless of any changes in prescription patterns during the observation period.
The study was approved by the ethics committee of Health Outcome Research Institute (submission ID 2024-06). Informed consent was waived by the ethics committee.
Outcomes
Complications related to T2D consisting of renal, neurological, vascular, retinal, and skin complications were comprehensively listed and adopted as a composite endpoint. In this study, we used this composite endpoint as the primary outcome for the incidence of diabetes-related complications. As indicators of the drug safety, hepatic events and cardiac events were included as the secondary outcome. All relevant event names and corresponding ICD codes are shown in Supplementary Table S1.
In addition to health-related outcomes, we calculated and compared the medical costs and their breakdown from the start of treatment until the incidence of an event or loss to follow-up. We adopted the healthcare payers’ perspective, under which all medical costs were included regardless of copayment. Costs were calculated by multiplying the actual usage of resources by the unit prices defined in Japan's National Medical Care Fee Schedule and the National Drug Tariff as of April 2021. For discontinued items, we used the latest available unit price. Under Japan's public health insurance system, all medical practices, except for hospitalization in tertiary medical facilities, are charged on a fee-for-service system. The government strictly regulates the unit prices of medical services to ensure they are uniform across all clinics [16].
Analysis
After the patients were split into the BG group and the DPP-4i group, one-to-one nearest-neighbor propensity score (PS) matching using logistic regression was conducted to mitigate influences of confounding factors between the groups. The following variables were incorporated to calculate propensity scores: age, sex, disease history (hepatic and cardiac), baseline renal function (eGFR), and baseline severity (HbA1c). The list of event names included in the disease history can be found in Supplementary Tables S2 and S3.
Considering the relatively small population size and number of events, we examined the impact of the type of medication and the prescription intervals during the initial treatment phase (baseline period) on the incidence of T2D-related complications using univariate survival analysis with the log-rank test. The safety of the medication, a secondary outcome, was also evaluated using the log-rank test based on the type of medication.
To further explore the relationship between T2D-related complications and various factors, we conducted a more advanced survival analysis: (1) bootstrap sampling (80%) from the dataset, (2) fit a cross-validated LASSO–Cox proportional hazards model incorporating the following explanatory variables: type of initial medication, initial prescription intervals, age, sex, HbA1c, eGFR, and medical history (3) repeat steps 1 and 2 a hundred times, count the number of times a non-zero coefficient was observed for each variable, and retain variables that were observed more than ten times, (4) fit a Cox proportional hazards model to the entire dataset using the variables retained from step 3 to investigate the impact of each variable on the outcome. Additionally, considering the nature of T2D, we conducted a sensitivity analysis by excluding events occurring or censoring within 180 days from the start of the baseline period, focusing on patients who had a relatively stable course.
Regarding the costs, we divided the patients into four groups based on the type of initial medication (BG or DPP-4i) and the length of the prescription interval (less than 40 days or 40 days and above). We then calculated and compared the annual cost per person for each group.
All statistical analyses were performed using Python version 3.7.9 and R version 4.2.0. Analysis items with a two-tailed P value of < 0.05 were considered statistically significant.
Results
Patients
A total of 865 patients were identified as eligible among 3788 patients who initiated treatment for T2D between January 2015 and September 2021. The number of patients in the group whose initial medication was BG was 289, and the number in the DPP-4i group was 576. After the PS matching, 208 patients for each group were included in the analysis (Fig. 1). The treatment for the first patient began on January 6, 2015, and the observation period for all patients finished on September 10, 2021.
Table 1 shows the baseline characteristics of the patients before and after the PS matching. During a total of 1180 person-years of follow-up, 36 T2D-related complications were observed. The median follow-up period was 2.69 years.
Health-Related Outcomes
Figure 2a, b presents the Kaplan–Meier plots for the primary outcome, i.e., the incidence of T2D-related complications. The confidence intervals for both the type of initial medication and the initial prescription interval overlapped considerably, showing no statistically significant difference in the univariate log-rank test.
As a result of variable selection using the LASSO-Cox proportional hazards model with a regularization term, out of 100 bootstrap sampling trials, non-zero coefficients were observed two times for age, 16 for sex, 20 for HbA1c, 99 for eGFR, 31 for history of hepatic diseases, eight for history of cardiac diseases, four for the type of initial medication, and nine for the initial prescription interval. Consequently, sex, HbA1c, eGFR, and the medical history of hepatic diseases were incorporated into the final Cox proportional hazards model. The hazard ratios for each variable are shown in Fig. 2c. A lower eGFR at baseline, indicating better renal function, significantly reduced the hazard ratio for complications, while having a history of hepatic diseases significantly increased the hazard ratio. These results are consistent with the findings of prior studies [17, 18]. Although the differences were not statistically significant, there was a trend toward lower hazard ratios in women and higher hazard ratios associated with higher initial HbA1c levels. The sensitivity analysis that focused on patients with stable course was consistent with the main analysis (Supplementary Figure S1).
The results of the survival analyses examining the impact of the initial medication on subsequent cardiac and hepatic events are presented in Supplementary Figures S2 and S3. In both cases, the survival curves of the two groups overlapped, and no statistically significant differences were observed.
Medical Costs
The costs for patients divided into four groups based on the type of initial medication and the length of the initial prescription interval (median less than 40 days or 40 days and over) are shown in Fig. 3. It was found that groups starting with high-cost treatments (DPP-4i with short intervals) incurred up to approximately 2.2 times, 1.9 times, 2.4 times, and 1.9 times higher costs, respectively, for consultation and management fees, dispensing fees, drug costs, and total medical costs compared to groups starting with low-cost treatments (BG with long intervals). The group that started treatment with BG and had longer initial prescription intervals incurred an annual cost per person that was JPY 66,879 lower compared to the group with DPP-4i and shorter intervals.
Discussion
The aim of this study was to investigate cost-effective T2D management methods in a Japanese patient population with T2D from the perspective of real-world true endpoints, i.e., T2D-related complications.
In our survival analysis, no clear benefit was confirmed in suppressing the long-term onset of T2D-related complications by using DPP-4is or by prescribing medications at short intervals (i.e., frequent visits) from the beginning of treatment. Previous research has reported that in patients with a lower BMI, DPP-4is are superior to BGs in terms of glycemic control [14]. It is considered that prescriptions made in actual clinical practice are based on a comprehensive judgment including BMI. Therefore, while these results do not suggest the superiority or inferiority of the drugs, given that the study population has BMIs around the borderline of obesity standards (BMI = 25) [19], it reconfirms that for patients with relatively high BMI, using DPP-4is as first-line medication does not provide any particular benefit over BGs, not only in terms of glycemic control but also in the suppression of complications. Furthermore, no statistically significant difference was observed in safety between DPP-4is and BGs. Considering the differences in safety profiles of drugs, it is reasonable to conclude that the results indicate that prescriptions are being appropriately made by physicians, taking safety considerations into account.
While no clear benefits to health were observed from the use of DPP-4is and the frequent visits at the beginning of treatment, it was found that the group starting with DPP-4is and having shorter prescription intervals incurred approximately 1.9 times the medical costs compared to the group starting with BGs and having longer prescription intervals.
Based on the above results, for Japanese patients with T2D, particularly those with a relatively high obesity, it is suggested that starting with BG drugs while making appropriate changes or additions to the prescription based on regular test results, and conducting follow-up observations with relatively longer intervals, can maintain the incidence of complications at the same level while significantly reducing medical costs. While in clinical settings, the selection of medications and the interval of follow-up need to be comprehensively determined based on factors such as the patient's age, degree of obesity, and hepatic and renal functions [19], this study is considered to provide new insights from the perspective of cost-effectiveness in treatment choices.
This study has several limitations. First, since we used EHR data from primary care clinics, it is anticipated that loss to follow-up could occur due to transfers to other clinics or hospitals. Similarly, there is a possibility that patients who had already received treatment at other clinics prior to being observed in the study may be included. Particularly, severe events, which are often treated at larger hospitals, may be underestimated in our analysis. While this study focused on the incidence of events and the costs up to the event, post-event costs and outcomes related to prognosis can be tracked using insurer-based claims data. In the future, it is desirable to conduct research with a more comprehensive framework. Nevertheless, this study has provided quantitative insights into the impact of initial treatment strategies on subsequent health outcomes and costs, aligning with its original objective. Secondly, BMI is a confounding factor that influences both the choice of medication and the prognosis of T2D [19, 20]. BMI should have been adjusted by using PS matching, but due to the high number of missing values in the dataset, it could not be included. In the matched dataset, BMI was available only for a subset of patients and the average BMI was 25.7 (n = 24) in the DPP-4i group and 27.0 (n = 23) in the BG group. It is unlikely that there is a systematic difference in the missing mechanism of BMI values between both groups, and the observed difference was not statistically significant nor considered clinically meaningful. Therefore, while this restricts the generalizability of this study to Japanese patients with T2D with relatively high BMI, it is not expected to affect the direction of the conclusions. Thirdly, this study had a limited sample size, which may have resulted in insufficient statistical power. Therefore, the absence of statistically significant differences in our analysis does not necessarily imply that the compared items are equivalent. It is necessary to continue conducting similar studies with larger sample sizes, and caution is required in interpreting the results of this study. With all the limitations, we believe that our results would contribute to the decision-making process of T2D pharmacotherapy.
Conclusions
The results of this study suggest that for Japanese patients with T2D with relatively high BMI, the use of BGs along with relatively long follow-up intervals in the beginning of treatment can remarkably reduce medical costs while providing a level of T2D-related complication suppression equivalent to that of the use of DPP-4is or frequent visits. We believe these findings offer new insights from a cost-effectiveness perspective to physicians in their decision-making process for T2D pharmacotherapy.
Data Availability
The data used in this study were provided by Allied Medical, K.K., and are subject to restrictions under the license agreement. Proposals and requests for data access should be directed to the corresponding author.
References
DeFronzo RA, Ferrannini E, Groop L, Henry RR, Herman WH, Holst JJ, et al. Type 2 diabetes mellitus. Nat Rev Dis Prim. 2015;1:15019.
Harding JL, Pavkov ME, Magliano DJ, Shaw JE, Gregg EW. Global trends in diabetes complications: a review of current evidence. Diabetologia. 2019;62:3–16.
Grover A, Sharma K, Gautam S, Gautam S, Gulati M, Singh SK. Diabetes and its complications: therapies available, anticipated and aspired. Curr Diabetes Rev. 2021;17:397–420.
Magliano DJ, Boyko EJ. IDF Diabetes Atlas 10th edition scientific committee. IDF DIABETES ATLAS. Brussels: International Diabetes Federation; 2021.
Bommer C, Sagalova V, Heesemann E, Manne-Goehler J, Atun R, Bärnighausen T, et al. Global economic burden of diabetes in adults: projections from 2015 to 2030. Diabetes Care. 2018;41:963–70.
Maruthur NM, Tseng E, Hutfless S, Wilson LM, Suarez-Cuervo C, Berger Z, et al. Diabetes medications as monotherapy or metformin-based combination therapy for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med. 2016;164:740–51.
ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, et al. 9. Pharmacologic approaches to glycemic treatment: standards of care in diabetes-2023. Diabetes Care. 2023;46:S140–57.
Fang M, Wang D, Coresh J, Selvin E. Trends in diabetes treatment and control in U.S. adults, 1999–2018. N Engl J Med. 2021;384:2219–28.
Urakami T, Kuwabara R, Yoshida K. Economic impact of diabetes in Japan. Curr Diab Rep. 2019;19:2.
Bouchi R, Sugiyama T, Goto A, Imai K, Ihana-Sugiyama N, Ohsugi M, et al. Retrospective nationwide study on the trends in first-line antidiabetic medication for patients with type 2 diabetes in Japan. J Diabetes Investig. 2022;13:280–91.
Fukushima M, Usami M, Ikeda M, Nakai Y, Taniguchi A, Matsuura T, et al. Insulin secretion and insulin sensitivity at different stages of glucose tolerance: a cross-sectional study of Japanese type 2 diabetes. Metabolism. 2004;53:831–5.
Kodama K, Tojjar D, Yamada S, Toda K, Patel CJ, Butte AJ. Ethnic differences in the relationship between insulin sensitivity and insulin response: a systematic review and meta-analysis. Diabetes Care. 2013;36:1789–96.
Kim YG, Hahn S, Oh TJ, Kwak SH, Park KS, Cho YM. Differences in the glucose-lowering efficacy of dipeptidyl peptidase-4 inhibitors between Asians and non-Asians: a systematic review and meta-analysis. Diabetologia. 2013;56:696–708.
Odawara M, Aoi S, Takeshima T, Iwasaki K. Comparative effects of metformin and dipeptidyl peptidase-4 inhibitors in Japanese obese patients with type 2 diabetes: a claims database study. Diabetes Ther. 2021;12:2165–77.
Prieto-Merino D, Mulick A, Armstrong C, Hoult H, Fawcett S, Eliasson L, et al. Estimating proportion of days covered (PDC) using real-world online medicine suppliers’ datasets. J Pharm Policy Pract. 2021;14:113.
Ishii M. DRG/PPS and DPC/PDPS as prospective payment systems. JMAJ. 2012;55:279–91.
Sheen Y-J, Hsu C-C, Kung P-T, Chiu L-T, Tsai W-C. Impact of chronic hepatitis on cardiovascular events among type 2 diabetes patients in Taiwan pay-for-performance program. Sci Rep. 2022;12:11720.
Babaliche P, Nadpara RA, Maldar A. Association between estimated glomerular filtration rate and microvascular complications in type II diabetes mellitus patients: a 1-year cross-sectional study. J Natl Med Assoc. 2019;111:83–7.
Araki E, Goto A, Kondo T, Noda M, Noto H, Origasa H, et al. Japanese clinical practice guideline for diabetes 2019. J Diabetes Investig. 2020;11:1020–76.
Tanaka S, Tanaka S, Iimuro S, Ishibashi S, Yamashita H, Moriya T, et al. Maximum BMI and microvascular complications in a cohort of Japanese patients with type 2 diabetes: the Japan Diabetes Complications Study. J Diabetes Complic. 2016;30:790–7.
Acknowledgements
We would like to thank Allied Medical, K.K., for processing and providing the pseudonymized data for the secondary analysis.
Authorship
All authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship.
Funding
Sponsorship for this study and Rapid Service Fee were funded by Allied Medical, K.K.
Author information
Authors and Affiliations
Contributions
Ataru Igarashi, Hiroshi Yoshihara, and Tohru Tonoike contributed to the study conception. Hiroshi Yoshihara performed the data analysis and drafted the manuscript. All authors contributed to the study design, data interpretation, revised the manuscript, and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Hiroshi Yoshihara is an employee of Aillis, Inc. Tohru Tonoike is the CEO of Allied Medical, K.K. Hiromitsu Ohno and Susumu Nishiuchi are executive officers of Allied Medical. Ataru Igarashi receives research funding from Allied Medical.
Ethical Approval
The study was approved by the ethics committee of Health Outcome Research Institute (submission ID 2024-06). Informed consent was waived by the ethics committee.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Yoshihara, H., Tonoike, T., Ohno, H. et al. Impact of Initial Treatment Policies on Long-term Complications and Costs in Japanese Patients with Type 2 Diabetes: A Real-World Database Study. Diabetes Ther 15, 1811–1820 (2024). https://doi.org/10.1007/s13300-024-01611-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-024-01611-9