Abstract
Introduction
Glucagon-like peptide-1 receptor agonists (GLP-1RAs) have attracted much attention because of their significant hypoglycemic and weight-loss effects. Previous preparations can only be subcutaneously injected. Oral administration of GLP-1RAs semaglutide helps to broaden treatment options, but its safety in the real world still needs to be observed. This study is based on FDA adverse event reporting system (FAERS) database to mine adverse drug events (ADE) of oral semaglutide, and provide references for the clinical safe use of this drug.
Methods
To analyze the signal quality of oral semaglutide, which is a drug used in the FAERS database from the third quarter of 2019 to the third quarter of 2023, we collected ADE data and performed data mining by using disproportionate analysis. Then, we standardized the data and used a variety of signal-quantification techniques, including reported odds ratio (ROR), proportional reporting ratio (PRR), Bayesian belief propagation neural network (BCPNN), and multiple empirical Bayesian gamma Poisson contractions (MGPS), for further analysis.
Results
We screened 2398 reports on the use of semaglutide tablets, involving a total of 5653 ADE. These reports were mainly submitted by consumers, and the reporting country was mainly the United States. A total of 23 system organ classes (SOC) and 93 preferred terms (PT) were mined for the signals of semaglutide tablets. The three most common SOC were gastrointestinal disorders, general disorders and administration site conditions, and investigations. At the PT level, metabolism and nutrition disorders exhibit the highest number of signals, with the top three being thyroid cyst, acute cholecystitis, and ketosis. Gastrointestinal disorders rank second, primarily involving eructation, pancreatitis, impaired gastric emptying, and regurgitation. In addition, vith nerve paralysis occurs and the signal intensity is high.
Conclusions
Our study provides a deeper and broader understanding of the safety of oral semaglutide. The results of the ROR, PRR, BCPNN, and MGPS algorithms exhibit high consistency, with metabolism and nutrition-related disorders having the highest number of signals. The conclusions align with the technical specifications of the product. Notably, other unexpected effects are reported, including acute cholecystitis, paralysis of the abducens nerve, and positional vertigo.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Glucagon-like peptide-1 receptor agonists (GLP-1RAs) are currently widely used for lowering blood sugar, weight loss, and reducing adverse cardiovascular events. Considering the dual nature of drug efficacy and toxic side effects, it is important to focus on its safety when used in the real world. |
Our study is the first to concentrate on the adverse reactions of oral semaglutide. We screened 2398 reports on the use of semaglutide tablets, involving a total of 5653 adverse drug events (ADEs). |
We identified signals for 23 system organ classes (SOC) and 93 preferred terms (PT) associated with semaglutide tablets, which differ from the adverse reactions listed in the instructions. |
Research indicates the need for medical professionals to prescribe oral semaglutide cautiously and to inform patients of possible adverse effects. |
Introduction
Type 2 diabetes mellitus (T2DM) is a progressive disease characterized by elevated blood glucose. Its etiology is mainly impaired insulin secretion, increased glucagon secretion, and insulin resistance. Obesity will aggravate and lead to hyperglycemia [1, 2]. Several studies have confirmed [3, 4] that T2DM and obesity are closely related to cardiovascular complications, which bring a heavy financial burden to the global health care system. In order to improve the two major drivers of cardiovascular disease, T2DM and obesity, a variety of new glucose-lowering therapies have emerged in the past 10 years, aiming to control blood glucose and reduce metabolic disease-related weight gain [5].
GLP-1RAs can effectively improve some key pathophysiological defects of T2DM, which has been proved to have significant hypoglycemic and weight-loss effects, and can reduce the major adverse cardiovascular events in adult patients with type 2 diabetes with cardiovascular disease [6], so it has become an important choice for the treatment of T2DM. However, GLP-1RAs usually require subcutaneous administration, limiting its use in patients. The availability of oral semaglutide may help broaden treatment options. It may also lead to the diabetes treatment group starting GLP-1 receptor agonist treatment earlier [5]. However, the drug itself has two sides: on the one hand it can play a therapeutic role in treating disease; on the other, the drug itself also has certain toxic and side effects, and may lead to an adverse drug reaction (ADR). Therefore, it is necessary to monitor its actual use and adverse events to ensure its safety and effectiveness. FAERS provides a platform for collecting and analyzing ADE related to drug utilization [7]. These data are important resources for evaluating drug safety and effectiveness. This paper aims to study the data of semaglutide oral dosage form in FAERS database, evaluate the data from different angles by using various signal quantification techniques, and provide more comprehensive and reliable results in order to provide references for the safe clinical application of semaglutide tablets.
Methods
Data Source
The research’s underpinning data were sourced from the FAERS database (FDA Adverse Event Reporting System), which is a publicly available database with de-identified data (https://fis.fda.gov/extensions/FPD-QDE-FAERS/FPD-QDE-FAERS.html). Considering the time of the drug's marketing, ADE data from the third quarter of 2019 to the third quarter of 2023 were downloaded in this study. Import the self-built database and process the data with R (4.3.2) software.
Data Extraction
Duplicate reports were removed. For data with the same case ID in the demo table, only the latest report based on date is retained. The primary ID field is used to establish the relationship between datasets, and the drug name is standardized through the Medex_UIMA_1.8.3 system. Reports that semaglutide was suspected to be the main drug associated with ADE were extracted. These reports cover a variety of information, such as the report date, the patient's age and gender, drug-use records, treatment results records, report sources, etc.
Signal Filtering and Categorization
Signal coding, classification, and localization of SOC and PT in the adverse drug reaction term were performed using the Medical Dictionary for Regulatory Activities (MedDRA) (version 26.0) to analyze the specific SOC involved in the signaling of adverse events.
Data Analysis
In this study, four disproportionate methods were used to mine drug ADE signals: reported odds ratio (ROR) [8], proportional reporting ratio (PRR) [9], Bayesian belief propagation neural network (BCPNN) [10], and multiple empirical Bayesian gamma Poisson contractions (MGPS) [11]. By applying these four technologies, we can give full play to their advantages, expand the detection range, verify the results from different angles, and use the unique functions of each algorithm to find more comprehensive and reliable safety signals. We also combined multiple algorithms for cross-validation to reduce false positives, and by adjusting the threshold and variance, we can detect more potential rare adverse reactions. All algorithms are based on 2 × 2 contingency table (supplementary Table 1), and specific formulas and thresholds can be referred to (supplementary Table 2). R software was used for statistical analysis.
According to the formula, ROR value, PRR value, BCPNN value, MGPS value, and the corresponding 95% confidence interval (95% CI) were calculated. By comparing the target ADE occurrence ratio of the target drug with that of all other drugs, if the ratio was greater than the set threshold, it was called imbalance, suggesting the generation of potential ADE signals [12]. These methodologies in the research by Jiang and others have provided us with important insights.
In this study, the detection threshold of the target drug-related risk signal is set to a ≥ 3 and 95% CI (lower limit) > 1 to generate a risk signal. The higher the ROR value, the higher the risk of corresponding ADE of the drug, the stronger the signal intensity, and the greater the correlation between the target drug and the risk signal (adverse event).
Results
The Basic Characteristics of Semaglutide-Related ADE
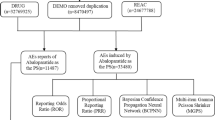
In the span delineated between Q3 2019 and Q3 2023, this study amassed an aggregate of 6,155,602 adverse event notations from the FAERS compendium. Among them, 2398 cases of target drug oral dosage form semaglutide were reported, involving a total of 5653 ADE (see Fig. 1 for details)
Flow diagram of selecting semaglutide-associated adverse events (AEs) from the FDA adverse event reporting system (FAERS) database. ROR reported odds ratio, PRR proportional reporting ratio, BCPNN Bayesian belief propagation neural network, MGPS multiple empirical Bayesian gamma Poisson contractions
In the realm of adverse event reporting for semaglutide tablets, the data skews towards female patients, who are implicated in 56.05% of cases, compared to 40.53% for male patients, while 3.42% of reports are silent on gender. A considerable swath of the data, 49.50%, is bereft of age particulars, thus stymieing a comprehensive grasp of the age-adverse event nexus. However, in instances where age is documented, the 45–75 year age bracket is predominantly represented. Since 2020, there has been a consistent annual swell in the number of reports; it is of particular import that a majority, 41.12%, originate from consumer accounts as opposed to 33.82% from medical professionals. The United States stands out as the primary source, accounting for 84.45% of all reports. In terms of outcomes, aside from indeterminate serious adverse events, hospitalization-inducing events were the most frequent at 36.45%, with mortality incidents at 3.82%. Also, 60.50% of the adverse events after medication were not provided, and the most common occurrence was within 7 days after medication (20.35%). Fifty percent were used for unknown product indications, and 27.63% were used for type 2 diabetes (Table 1).
Semaglutide Signal Mining
In this study, according to the set threshold as the screening condition, four algorithms were used for signal detection one by one. By analyzing the adverse event reports involving semaglutide tablets, it was found that the adverse reactions related to the drug involved 23 SOC (Table 2, Fig. 2). According to the results of the study, the top ranked according to the number of SOC reports were gastrointestinal diseases (n = 1735), general diseases and administration site conditions (n = 549), investigations (n = 539), neurological diseases (n = 465), metabolic and nutritional diseases (n = 363). Moreover, according to the signal intensity, semaglutide tablets were highly associated with the following adverse events of SOC: gastrointestinal diseases (n = 1735, ROR 5.28, PRR 3.93, IC 1.97, EBGM 3.92), metabolic and nutritional disorders (n = 363, ROR 3.52, PRR 3.35, ic1.75, EBGM 3.35), hepatobiliary diseases (n = 103, ROR 2.27, PRR 2.24, IC 1.17, EBGM 2.24), ocular diseases (n = 181, ROR 1.69, PRR 1.67, IC 0.74, EBGM 1.67) Survey (n = 539, ROR 1.68, PRR 1.61, IC 0.69, EBGM 1.61). The results of the four methods have high consistency (See Table 2 for details).
At the PT level, this study used four algorithms to analyze the ADE signal of semaglutide tablets as the target drug for screening, and 93 PT were obtained (supplementary Table 3). According to the algorithm, the top 30 PT involved in the 13 SOC ranking have high signal intensity (Table 3, Fig. 3). The screening results of the four algorithms are highly consistent, and the number of signals of metabolic and nutritional diseases is the largest. In order, they mainly include ketosis, weight loss poor, food craving, hyperglycemic hyperosmolar non-ketotic syndrome, abnormal weight loss, and inadequate diabetes control. Among them, the number of reports of reduced match (n = 145) is higher, but the signal intensity is not among the top 30. The top three signal categories were: thyroid cyst (n = 3, ROR 70.04, PRR 70.00, IC 6.1, EBGM 68.55), acute cholecystitis (n = 18, ROR 50.41, PRR 50.24, IC 5.63, EBGM 49.49), and ketosis (n = 4, ROR 46.87, PRR 46.84, IC 5.53, EBGM 46.18). Pancreatitis (n = 117, ROR 23.12, PRR 22.64, IC 4.49, EBGM 22.49), eructation (n = 35, ROR 26.43, PRR 26.27, IC 4.70, EBGM 26.06), and blood glucose reduction (n = 42, ROR 13.63, PRR 13.54, IC 3.75, EBGM 13.49) that were closely watched by the drug instructions also had higher frequency and signal intensity. This study also found that although the frequency of adverse reactions such as pancreatic cancer, early satiety, and dizziness location was not high, the signal intensity was high. It is worth noting that after treatment with semaglutide tablets, there was vith nerve paralysis and the signal intensity was high.
Discussion
Characteristics of Semaglutide Tablets
In December of 2017, the U.S. Food and Drug Administration (FDA) approved semaglutide injection to be marketed for glycemic control in adult patients with type 2 diabetes. In September of 2019, the more convenient oral semaglutide was approved for marketing to combine diet and exercise to improve glycemic control in patients with type 2 diabetes. Because of its excellent curative effect, it is recommended by many diabetes prevention and treatment guidelines [13,14,15]. Semaglutide is an oral glucagon-like peptide (GLP-1) receptor protein therapeutic drug [16]. Based on PIONEER 1–10 global clinical trials [17,18,19,20], it has been confirmed that it can significantly improve blood glucose control and weight loss in patients with type 2 diabetes, and can also improve cardiovascular outcomes, which has attracted much attention at present.
How can oral preparations based on tablets achieve the same efficiency and specificity as therapeutic peptides for injection? Studies have found that the absorption of oral semaglutide, a glucagon-like peptide-1 analog, is co formulated in tablets with the absorption enhancer n-[8-(2-hydroxybenzoyl) sodium aminooctanoate] (SNAC) [21]. Therefore, although oral semaglutide has different absorption characteristics, once the drug is absorbed, the pharmacokinetic properties and effects of semaglutide are similar regardless of the route of administration. Pharmacokinetic analysis showed that due to the long half-life of oral semaglutide, once-daily administration can achieve a stable steady-state concentration [22]. Upper gastrointestinal disease and kidney and liver damage did not affect the pharmacokinetic profile.
Semaglutide Tablets-Related ADE
By conducting a meticulous examination of the FAERS database spanning from Q3 2019 to Q3 2023, this inquiry has methodically appraised the adverse reactions associated with oral semaglutide. In the course of this analysis, the research not only corroborated extant safety data but also unearthed novel potential hazards. Such revelations furnish a more expansive and precise data foundation for clinical application and the formulation of public health policies. An elaborate discourse on the findings of this research ensues below.
Clinical Characteristics of Adverse Event Reports
Our analysis discerned a year-on-year escalation in the frequency of adverse event reports for oral semaglutide, a trend that appears to be in lockstep with the uptick in the drug’s cumulative user base. Notably, a substantial fraction of the collected data is devoid of precise age-specific details, thereby impeding a thorough understanding of the prevalence of adverse events across various age segments. Future research endeavors should aim to harness accurate age-related data to thoroughly investigate the differential responses to the drug among distinct age groups. It is also significant to highlight that a majority of the adverse reaction reports, accounting for 41.12%, were furnished by consumers rather than medical professionals, which may have implications for the data’s reliability and scope.
This may indicate that patients are more inclined to report adverse reactions directly after the use of oral semaglutide or reflect deficiencies reported by medical professionals. Since most of the reports came from the United States (84.45%), this can also reflect the reporting trends of specific regions or cultures. This requires further investigation to confirm potential regional or cultural biases. Most reports do not provide the occurrence time of adverse events after medication. Due to the indications approved by FDA, oral semaglutide is mainly used for type 2 diabetes (27.63%), and a few are used for weight control and weight decreased (both less than 1%).
SOC and PT Induced by Semaglutide Tablets
GLP-1RAs drugs can delay gastric emptying and inhibit appetite, so the most common adverse events are gastrointestinal adverse events [23], including nausea, vomiting diarrhea and constipation. Studies have shown that the severity of such adverse events of oral semaglutide is mostly mild to moderate [24, 25], which also reflects the consistency in our study.
Although the clinical trial clearly reported that semaglutide tablets have the risk of acute pancreatitis and pancreatic cancer [24, 26], another study [27] did not observe clear evidence of pancreatitis risk. A systematic review also showed that semaglutide tablets do not increase the risk of pancreatitis [28, 29]. The bias in these conclusions may be due to the limited sample size and the lack of updated real-world evidence. However, our post-marketing pharmacovigilance analysis based on big data showed that there was a strong correlation between semaglutide exposure and the occurrence of pancreatitis and pancreatic cancer. Pancreatitis (n = 117, ROR 23.12, PRR 22.64, IC 4.49, EBGM 22.49) had high frequency and signal intensity; pancreatic cancer (n = 3, ROR 34.16, PRR 34.14, IC 5.08, EBGM 33.80) had high signal intensity, although the frequency of adverse reactions was not high. This also further confirms the results of clinical trials. Moreover, in hepatobiliary disorders, we observe that acute cholecystitis (n = 18, ROR 50.41, PRR 50.24, IC 5.63, EBGM 49.69) and bile duct stones (gallstones) (n = 6, ROR 25.33, PRR 25.31, IC 4.65, EBGM 25.12) also present strong signals that warrant our vigilance in routine clinical practice. In conclusion, as more reports are submitted, the safety signal spectrum of semaglutide tablets may change over time.
The study showed that the total number of hypoglycemic episodes of oral semaglutide, subcutaneous semaglutide, and placebo was low and similar [16]. A systematic review and meta-analysis of randomized trials also suggested that there was a risk of key safety outcomes (severe hypoglycemia, retinopathy, or pancreatic adverse reactions) with GLP-1RAs, but there was no increase [30]. At the PT level, our study also found that the pancreatitis, diabetic retinopathy, and blood glucose decrease mentioned in the manual all showed strong signals. This may lead to retinopathy progression on the basis of baseline retinopathy status. Particularly in metabolism and nutrition disorders, both ketosis (ROR 46.87, PRR 46.84, IC 5.53, EBGM 46.18) and hyperglycemic hyperosmolar nonketotic syndrome (ROR 22.43, PRR 22.42, IC 4.48, EBGM 22.27) show strong signals, indicating a high potential risk of diabetic ketoacidosis. Due to the risks of these adverse reactions, close monitoring is required during administration.
The manual warns that the personal or family history of patients with medullary thyroid cancer is prohibited. Our study suggests that semaglutide tablets do affect the thyroid system, such as the strongest signal of adverse reactions of thyroid cysts (ROR 70.04, PRR 70.00, IC 6.1, EBGM 68.55). In addition, although ketosis and adenocarcinoma pancreas are rare, they have strong signal intensity and deserve special attention.
Patients with type 2 diabetes mellitus are prone to developing complications involving impaired renal function. Renal dysfunction complicates medication management in these patients. Patients with T2DM with CKD are affected by renal function status, and there are certain limitations in drug selection, which increases the difficulty of treatment in clinical practice. Semaglutide tablets are the first oral GLP-1RAs to fully confirm its efficacy and safety in clinical studies. Studies have shown that different degrees of impaired renal function have no significant impact on its pharmacokinetic characteristics, and there is no need to adjust the dose for patients with T2DM with mild, moderate, and severe renal dysfunction [31]. Another study showed that GLP-1RAs reduced the composite renal outcome (including the occurrence of macroalbuminuria, doubling of serum creatinine, and deterioration of renal function based on EGFR changes) by 21% (HR 0.79 [95% CI 0.73–0.87]; p < 0.0001) [30]. In this study, there was only renal pain on the PT level, indicating that the renal safety risk of oral semaglutide was not high, but there might be renal benefit.
Oral semaglutide has been confirmed to have a clear cardiovascular benefit [32]. Our study has not seen reports of major adverse cardiovascular events (death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke). Furthermore, according to the SOC report on cardiac disorders, n = 80, ROR 0.71 (0.57, 0.88), PRR 0.71 (0.57, 0.88), IC – 0.49 ( – 0.8), EBGM 0.71 (0.59), all four algorithms indicate a low signal for adverse reactions, confirming its cardiovascular safety and effectiveness.
At the same time, the related adverse reactions and allergic reactions caused by subcutaneous injection have not been reported in this study, which is also the advantage of the oral dosage form.
This study also found adverse events that were not mentioned in the drug instructions, such as acute cholecystitis, nerve palsy, vertigo location, etc. This indicates the value of quantitative signal detection technology in the monitoring of adverse drug events, which can provide potential risk information, and is worthy of further attention and investigation.
In conclusion, oral semaglutide does have multi-system adverse reactions with varying severity. These adverse reactions should be monitored when using oral semaglutide, and medical professionals should be immediately consulted if there are any symptoms.
Although the study in question has provided reliable empirical evidence for the safety assessment of oral semaglutide from several perspectives, it is not without certain limitations. Firstly, the main limitation is the reporting of adverse events, which often relies on consumer reporting and may therefore compromise objectivity. Secondly, there are limited data available to adjust for factors such as age, BMI, HbA1c, and comorbidities, or to provide clinical context for the findings. This also limits the ability to understand the exact adverse event observed. Furthermore, the paucity of information accessible via adverse event reporting systems is insufficient to tackle pivotal clinical inquiries but serves merely as a directional tool for specialized clinical research endeavors. To cultivate an encompassing and precise comprehension, forthcoming studies are advised to integrate stringent prospective methodologies amalgamated with clinical experiments and epidemiological scrutiny to enhance the precision in evaluating safety hazards linked with oral semaglutide consumption.
Conclusions
Broadly speaking, the research on hand furnishes a robust empirical foundation for appraising the safety profile of orally administered semaglutide, employing a comprehensive, multi-faceted analytical approach. It merits emphasis that, notwithstanding the relative infrequency of certain adverse events—such as ketosis and pancreatic adenocarcinoma—the pronounced signal strength they exhibit warrants heightened vigilance and investigative scrutiny. Moreover, the investigation has unearthed a spectrum of distinctive adverse reactions that are not delineated in the pharmaceutical guidelines, including acute cholecystitis, abducens nerve paralysis, and positional vertigo, among others. This underscores the instrumental role of quantitative signal detection methodologies in the surveillance of pharmacological adverse effects. The insights gleaned from this study advocate for a more circumspect stance among healthcare practitioners regarding the therapeutic deployment of oral semaglutide, and underscore the imperative for patient cognizance of these potential adverse manifestations. While acknowledging the constraints inherent in the data, these nascent findings incontrovertibly serve as a pivotal touchstone for ensuing inquiries and the oversight of drug safety. Specifically, the identification of emergent potential hazards should command earnest consideration within the medical fraternity and regulatory bodies.
Data Availability
The research's underpinning data were sourced from the publicly available FAERS database (https://fis.fda.gov/extensions/FPD-QDE-FAERS/FPD-QDE-FAERS.html). The dataset generated during and analyzed in the current study are available from the corresponding author upon reasonable request.
References
Yan J, et al. Obesity- and aging-induced excess of central transforming growth factor-β potentiates diabetic development via an RNA stress response. Nat Med. 2014;20:1001–8.
A 16-year prospective cohort study to evaluate effects of long-term fluctuations in obesity indices of prediabetics on the incidence of future diabetes - PubMed. https://pubmed.ncbi.nlm.nih.gov/34079024/. Accessed 3 Mar 2024.
Vaduganathan M, Mensah GA, Turco JV, Fuster V, Roth GA. The global burden of cardiovascular diseases and risk. J Am Coll Cardiol. 2022;80:2361–71.
Hess DA, et al. Vascular repair and regeneration in cardiometabolic diseases. Eur Heart J. 2022;43:450–9.
Cardiometabolic-based chronic disease, adiposity and dysglycemia drivers: JACC state-of-the-art review - PubMed. https://pubmed.ncbi.nlm.nih.gov/32029136/. Accessed 3 Mar 2024.
Davies MJ, et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American diabetes association (ADA) and the European association for the study of diabetes (EASD). Diabetes Care. 2022;45:2753–86.
Sarangdhar M, et al. Data mining differential clinical outcomes associated with drug regimens using adverse event reporting data. Nat Biotechnol. 2016;34:697–700.
Rothman KJ, Lanes S, Sacks ST. The reporting odds ratio and its advantages over the proportional reporting ratio. Pharmacoepidemiol Drug. 2004;13:519–23.
Heeley E, Waller P, Moseley J. Testing and implementing signal impact analysis in a regulatory setting. Drug Saf. 2005;28:901–6.
A Bayesian neural network method for adverse drug reaction signal generation - PubMed. https://pubmed.ncbi.nlm.nih.gov/9696956/. Accessed 3 Mar 2024.
Heo S, Jung I. Extended multi-item gamma Poisson shrinker methods based on the zero-inflated Poisson model for postmarket drug safety surveillance. Stat Med. 2020;39:4636–50.
Jiang Y, et al. Safety assessment of brexpiprazole: real-world adverse event analysis from the FAERS database. J Affect Disord. 2024;346:223–9.
Navaneethan SD, et al. Diabetes management in chronic kidney disease: synopsis of the KDIGO 2022 clinical practice guideline update. Ann Intern Med. 2023;176:381–7.
Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) - PubMed. https://pubmed.ncbi.nlm.nih.gov/36148880/. Accessed 3 Mar 2024.
American Diabetes Association Professional Practice Committee. 9. Pharmacologic approaches to glycemic treatment : standards of care in diabetes—2024. Diabetes Care. 2024;47:S158–78.
Davies M, et al. Effect of oral semaglutide compared with placebo and subcutaneous semaglutide on glycemic control in patients with type 2 diabetes. JAMA. 2017;318:1460.
Safety and efficacy of oral semaglutide versus dulaglutide in Japanese patients with type 2 diabetes (PIONEER 10): An open-label, randomised, active-controlled, phase 3a trial - PubMed. https://pubmed.ncbi.nlm.nih.gov/32333876/.
Aroda VR, et al. PIONEER 1: randomized clinical trial of the efficacy and safety of oral semaglutide monotherapy in comparison with placebo in patients with type 2 diabetes. Diabetes Care. 2019;42:1724–32.
Efficacy and safety of once-daily oral semaglutide 25 mg and 50 mg compared with 14 mg in adults with type 2 diabetes (PIONEER PLUS): A multicentre, randomised, phase 3b trial - PubMed. https://pubmed.ncbi.nlm.nih.gov/37385279/. Accessed 3 Mar 2024.
Pratley R, et al. Oral semaglutide versus subcutaneous liraglutide and placebo in type 2 diabetes (PIONEER 4): a randomised, double-blind, phase 3a trial. The Lancet. 2019;394:39–50.
Transcellular stomach absorption of a derivatized glucagon-like peptide-1 receptor agonist - PubMed. https://pubmed.ncbi.nlm.nih.gov/30429357/. Accessed 3 Mar 2024.
A new era for oral peptides: SNAC and the development of oral semaglutide for the treatment of type 2 diabetes - PubMed. https://pubmed.ncbi.nlm.nih.gov/35838946/. Accessed 3 Mar 2024.
Shu Y, et al. Gastrointestinal adverse events associated with semaglutide: a pharmacovigilance study based on FDA adverse event reporting system. Front Public Health. 2022;10: 996179.
Effect of oral semaglutide compared with placebo and subcutaneous semaglutide on glycemic control in patients with type 2 diabetes: a randomized clinical trial - PubMed. https://pubmed.ncbi.nlm.nih.gov/29049653/.
Efficacy and safety of once-weekly semaglutide monotherapy versus placebo in patients with type 2 diabetes (SUSTAIN 1): A double-blind, randomised, placebo-controlled, parallel-group, multinational, multicentre phase 3a trial - PubMed. https://pubmed.ncbi.nlm.nih.gov/28110911/. Accessed 3 Mar 2024.
Marso SP, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834–44.
PIONEER 1: Randomized clinical trial of the efficacy and safety of oral semaglutide monotherapy in comparison with placebo in patients with type 2 diabetes - PubMed. https://pubmed.ncbi.nlm.nih.gov/31186300/. Accessed 3 Mar 2024.
Storgaard H, Cold F, Gluud LL, Vilsbøll T, Knop FK. Glucagon-like peptide-1 receptor agonists and risk of acute pancreatitis in patients with type 2 diabetes. Diabetes Obes Metab. 2017;19:906–8.
Shi F-H, et al. Efficacy and safety of once-weekly semaglutide for the treatment of type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Front Pharmacol. 2018;9:576.
Sattar N, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol. 2021;9:653–62.
Mosenzon O, et al. Efficacy and safety of oral semaglutide in patients with type 2 diabetes and moderate renal impairment (PIONEER 5): a placebo-controlled, randomised, phase 3a trial. Lancet Diabetes Endocrinol. 2019;7:515–27.
Husain M, et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2019;381:841–51.
Acknowledgements
This study was performed using the FAERS source that was provided by the FDA. The information, results, or interpretation of the current study do not represent any opinion of the FDA. We thank the FDA for their valuable contributions. Thanks to Zhang Jing (Second Department of Infectious Disease, Shanghai Fifth People's Hospital, Fudan University) for his work on the FAERS database.
Funding
This work and the journal’s Rapid Service Fee were supported by the National Natural Science Foundation of China (Grant No. 82360052).
Author information
Authors and Affiliations
Contributions
Si Xiong, Ruoyu Gou, Xudong Liang, and Changjun Luo had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Hao Wu, Shuitao Qin, Bing Li, and Junan Chen contributed methodology and Changjun Luo and Junan Chen supervised the manuscript. All authors contributed to the article and approved the submitted version.
Corresponding authors
Ethics declarations
Conflict of Interest
There are no competing interests. Si Xiong, Ruoyu Gou, Xudong Liang, Hao Wu, Shuitao Qin, Bing Li, Changjun Luo, and Junan Chen declare that they have no conflicts of interest.
Ethical Approval
The research’s underpinning data was sourced from the FAERS database (FDA Adverse Event Reporting System) which is a publicly available database with de-identified data (https://fis.fda.gov/extensions/FPD-QDE-FAERS/FPD-QDE-FAERS.html).
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Xiong, S., Gou, R., Liang, X. et al. Adverse Events of Oral GLP-1 Receptor Agonist (Semaglutide Tablets): A Real-World Study Based on FAERS from 2019 to 2023. Diabetes Ther (2024). https://doi.org/10.1007/s13300-024-01594-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13300-024-01594-7