Abstract
Introduction
This study assessed experiences, attitudes, and behaviors of people with diabetes (PwD) regarding diabetes self-management and glucose control, and their level of awareness, knowledge, and attitudes toward time in range (TIR).
Methods
This quantitative survey was conducted using an online questionnaire across seven countries. Respondents were PwD classified into three subgroups: type 1 (T1), type 2 insulin (T2 insulin), and type 2 not on insulin (T2 N/insulin).
Results
Respondents included 621 people in the T1, 780 people in the T2 insulin, and 735 people in the T2 N/insulin subgroups. Awareness of TIR was low, particularly in the T2 N/insulin subgroup (T1 53%, T2 insulin 29%, T2 N/insulin 9%). Despite a lower current use of continuous glucose monitoring (CGM) among the T2 insulin and T2 N/insulin participants (38% and 9%, respectively), versus T1 participants (64%), most (> 70%) were positive toward utilizing new tools and measures to self-manage blood glucose. Recommendations from their healthcare professionals (HCPs) were cited as a strong motivator to try new measures for analyzing glucose levels. The main barriers cited were limited access to CGM and lack of understanding of TIR benefits. Cost was the main reason given by ≥ 40% of respondents for stopping CGM use.
Conclusions
There is an unmet need in diabetes management, and TIR and CGM offer a potential solution. PwD are motivated to manage their blood glucose levels and are positive toward utilizing new tools and measures to achieve this goal. HCPs play a pivotal role in informing and guiding PwD on new measures for analyzing glucose.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? | |
The metric time in range (TIR), derived from continuous glucose monitoring (CGM), provides more information about glycemic control than glycated hemoglobin, but there has been little research on patient knowledge and attitudes concerning TIR. | |
The aim of this quantitative market survey was to assess the experiences, attitudes, and behaviors of people with diabetes regarding diabetes self-management, specifically the measurement and interpretation of glucose levels. | |
What was learned from the study? | |
This international online survey revealed a low level of knowledge about TIR, yet a high level of interest in new tools and measures to self-manage blood glucose. | |
Perceived benefits of TIR included better understanding of how food choices, physical activity, and medication adjustments impact glycemia, better management of hypoglycemia and improved ability to set tangible goals for self-management of a person’s own diabetes. |
Introduction
People with diabetes (PwD) and healthcare professionals (HCPs) involved in their care are faced with an increasing number of therapeutic interventions and complex treatment regimens, both pharmaceutical and technological [1]. Recently, we surveyed HCPs about their knowledge of and attitudes to using the continuous glucose monitoring (CGM) metric of time in range (TIR) to assist in diabetes management [2]. However, understanding PwD, their views on self-management, and their willingness to embrace changes to self-management methods will be critical in determining whether TIR will become widely accepted and used.
For several decades, glycated hemoglobin (HbA1c/A1c) has been the key metric of diabetes management for monitoring long-term average glucose control and assessing the risk of diabetes-related complications [3, 4]. However, HbA1c cannot be used to assess day-to-day variability and glucose fluctuations, such as hypoglycemia or hyperglycemia [5]. Conversely, CGM devices measure glucose levels in the interstitial fluid every 1–5 min, providing a more detailed picture of glucose excursions than HbA1c/A1c [6]. A standardized report such as the Ambulatory Glucose Profile provides a visual representation of glucose levels in PwD who use CGM and can aid communication between PwD and HCPs [6].
Guidelines recommend real-time CGM (rt-CGM) or intermittently scanned CGM (is-CGM) for the management of diabetes in adults with diabetes on multiple daily injections or continuous subcutaneous insulin infusion [7]. TIR, time below range (TBR), and time above range (TAR) are some of the key glucose management metrics available to CGM users. Recommendations from an international consensus on TIR (IC-TIR) set out guidance on target ranges and time spent within target ranges for different diabetes populations. The recommended target range for most people with type 1 or type 2 diabetes is 70–180 mg/dL (3.9–10.0 mmol/L) with a TIR > 70% [8]. However, the consensus also states that targets should be individualized depending on personal needs, patient profile (e.g., elderly, frail, or pregnant), and their circumstances [8].
A major barrier to the widespread adoption of TIR is that many PwD do not use CGM [9]. In a survey of 1503 adult T1D Exchange participants, the most commonly reported barriers to CGM use were cost-related and included the cost of supplies, cost of the device, and insurance coverage [10]. Healthcare disparities in the use of CGM within racially and ethnically diverse and lower socioeconomic populations have also been identified, limiting access and resulting in lower use of CGM in these populations [11].
The aim of this quantitative market survey was to assess the experiences, attitudes, and behaviors of PwD regarding diabetes self-management, specifically the measurement and interpretation of glucose levels. In addition, we sought to identify unmet needs and areas of dissatisfaction within diabetes management. We also investigated how PwD obtain information about new resources, tools, and measures for diabetes management, and what motivates PwD to integrate new tools into their daily management routines. Lastly, we investigated the usefulness of HbA1c/A1c measurements and TIR in helping PwD manage their diabetes.
Methods
Study Design
This patient quantitative survey was conducted using an online questionnaire across seven countries (Brazil, Canada, South Korea, Spain, Sweden, UK, and USA) between 22 April and 14 July 2022. The Harris Poll designed the survey in collaboration with Novo Nordisk. The survey was also reviewed by an expert panel. The Harris Poll hosted the survey on a secure website; the average time expected to complete the survey was ~ 20 min. Participants were sampled from online consumer panels maintained by The Harris Poll’s trusted partners. All panelists had opted into receiving survey invitations and had agreed to take part in online research. Participants received a unique password-protected survey link to ensure anonymity and prevent the completion of the survey more than once. All participants who completed the survey received redeemable panel reward points.
Participants
Respondents eligible to participate in the survey were aged ≥ 18 years and diagnosed with type 1 or type 2 diabetes for ≥ 1 year. Participants included in the study were classified into three subgroups: type 1 (T1), type 2 insulin-treated (T2 insulin) or type 2 not on insulin (T2 N/insulin), according to participant self-identification in response to a series of screening questions at the start of the survey (see Q1000 section of the questionnaire in the Supplementary Material: full questionnaire). Participants in the T1 subgroup had been diagnosed with type 1 diabetes primarily before the age of 30 years, prescribed insulin immediately or within 3 months of diagnosis, and were taking insulin using an insulin pump and/or two or more insulin injections a day at the time of participation. Participants in the T2 subgroups had been diagnosed with type 2 diabetes primarily after the age of 30 years and were using insulin (T2 insulin subgroup) or not using insulin (T2 N/insulin subgroup) at the time of participation. See Supplementary Table 1 for the full criteria of each PwD subgroup.
Ethics Approval
Participants provided consent to participate in the study and confidentiality was maintained with appropriate measures at all stages of the research. This study adhered to the European Pharmaceutical Market Research Association Code of Conduct 2019, page 15, Sect. 1.3, meaning it did not require clinical research ethics committee or independent/institutional review board approval for its conduct in the following countries: Brazil, Canada, Denmark, Finland, France, Germany, Greece, Italy, Japan, Mexico, the Netherlands, Norway, Poland, Russia, South Korea, Spain, Sweden, Turkey, the UK, and the USA [12].
Survey Questionnaire
Within the survey, questions were asked relating to the participant’s age at diagnosis, length of time since diagnosis, currently used treatments for diabetes, duration of insulin use, method of monitoring glucose levels, and awareness of different measures for monitoring glucose levels. Also included were questions relating to communication with HCPs and the information and resources that participants used to learn about new developments and tools in diabetes management. The full questionnaire is available in the Supplementary Material. For the purposes of this study, CGM was defined as rt-CGM (such as Dexcom G5 or G6, Guardian 3 or 4, Enlite, Eversense) or is-CGM (such as FreeStyle Libre 1 or 2). For the purposes of this study, TIR was defined as the amount of time spent in range, below range, and above range. The commonly used term “sugar” was used in the survey when referring to glucose levels.
Statistical Methods
Results were not weighted and are only representative of those who took part in the study. Data were collected and analyzed by diabetes type subgroup: T1, T2 insulin, or T2 N/insulin. Statistical testing was performed to compare differences between the subgroups. Subgroup differences were tested at the 95% level of confidence. Means were analyzed using the T test and proportions were analyzed using the Z test. Adjustments for multiple comparisons were not made.
Results
Participant Characteristics
A total of 2136 participants completed the questionnaire: 621 in the T1 subgroup, 780 in the T2 insulin subgroup, and 735 in the T2 N/insulin subgroup. PwD used a variety of methods to measure their glucose levels. However, a greater proportion of participants among T1 (64%, P < 0.05 versus T2 insulin and T2 N/insulin) currently use CGM to measure glucose levels compared with the T2 insulin (38%, P < 0.05 versus T2 N/insulin) and T2 N/insulin (9%) subgroups. (Table 1). In contrast, 74% of participants among the T2 insulin (P < 0.05 versus T1 and T2 N/insulin) and 67% of those in the T2 N/insulin (P < 0.05 versus T1) subgroup currently use finger stick glucose monitoring compared with 62% of participants in the T1 subgroup (Table 1).
Usefulness of HbA1c/A1c in Helping PwD Manage their Diabetes
While 84% of participants in the T1 (P < 0.05 versus T2 insulin and T2 N/insulin) and 76% in the T2 insulin (P < 0.05 versus T2 N/insulin) subgroups reported that they were “somewhat well equipped/very well equipped” to bring HbA1c level to where it needs to be, only 66% of T2 N/insulin participants did. However, at least 50% of participants across all three subgroups agreed that they were not clear on how to use their HbA1c/A1c results to help them manage their diabetes. Furthermore, > 70% of participants wished their HCP(s) provided them with specific instructions on how they can best manage their diabetes on their own (data not shown).
Behaviors and Attitudes of PwD toward Using New Technology for Managing their Diabetes and Role of HCPs in Facilitating the Use of New Tools
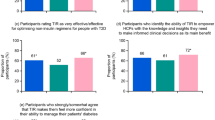
Most participants (> 70%) expressed an interest in new tools and ways to manage their diabetes, and specifically for monitoring and analyzing glucose levels (Fig. 1). However, people in the T1 subgroup were more likely than those in the T2 subgroup to engage in proactive behaviors to learn about and try new ways and tools to manage their diabetes (especially compared with the T2 N/insulin subgroup). A total of 74% of participants among the T1 (P < 0.05 versus T2 insulin and T2 N/insulin) and 65% of those in the T2 insulin (P < 0.05 versus T2 N/insulin; Fig. 1) subgroup brought information they found about new ways and tools to manage their diabetes to discuss with their HCPs during visits, while only 48% of T2 N/insulin participants did so (Fig. 1). Between 71 and 79% of participants across all subgroups cited “recommendation from HCP” as the top motivator to try new measures for analyzing glucose levels (Fig. 1). At least half of participants across all three subgroups (T1: 50%, T2 insulin: 52%, T2 N/insulin: 53%) reported “seeing sugar levels where they need to be” as the top motivating factor for continuing use of a new measure for analyzing glucose levels. Most participants (across all three subgroups) expect HCPs to keep them informed about new tools and consider it important for HCPs to help them decide which tools would be most suitable (Fig. 1).
Behavior and attitudes of PwD toward using new technology for managing their diabetes and the role of HCPs in facilitating the use of new tools. HCP healthcare professional, PwD people with diabetes, T2 insulin type 2 diabetes on insulin, T2 N/insulin type 2 diabetes not on insulin. aP < 0.05 for difference versus T2 insulin and T2 N/insulin. bP < 0.05 for difference versus T2 N/insulin
Awareness and Attitudes toward TIR among PwD
A greater proportion of participants in the T1 insulin subgroup (53%, P < 0.05 versus T2 insulin and T2 N/insulin) were aware of TIR (including TBR and TAR), compared with the T2 insulin (29%, P < 0.05 versus T2 N/insulin) and T2 N/insulin subgroups (9%; Table 1). Among those aware of TIR, knowledge of the measure is somewhat limited, yet many say they would be “comfortable/very comfortable” using it, particularly among the T1 subgroup (73%, P < 0.05 versus T2 insulin and T2 N/insulin; Fig. 2). Among those aware of TIR, the most common source of introduction was from their HCP (reported between 41 and 48% of participants for all three subgroups). The next most common sources were social media, manufacturers’ websites, and online videos (1–14%). PwD who are aware of TIR cited the primary benefits of using TIR as identifying and tracking glucose level patterns over time to help them better understand how daily routines impact glucose levels and immediately being able to see the impact of diet/exercise adjustments on glucose levels. In addition, a high percentage of participants agreed that (1) TIR provides a more accurate picture of glucose levels than other measures, (2) they have greater confidence in their ability to correct hypoglycemia, and (3) TIR is easier to understand than other measures to analyze glucose levels (Fig. 2).
Knowledge of and attitudes toward TIR among PwD. HCP healthcare professional, PwD people with diabetes, TIR time in range, T1 type 1, T2 insulin type 2 diabetes on insulin, T2 N/insulin type 2 diabetes not on insulin. aP < 0.05 for difference versus T2 insulin and T2 N/insulin. bP < 0.05 for difference versus T2 N/insulin. cP < 0.05 for difference versus T1. dSmall base size (n = 67)
Barriers to Using TIR
Among those aware of TIR, limited access to CGM and lack of understanding of TIR benefits were often cited by PwD as the main barriers for using TIR to manage their diabetes (Fig. 3). Lack of comprehensive education programs and guidance from HCPs could be contributing factors (Fig. 3). In addition, “out-of-pocket costs too high” was the main reason given by ≥ 40% of participants across all three subgroups who used CGM in the past for stopping its use, followed by “sensor uncomfortable to wear” and “CGM not covered by their health insurance” (Fig. 3).
Discussion
In this study, the experiences, attitudes, and behaviors of PwD to diabetes self-management and glucose control, and their level of awareness, knowledge, and general attitudes toward TIR were investigated. Overall, the survey highlighted unmet needs in the self-management of diabetes in terms of glucose management in all three diabetes subgroups. Most of the participants reported that they were not always clear on how to use HbA1c/A1c results to help manage their diabetes and only a small proportion felt that they were very well equipped to bring their HbA1c/A1c to the level where it needs to be. In contrast, participants who were aware of TIR perceived it to provide beneficial information, and many of them were already making use of this metric in their diabetes management. Perceived benefits included the ability to understand how daily routines, food choices, physical activity, and medication adjustments impacted glycemic control, as well as the ability to better manage hypoglycemia and set tangible goals for improved self-management of their diabetes. However, awareness of TIR was low overall, particularly among those in the T2 N/insulin subgroup. There are different methods available for PwD to check their glucose levels, and CGM was most often used by participants in the T1 subgroup (64%, compared with 38% and just 9% in the T2 insulin and T2 N/insulin subgroups, respectively). Despite the comparatively lower use of CGM by people with T2 diabetes, this survey showed that most participants are nevertheless motivated to manage their blood glucose levels and felt positive toward utilizing new tools and measures to achieve this goal. However, they relied heavily on their HCPs for such information, and recommendation and guidance from their HCPs are strong motivators for using new measures.
As apparently recognized by the participants in this survey who were aware of TIR, TIR is a particularly accessible and intuitive metric for PwD that offers a more nuanced, cause-and-effect-related understanding of glucose fluctuations that can illustrate how certain behaviors and decisions may impact glucose levels. As such, TIR has previously been identified by PwD as the top outcome measure for impacting quality of daily life [13]. Furthermore, TIR has prognostic value; in several studies, a lower TIR has been associated with microvascular and macrovascular complications, hypoglycemia, and ketoacidosis [8, 14,15,16,17,18]. TIR can also be useful in circumstances when there may be discrepancies between HbA1c levels and mean glucose, such as chronic kidney disease or hemoglobinopathy [19, 20]. Racial differences in HbA1c have also been observed, with HbA1c reported to be 0.4% higher in Black than in White persons for the same mean glucose concentration [21], further demonstrating the potential importance of using alternative measures for glycemic control. However, rather than a replacement for HbA1c measurement, TIR can be viewed as complementary information showing the quality of overall glucose control.
A recent survey investigating HCP attitudes toward, and knowledge of, TIR in diabetes management [2] found that > 90% of surveyed HCPs agreed that TIR is likely/somewhat likely to become the standard metric for diabetes management in the future and that TIR empowers PwD with the information they need to successfully manage their diabetes [2]. However, results from the survey also showed that education is needed to increase awareness of TIR among HCPs and to optimize its use in clinical practice. In the study presented here, TIR awareness was relatively low among PwD, especially among people with type 2 diabetes, although most participants were interested in using TIR. Those aware of TIR cited several benefits to its use, including ease of use compared with other measures for analyzing glucose levels. As might be expected, it is a HCP who most commonly introduces TIR to PwD. However, limited access to CGM and lack of understanding of the benefits of TIR—which may in part be related to a greater need for education of HCPs, who in turn can educate PwD—were frequently cited by PwD as key barriers to using TIR in the management of their diabetes. Cost continued to be a major barrier to PwD using CGM and is one of the main reasons given for stopping CGM. Reimbursement by payers (policy makers and budget holders) as well as addressing the healthcare disparities within racially and ethnically diverse and lower socioeconomic populations will be essential for the uptake and use of new diabetes technologies such as CGM [11, 22].
After out-of-pocket costs, the next most common reason for PwD stopping using a CGM was “my sensor is uncomfortable to wear.” Findings from the T1D Exchange survey study showed that the hassle of wearing devices and disliking devices on one’s body were significant physical barriers to the uptake of CGM. Therefore, efforts to increase CGM use may also need to focus on improving the physical experience of wearing these devices [10].
The strengths of this study include its global design involving countries from Europe, the Americas, and Asia. Data from three different PwD subgroups were analyzed, enabling the assessment of differences (and similarities) in diabetes management by people with type 1 diabetes, type 2 diabetes using insulin, and type 2 diabetes not using insulin. The detailed findings from this study should help inform HCPs on how best to empower PwD to self-manage their diabetes.
As with all survey analyses, there are several limitations in this study. Firstly, reporting bias may have been introduced regarding knowledge of TIR because the participants would have already gained some knowledge from earlier questions in the survey. Furthermore, awareness and knowledge of new technologies and metrics will continue to improve over time, meaning that some results from the survey will inevitably become less relevant. In addition, differences between the responses of the three diabetes subgroups are likely to be influenced by demographic differences, the education received in the context of starting insulin therapy, and perhaps even the level of encouragement given by HCPs on the basis of their preconception of their patient’s ability to assimilate information or manage a CGM device. The study did not consider differences in reimbursement for CGM between the different countries and diabetes subtypes. It is possible that experiences, attitudes, and behaviors could differ depending on whether CGM is reimbursed or not. Finally, participants who completed the survey received redeemable panel reward points. It cannot be stated if this impacted motivation to participate in the survey and hence whether the sample was representative of the broader population of PwD.
Conclusions
Although the value of reaching HbA1c target is recognized, the utility of HbA1c to guide diabetes self-management is low. Other tools (CGM) and glycemic measures (TIR) can offer solutions. PwD expressed optimism about using CGM to monitor TIR and were motivated by seeing how adjustments they make to their diet, physical activity, and medication could positively impact their blood glucose levels. This information could also prove useful for HCPs to adjust diabetes management on a personal level. However, for successful implementation, PwD believe that comprehensive education programs and guidance from HCPs are essential, along with addressing the barrier of high cost. Given the findings in this study, HCPs should continue to inform, educate, and guide PwD on the use and interpretation of CGM, TIR, and other glycemic measures to increase empowerment, regardless of diabetes or treatment type. In addition, HCPs should continue to keep up to date with the latest advancements in diabetes management and advocate for better access to novel technologies, such as CGM, as the potential benefits of independent diabetes management by PwD and personalized healthcare can be substantial.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Davies MJ, Aroda VR, Collins BS, Management of hyperglycaemia in type 2 diabetes, et al. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2022;2022(65):1925–66.
De Block C, Cheng AY, Christensen TB, Patted URH, Ginovker A. Healthcare professionals’ knowledge of and attitudes towards the use of time in range in diabetes management: online survey across seven countries. Diabetes Therapy. 2023;14:1399–413.
Diabetes Control and Complications Trial Research Group, Nathan DM, Genuth S, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–86.
Nathan DM, DCCT/EDIC Research Group. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: overview. Diabetes Care. 2014;37:9–16.
Danne T, Nimri R, Battelino T, et al. International consensus on use of continuous glucose monitoring. Diabetes Care. 2017;40:1631–40.
Dovc K, Battelino T. Time in range centered diabetes care. Clin Pediat Endocrinol Case Rep Clin Investig Off J Jpn Soc Pediat Endocrinol. 2021;30:1–10.
American Diabetes Association Professional Practice Committee. 7. Diabetes technology: standards of medical care in diabetes-2022. Diabetes Care. 2022;45:S97–112.
Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the International Consensus on Time in Range. Diabetes Care. 2019;42:1593–603.
Advani A. Positioning time in range in diabetes management. Diabetologia. 2020;63:242–52.
Tanenbaum ML, Hanes SJ, Miller KM, Naranjo D, Bensen R, Hood KK. Diabetes device use in adults with type 1 diabetes: barriers to uptake and potential intervention targets. Diabetes Care. 2017;40:181–7.
Isaacs D, Bellini NJ, Biba U, Cai A, Close KL. Health care disparities in use of continuous glucose monitoring. Diabet Technol Ther. 2021;23:S81–7.
EPHMRA. EPHMRA standards. 2022. https://www.ephmra.org/ephmra-standards. Accessed October 5, 2023.
Runge AS, Kennedy L, Brown AS, et al. Does time-in-range matter? Perspectives from people with diabetes on the success of current therapies and the drivers of improved outcomes. Clin Diabet Publicat Am Diabet Assoc. 2018;36:112–9.
Chehregosha H, Khamseh ME, Malek M, Hosseinpanah F, Ismail-Beigi F. A view beyond HbA1c: role of continuous glucose monitoring. Diabet Therapy Res Treat Educat Diabet Relat Disord. 2019;10:853–63.
Beck RW, Bergenstal RM, Riddlesworth TD, et al. Validation of time in range as an outcome measure for diabetes clinical trials. Diabetes Care. 2019;42:400–5.
El Malahi A, Van Elsen M, Charleer S, et al. Relationship between time in range, glycemic variability, HbA1c, and complications in adults with type 1 diabetes mellitus. J Clin Endocrinol Metab. 2022;107:e570–81.
Lu J, Ma X, Zhou J, et al. Association of time in range, as assessed by continuous glucose monitoring, with diabetic retinopathy in type 2 diabetes. Diabetes Care. 2018;41:2370–6.
Lu J, Ma X, Shen Y, et al. Time in range is associated with carotid intima-media thickness in type 2 diabetes. Diabetes Technol Ther. 2020;22:72–8.
Vos FE, Schollum JB, Coulter CV, Manning PJ, Duffull SB, Walker RJ. Assessment of markers of glycaemic control in diabetic patients with chronic kidney disease using continuous glucose monitoring. Nephrology (Carlton, Vic.). 2012;17:182–8.
Beck RW, Connor CG, Mullen DM, Wesley DM, Bergenstal RM. The fallacy of average: how using HbA(1c) alone to assess glycemic control can be misleading. Diabetes Care. 2017;40:994–9.
Bergenstal RM, Gal RL, Connor CG, et al. Racial differences in the relationship of glucose concentrations and hemoglobin A1c levels. Ann Intern Med. 2017;167:95–102.
Seidel D, Boggio Mesnil F, Caruso A. Reimbursement pathways for new diabetes technologies in Europe: top-down versus bottom-up. J Diabetes Sci Technol. 2019;13:118–22.
Medical Writing, Editorial and Other Assistance
The authors thank the ex-Novo Nordisk employees Trine Brandt Christensen and Amitkumar Agrawal for planning and driving the execution of the market research and their involvement in conceptualization, design, and driving the expert review panel. Amitkumar Agrawal also supported the manuscript development. The authors also thank Magda Dutkeiwicz Vaughan from The Harris Poll for supporting the planning and execution of the survey. Medical writing support for the development of this manuscript, under the direction of the authors, was provided by Katy Adams, PhD, of Ashfield MedComms, an Inizio company, and funded by Novo Nordisk.
Authorship
All authors contributed to critically reviewing and editing the manuscript drafts. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship in this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Funding
This survey, analysis, and medical writing support, and the Rapid Service Fee were funded by Novo Nordisk A/S.
Author information
Authors and Affiliations
Contributions
Christophe De Block, Alice YY Cheng, Gayathri Anil and John Michael D’Cruz contributed to the study conception, design and interpretation of results. End-to-end research, analytics, reporting and data collection were led by Anna Ginovker. All authors contributed to critically reviewing and editing the manuscript drafts, and all authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Christophe De Block has participated in advisory boards for Abbott Diagnostics, A. Menarini Diagnostics, Insulet, Roche Diagnostics, Eli Lilly and Company, Novo Nordisk, and Novartis; has received search support from AstraZeneca, Boehringer Ingelheim, Indigo NV, and Novo Nordisk; and declares participation in speaker bureaus for Eli Lilly and Company, Novo Nordisk, A. Menarini Diagnostics, Abbott Diagnostics, and Insulet. Alice YY Cheng has received honoraria for consulting and/or speaking from Abbott, Amgen, AstraZeneca, Bayer, Bausch, Boehringer Ingelheim, Eisai, Eli Lilly, GSK, HLS Therapeutics, Insulet, Janssen, Medtronic, Novo Nordisk, Pfizer, Sanofi, and Takeda. Gayathri Anil and John Michael D'Cruz are employees of Novo Nordisk Service Centre India Private Limited. Anna Ginovker is a former employee of The Harris Poll, Princeton, NJ, USA (affiliation at the time of the study) and a current employee of Atomik Research, Bentonville, AR, USA.
Ethics Approval
Participants provided consent to participate in the study and confidentiality was maintained with appropriate measures at all stages of the research. This study adhered to the European Pharmaceutical Market Research Association Code of Conduct 2019, page 15, Sect. 1.3, meaning it did not require Clinical Research Ethics Committee or Independent/Institutional Review Board approval for its conduct in the following countries: Brazil, Canada, Denmark, Finland, France, Germany, Greece, Italy, Japan, Mexico, the Netherlands, Norway, Poland, Russia, South Korea, Spain, Sweden, Turkey, the UK, and the USA [12].
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
De Block, C., Cheng, A.Y.Y., Anil, G. et al. Perspectives and Behaviors of People with Diabetes toward Time in Range and Glucose Control in Diabetes Management: An Online Survey. Diabetes Ther (2024). https://doi.org/10.1007/s13300-024-01603-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13300-024-01603-9