Abstract
Introduction
The prevalence of diabetes mellitus and its sequelae has been on the rise, and diabetic foot ulcer (DFU) is the leading cause of non-traumatic lower limb amputation globally. The rising occurrence and financial burden associated with DFU necessitate improved clinical assessment and treatment. Diabetes has been found to enhance the formation of neutrophil extracellular traps (NETs) by neutrophils, and excessive NETs have been implicated in tissue damage and impaired wound healing. However, there is as yet insufficient evidence to clarify the value of NETs in assessing and predicting outcomes of DFU.
Methods
We designed this prospective study with three cohorts formed from type 2 diabetes mellitus (T2DM) patients with DFU (n = 200), newly diagnosed T2DM patients (n = 42), and healthy donors (n = 38). Serum levels of NETs were detected for all groups, and the prognostic value for DFU-related amputation was analyzed.
Results
The results showed that serum NET levels of the DFU group were significantly higher than in the T2DM group (P < 0.05), which also had significantly elevated serum NET levels compared to healthy donors (P < 0.05). Multivariate Cox regression showed that serum NET levels, diabetic foot surgical history, and Wagner grade were the risk factors for amputation (P < 0.05), and these three variables also exhibited the highest coefficient values in additional Lasso Cox regression. For patients with DFU, Kaplan-Meier curves showed that high serum NET levels associated with higher amputation probability (HR = 0.19, P < 0.01) and ROC curve based on NET value showed good validity for amputation (AUC: 0.727, CI 0.651–0.803).
Conclusion
Elevated serum NET levels serve as an easily accessible serological prognostic marker for assessing the risk of DFU-related amputation, thereby offering evaluation metrics for healthcare providers. Further investigations are necessary to understand the mechanisms driving this relationship.
Similar content being viewed by others
Why carry out this study? |
Diabetic foot ulcers (DFUs) represent a significant source of morbidity and mortality. Yet there is a paucity of objective prognostic markers for DFU outcomes. |
Existing literature highlights a role for neutrophil extracellular traps (NETs) in diabetes and associated tissue injury. |
What was learned from the study? |
Serum NET concentrations are significantly higher in patients with DFUs than in those with type 2 diabetes mellitus without ulcers. |
Elevated serum NET levels have prognostic utility for anticipating amputation risk in the context of diabetic foot ulcers. |
Introduction
Among the various complications associated with diabetes, diabetic foot ulcer (DFU) has emerged as a significant contributor to both disability and mortality. The prevalence of DFU among individuals aged 50 years and above in China is alarmingly high at 8.1% [1]. Furthermore, the mortality rate for patients afflicted with diabetic foot (DF) ulcers and infections is as high as 11%, while people with an amputation may face a mortality rate of up to 22%. The enormous treatment costs account for about one-third of the entire diabetic medical expenses [2, 3]. Anomalies in the inflammatory response play a crucial role in delayed wound healing among patients with DF.
As neutrophils are commonly recruited to sites of inflammation, their aggregation has been observed in the initial phase of DFU [4]. Their secretion of neutrophil extracellular traps (NETs) may have an immunological role and potentially induce tissue damage, impair angiogenesis, and prolong the healing process of diabetic wounds [5, 6]. The contradictory effect of NETs has initiated a novel avenue of investigation into the pathogenesis of diabetic foot. Related research indicated that an elevated glucose environment of diabetes can stimulate the release of NETs, potentially delaying wound healing of DFU. Conversely, inhibiting NET release may accelerate the healing process. NETs have thus become a potential indicator linking diabetes to inflammation and tissue damage [7, 8]. Previous studies also have demonstrated a considerable increase of NETs in individuals with non-healing DFU and a substantial association between NETs and the likelihood of wound infection [8, 9].
As the pathogenesis of DFU is complex and multifactorial, with a high recurrence rate of 42% [10], few studies have reported objective indices for prognosis assessment [11]. Furthermore, DFU exhibits a higher incidence among individuals of lower socioeconomic status and residents of rural areas [12]. Kashi, the location of our study population, shares the characteristics of an underdeveloped and poorly resourced area. Therefore, we undertook this study to investigate novel and valuable evaluation metrics for clinical applications by detecting and analyzing serum levels of NETs in patients with type 2 diabetic foot ulcers and verifying their significance and changes in the prognosis of this disease.
Methods
Study Cohort
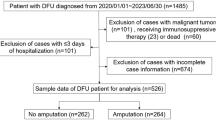
This study prospectively recruited 42 newly diagnosed type 2 diabetes mellitus (T2DM) patients and 200 patients with diabetic foot who were admitted to the Department of Endocrinology in the First People's Hospital of Kashi from June 2019 to June 2022. Patients who met the diagnostic criteria for diabetic foot published by the International Working Group on The Diabetic Foot [13] or the Chinese Guideline for Prevention and Treatment of Diabetic Foot (2019) were enrolled in this study [14]. Patients with venous ulceration, lower extremity ulcers due to trauma, acute vascular occlusion, and skin tumors were excluded from the study. Additionally, 38 healthy individuals were included as the control group. Informed consent was obtained from all participants, and 10 ml peripheral blood samples were collected for follow-up testing. A multidisciplinary team consisting of surgeons, endocrinologists, wound care specialists, pharmacologists, and physical therapists was involved in the management of diabetic foot ulcers (DFU). Initial assessments included evaluating vascular status, ischemic condition, osteomyelitis, and glycemic control. Continuous wound care, including hypoglycemic therapy, antibiotics for infections, and wound debridement, was provided as necessary. Surgeons evaluated the need for amputation on a case-by-case basis. Inflammatory markers and wound culture analyses were used to differentiate diabetic foot infections and optimize antibiotic usage. Antibiotics were administered until infection resolution, regardless of wound healing progress. Patient information, such as age, sex, diabetes duration, and laboratory results, including fasting blood glucose, glycated hemoglobin (HbA1c), hemoglobin (HB), white blood cell count (WBC), neutrophil count, CRP (C-reactive protein), and blood lipids, and physical examination findings, such as temperature, blood pressure, body mass index (BMI), Wagner grade, and ankle-brachial index (ABI), were collected.
Ethical Approval
The presented study received approval from the Ethics Committee of The First People’s Hospital of Kashi Prefecture (KDYY-202023) and conformed with the Helsinki Declaration of 1964 (as re-vised in 2013) concerning human and animal rights. Written consent was required for all participants.
NET Quantification
Peripheral blood was obtained from all patients at the start of our multidisciplinary management program. Blood samples were collected from patients in the newly diagnosed diabetes group prior to hypoglycemic treatment and from patients in the DFU group prior to pharmacological or surgical intervention. Myeloperoxidase-DNA (MPO-DNA) levels were quantified using commercially available ELISA kits as previously described in related literature [9]. A 96-well plate was coated with anti-MPO mAb (Abcam) and incubated with patient samples containing peroxidase-labeled anti-DNA mAb (Roche, Spain). After incubation, absorbance at 405 nm was measured, and values for soluble NET formation were expressed as percentage increases in absorbance above the control.
Endpoint Event and Follow-Up
To accurately assess the risk factors for amputation among patients with DFU, we conducted a prospective study with a clearly defined follow-up period. For all study participants, we initiated follow-up at the time of their first presentation to our clinic and continued monitoring their clinical outcomes for a duration of 2 years. We continued this follow-up until June 2023, ensuring that we captured all occurrences of amputation within this time frame.
Statistical Analysis
Statistical analysis was performed using SPSS 26.0 and R 4.2.2 software. GraphPad Prism 8.0 was used to create graphics. Mean and standard deviation (mean ± SD) were used to describe normally distributed quantitative data, while Student’s t-test was used for group comparisons. The Mann-Whitney U test was used for non-normally distributed quantitative data, reported as median and interquartile range values (M, Q25, Q75). Missing data were not imputed, and the analysis was performed only on the available data. Categorical data were compared using the chi-square test. Cox regression analysis was used to study the 2-year amputation outcome, reporting hazard ratio (HR) and 95% confidence interval (CI) [15]. The LASSO approach was employed to examine the impact of various factors, utilizing the “glmnet” package in R. A significance level of P < 0.05 was considered statistically significant.
Results
Variations of Serum NET Levels in Patients with Newly Diagnosed T2DM
A total of 80 subjects, 42 patients with newly diagnosed T2DM without complications or comorbidities and 38 healthy volunteers, were analyzed for serum NET levels. Baseline characteristics show no statistical differences were found regarding age, gender, triglyceride, and neutrophil count; significant statistical differences were seen in BMI, fasting blood glucose, and HbA1c between the two groups (P < 0.05) (Table 1).
Fasting blood glucose and HbA1c levels were significantly higher among patients with T2DM (Fig. 1A, B). Furthermore, the diabetes group exhibited significantly elevated serum levels of NET indicator compared to non-diabetic individuals (P < 0.001) (Fig. 1C). Spearman correlation analysis revealed a positive correlation between serum NET levels and HbA1c in the T2DM group (r = 0.857, P < 0.001) (Fig. 1D).
Scattergram (median and interquartile range) of 42 patients with type 2 diabetes mellitus (T2DM) and 38 healthy controls on A fasting blood glucose (FBG), mmol/l, B HbA1c levels, C serum NETs (MPO-DNA complex) levels, Wilcoxon rank sum test was utilized. D Plot demonstrating the Spearman correlation for NET (MPO-DNA complex) levels and HbA1c, with Spearman correlation coefficient of 0.857 and P < 0.001. NETs neutrophil extracellular traps, MPO-DNA myeloperoxidase-DNA. ***P < 0.001
Variations of Serum NETs in Patients with DFU
A total of 200 patients with diabetic foot, with an average age of 62 (IQR 56–66) years, were analyzed. Patients with DFU demonstrated a statistically significant increase in age, WBC, and neutrophil count as well as a decrease in BMI indices, triglycerides, and HB compared to T2DM (Table 1). Notably, significantly elevated serum levels of NETs were observed in the DFU group compared to T2DM without DFU (P < 0.001) (Fig. 2A). Of those patients with DFU, 52 had amputatations, among which 14 were major amputations (7.0%) and 38 were minor amputations (19.0%). There were 148 cases without amputation; among them 68 patients (34.0%) underwent debridement surgery and 80 patients (40.0%) received standard wound care.
A Box plot displaying the NET (MPO-DNA complex) levels between patients with diabetic foot ulcer (DFU) and type 2 diabetes mellitus (T2DM), Wilcoxon rank sum test was utilized. B Box plot displaying the MPO-DNA complex levels among patients with DFU-related amputation (DFU + amputation), patients with DFU and no amputation (DFU—amputation), T2DM, and healthy controls, Dunn’s test was utilized. C Box plot displaying the MPO-DNA complex levels between patients with infected DFU and non-infected DFU, Wilcoxon rank sum test was utilized. D Box plot displaying the MPO-DNA complex levels of patients with DFU grouped by Wagner grades > 2 and ≤ 2, Student’s t-test was utilized. NETs neutrophil extracellular traps, MPO-DNA myeloperoxidase-DNA, **P < 0.01; ***P < 0.001
Additionally, we have analyzed patients with and without amputations as two subgroups (Table 2). No significant difference was found in gender distribution between the two groups (28/24 vs 77/71, P = 0.848). Subjects with DFU had an age range of 46–79 years, and there was no significant difference in the distribution of patients over 60 years of age between the two subgroups (P = 0.663). The duration of T2DM of the included patients ranged from 6 to 35 years, with an average of 18.6 ± 6.7 years. The proportion of patients with infected DFU and diabetes duration > 10 years was significantly higher in the amputated group than in the non-amputated group (P < 0.001). The body mass index ranged from 20.7 to 34.5 kg/m2, with a mean of 25.6 ± 2.9 kg/m2, and the difference was not statistically significant between groups (P = 0.895). Hypertension, HbA1c, Wagner grade, fever > 38.5℃, ABI, HB, WBC, neutrophil count, and other indicators were compared between the two groups and are shown in Table 2.
The comparison of people with a DFU-related amputation, people with DFU and no amputation, newly diagnosed type 2 diabetes, and healthy individuals, as shown in Fig. 2B, showed that serum NET levels of whole patients with DFU were significantly higher than those of the type 2 diabetics, both of which were higher than in the healthy controls (P < 0.05). In addition, serum NET levels were significantly higher in the group of DFU patients with amputations compared to DFU patients without amputations (Fig. 2B) (P < 0.05). Moreover, among the DFU group, patients with infections and higher Wagner grade exhibited significantly elevated serum levels of NETs compared to those without infections and those with lower Wagner grade (Fig. 2C, D) (P < 0.05).
Endpoint Analysis of Patients with DFU
Risk factors for diabetic foot amputation were analyzed using univariate Cox proportional hazard models, with 24-month follow-up and amputation as the endpoint event. As Table 3 presents, the model was set with 13 independent variables. Among these, high serum NET levels were the feature most significantly associated with amputation (P < 0.001). Additionally, longer duration of illness, previous DFU surgery, Wagner grade > 2, ABI < 0.9, and low hemoglobin level were found to have a regression relationship with the endpoint event (P < 0.05).
LASSO and multivariate Cox regression were used to select predictive variables from the univariate Cox regression. Multivariate Cox regression showed that serum NET levels, previous DFU surgery, and Wagner grade were the risk factors for amputation [AIC: 449.34, C-Index: 0.807 (0.779–0.836)] (Fig. 3). Furthermore, we applied a LASSO regression algorithm based on each feature in Table 3. Figure 4A shows that the most appropriate tuning parameter λ for LASSO regression was 0.012667 when the partial likelihood binomial deviance reached its minimum value. As shown in Fig. 4B, serum NET levels, previous DFU surgery, and Wagner grade exhibit the highest coefficient values, which imply high predictive probabilities for endpoint events.
Forest plot showing risk factors of diabetic foot ulcer (DFU)-related amputation based on multivariate Cox regression analysis. HR hazard ratio, CI confidence interval, ABI ankle-brachial index, T2DM type 2 diabetes mellitus, HbA1c glycated hemoglobin, HB hemoglobin, NETs neutrophil extracellular traps
Feature selection using least absolute shrinkage and selection operator (LASSO) Cox regression. A Selection of tuning parameter (λ) in the LASSO regression. The partial likelihood binomial deviance is plotted vs log (λ). At the optimal value log (λ), where features are selected, dotted vertical lines are set using the minimum criteria and the one standard error of the minimum criteria. B Hormonal parameters display coefficient values for the eight key variables. HB hemoglobin, T2DM type 2 diabetes mellitus, ABI ankle-brachial index, HbA1c glycated hemoglobin, NETs neutrophil extracellular traps, DFU diabetic foot ulcer
We calculated the risk score according to the multivariate Cox regression. Subjects with DFU were grouped by the median level of the risk score and NET levels and analyzed for amputation probability. The Kaplan-Meier curves showed that high risk score and high serum NET levels were potential predictive factors of amputation in 2 years (Fig. 5). Furthermore, we also constructed the receiver-operating characteristic (ROC) curve based on NET value and risk score for amputation. For NETs level, the area under the curve (AUC) was 0.727 (Fig. 6A), and for the risk score, the AUC was 0.822 (Fig. 6B).
Receiver-operating characteristic curve analysis for the predictive power of A serum NET levels and B risk score for DFU-related amputation, with areas under the curve of 0.727 (0.651–0.803) and 0.822 (0.754–0.890), respectively. DFU diabetic foot ulcer, NETs neutrophil extracellular traps, TPR true-positive rate, FPR false-positive rate, ROC receiver-operating characteristic, AUC area under the curve, CI confidence interval
Discussion
In our study, when analyzing the four cohorts formed by DFU with amputation, DFU without amputation, T2DM without DFU, and healthy volunteers, serum NET levels of the former were significantly higher than those of the latter, and the differences showed statistical significance (P < 0.01). Furthermore, patients with infected DFU presented higher NET levels than those without infections (P < 0.01), which is similar to previous studies [9].
Neutrophil cells are fundamental constituents of innate immunity, our body's first line of defense. Upon pathogenic incitement, neutrophils expel a mesh-like array formed from unraveling chromatin, histones, and assorted proteins from neutrophil granules, coalescing into structures known as neutrophil extracellular traps (NETs) [16]. In 2004, Brinkmann and colleagues discovered NETs, and the process of NET formation was named NETosis [17]. Within these NETs, DNA filaments are interwoven with histones and various proteins such as myeloperoxidase (MPO), proteinase 3, neutrophil elastase (NE), and cathepsin G, along with antimicrobial proteins including calprotectin, cathelicidins, and defensins.[18]
Recently, NETs have been involved in a variety of infectious and noninfectious diseases, such as diabetes and its complications, sepsis, atherosclerosis, thrombosis, and autoautoimmune diseases [19,20,21,22]. Besides their crucial role in host defense, their involvement contributes to organ tissue damage and disease pathology. For example, in sepsis and deep vein thrombosis, NETs bind platelets and red blood cells to promote thrombogenesis, and markedly elevated plasma concentrations of DNA, NE, and MPO were observed [23]. In a study conducted by Yang and colleagues, a group of patients with diabetes and active foot ulcers was assessed to determine the relationship between NET markers and the extent of DFU pathology. Their findings indicated a pronounced elevation of NETs in those with DFU relative to diabetic individuals without foot ulcers or non-diabetic healthy subjects, showing a direct association with both diabetic ulcer severity and wound, ischemia, and foot infection (WIfI) severity scores [9]. Additionally, neutrophil elastase levels of diabetic foot wound tissue were significantly elevated in cases with infection and delayed healing. In a separate study, Fadini et al. reported an association between higher NE and proteinase-3 levels and increased risk of wound infection [8]. Lastly, NETs-specific marker citrullinated histone H3 (cit-H3) was identified as an independent risk factor for impaired wound healing and amputation. In certain aspects, these findings are consistent with our results. The precise mechanistic contributions of NETs in a wide range of pathological conditions, including diabetes-related foot ulcers and infections, have yet to be comprehensively elucidated [24].
Common etiologies of DFU include neuropathic, arterial, and neuroischemic causes. The aforementioned triad etiologies, which are mutually causal, independently or cooperatively influenced the progression and prognosis of DFU [10, 25]. Peripheral artery disease (PAD) is an independent risk factor for diabetic foot complications, leading to lower limb ischemia. Importantly, the prognosis of DFU in the presence of PAD may surpass that of several common malignancies [26]. Furthermore, individuals suffering from lower extremity ischemia often exhibit a prothrombotic state, characterized by increased blood coagulation and a propensity for thrombotic events [27]. If the DFU is complicated by infection and ischemia, prompt evaluation and treatment are imperative, as the rate of amputation increases nearly 90 times and, in severe cases, entails high mortality rates [28, 29]. The wide range of pathological conditions in which NETs have been implicated include infections, PAD, ischemia, and thrombosis. It is indeed noteworthy that these conditions are all relevant within the context of DFUs. Despite the complexity and diversity of NET-influencing factors, our findings still demonstrate a significant correlation between NETs and the prognosis and amputation rates of DFUs as a whole. This highlights the potential importance of considering NETs as a biomarker or therapeutic target in the management of DFUs.
Another noteworthy aspect is the occurrence of infection. It is estimated that around 50% to 60% of ulcers undergo infection, while a considerable 20% of infections categorized as moderate to severe ultimately culminate in the unfortunate outcome of lower extremity amputations. A diagnosis of infection is based on microbiological examination of bacterial cultures, the severity of which is assessed by the extent and depth of the wounds and the systemic conditions following the debridement of nonviable and necrotic tissue [30]. In our results, the levels of WBC and neutrophils did not show significant changes in individuals with DFU-related amputations. Furthermore, these markers were not found to be associated with the probability of amputation in the Cox regression analysis. However, the inflammatory marker CRP was found to be significantly elevated in individuals with DFU-related amputations. Despite this, the Cox regression analysis did not show a statistically significant impact of CRP on amputation within 2 years. However, it is worth noting that NETs showed promising clinical value in assessing the progression of DFU and the likelihood of amputation within a 2-year timeframe.
Related research shows that residents in economically underdeveloped areas have a higher incidence of DFU [31]. Kashi Prefecture, where the patients enrolled in our study came from, is a border city in northwestern China and represents an undeveloped and poorly resourced area. In our study population, rural residents and less than high school-educated individuals compose 74% and 82% of all patients with DFU, respectively, and our amputation rate was 26%. This rate is much higher than in the economically developed area of the country of 7.3%, as reported by Gong et al.[32].
To determine the severity of diabetic foot ulcers, the Wagner grading system is utilized. It classifies ulcers based on the depth and extent of tissue involvement. This approach aids medical professionals in determining the best course of treatment and anticipating the possibility of amputation. A higher Wagner grade indicates a more severe ulcer with an increased risk of complications, including infection and potential amputation. Our results showed that Wagner grade > 2 is a risk factor for amputation, which is in line with previous studies [33, 34].
The findings of the current study indicate that previous DFU surgery is also a risk factor for amputation (95% CI 1.735—7.039, P < 0.001). It is generally accepted and proved that patients who have experienced debridement or amputations for DFU are more likely to need additional amputations in the future [32]. An earlier amputation indicates that the patient has already gone through serious diabetic foot condition-related consequences. Previous surgical intervention and subsequent amputation may be correlated with increased vulnerability, residual limb complications, and disease progression [35, 36].
In our results, the T2DM group demonstrated significantly elevated HbA1c compared to the healthy control group, and HbA1c was positively correlated with NETs. However, the aforementioned difference was not observed between the T2DM and DFU groups, and the correlation of HbA1c and NETs was revealed in neither the DFU group nor healthy controls (Supplementary Fig. 1A, B). Furthermore, in the analysis of amputation risk factors among patients with DFU, the hazard ratio of HbA1c in univariate analysis was determined to be 2.224 (95% CI 1.220–4.055), with a corresponding P value of 0.009, but no statistical difference was found in multivariate analysis with HR 1.781 (95% CI 0.959–3.308) and P value 0.068, which is in line with the existing study [33]. This indicated that after experiencing long-term diabetes and administrating various hypoglycemic treatments, HbA1c is not a sensitive indicator for assessing the prognosis of DFU, which is a complex multifactorial disease.
ABI is widely used in clinical practice as an indicator of peripheral artery disease. A decreased ABI value indicates significant artery disease in the lower limbs, which compromises the blood flow to the foot. Due to impaired blood flow, wounds have a slower rate of healing and are more likely to become infected, which raises the risk of amputation in patients with diabetes [9, 37]. However, our results showed that ABI does not correlate with amputation.
In addition, the LASSO-Least Absolute Shrinkage and Selection Operator was applied to select the most valuable predictive variables, and the most appropriate tuning parameter λ for LASSO regression was 0.012667. Moreover, the NET levels, previous DFU surgery, and Wagner grade have the highest coefficients, which is in accordance with multivariate Cox regression results, which implies a strong correlation of these variables with amputation.
Limitations
Although some promising results were achieved in our prospective study, several limitations still exist. Our findings came from individuals sampled from a single facility. More severe cases in patients with lower socioeconomic status lead to higher amputation rates. Hence, potential biases exist in patient and treatment selection, and our outcomes lack long-term follow-up. In addition, we present the main consequences seen in 2 years. It is possible that our study neglected postponed diabetic foot amputations. Besides, we have not provided an in-depth analysis of the contributions of the factors influencing NETs which are also in the context of diabetic foot ulcers. Despite these limitations, our results underscore the clinical value of NETs as a prognostic tool in DFU.
Conclusion
Among patients with DFU, high serum NET levels are associated with increased amputation rate, suggesting that NETs have potential clinical implication value for assessing DFU prognosis. Serum NETs could be important when monitoring and managing DFU. Multicenter, large-scale, long-term observational studies are needed for further validation and support of NETs' predictive value in the wound healing process and amputation.
Data Availability
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.
References
Du F, Ma J, Gong H, et al. Microbial infection and antibiotic susceptibility of diabetic foot ulcer in China: literature review. Front Endocrinol (Lausanne). 2022;13: 881659. https://doi.org/10.3389/fendo.2022.881659.
International Diabetes Federation. IDF Diabetes Atlas teB, Belgium. 2017. https://www.diabetesatlas.org
Meloni M, Izzo V, Giurato L, Lazaro-Martinez JL, Uccioli L. Prevalence, clinical aspects and outcomes in a large cohort of persons with diabetic foot disease: comparison between neuropathic and ischemic ulcers. J Clin Med. 2020;9(6):1780. https://doi.org/10.3390/jcm9061780.
Martin P, Leibovich SJ. Inflammatory cells during wound repair: the good, the bad and the ugly. Trends Cell Biol. 2005;15(11):599–607. https://doi.org/10.1016/j.tcb.2005.09.002.
Njeim R, Azar WS, Fares AH, Azar ST, Kfoury Kassouf H, Eid AA. NETosis contributes to the pathogenesis of diabetes and its complications. J Mol Endocrinol. 2020;65(4):R65–76. https://doi.org/10.1530/JME-20-0128.
Yang S, Wang S, Chen L, et al. Neutrophil extracellular traps delay diabetic wound healing by inducing endothelial-to-mesenchymal transition via the hippo pathway. Int J Biol Sci. 2023;19(1):347–61. https://doi.org/10.7150/ijbs.78046.
Menegazzo L, Ciciliot S, Poncina N, et al. NETosis is induced by high glucose and associated with type 2 diabetes. Acta Diabetol. 2015;52(3):497–503. https://doi.org/10.1007/s00592-014-0676-x.
Fadini GP, Menegazzo L, Rigato M, et al. NETosis delays diabetic wound healing in mice and humans. Diabetes. 2016;65(4):1061–71. https://doi.org/10.2337/db15-0863.
Yang S, Gu Z, Lu C, et al. Neutrophil extracellular traps are markers of wound healing impairment in patients with diabetic foot ulcers treated in a multidisciplinary setting. Adv Wound Care (New Rochelle). 2020;9(1):16–27. https://doi.org/10.1089/wound.2019.0943.
Armstrong DG, Boulton AJM, Bus SA. Diabetic foot ulcers and their recurrence. N Engl J Med. 2017;376(24):2367–75. https://doi.org/10.1056/NEJMra1615439.
Armstrong DG, Tan TW, Boulton AJM, Bus SA. Diabetic foot ulcers: a review. JAMA. 2023;330(1):62–75. https://doi.org/10.1001/jama.2023.10578.
Hurst JE, Barn R, Gibson L, et al. Geospatial mapping and data linkage uncovers variability in outcomes of foot disease according to multiple deprivation: a population cohort study of people with diabetes. Diabetologia. 2020;63(3):659–67. https://doi.org/10.1007/s00125-019-05056-9.
Bus SA, Van Netten JJ, Hinchliffe RJ, et al. Standards for the development and methodology of the 2019 International Working Group on the Diabetic Foot guidelines. Diabetes Metab Res Rev. 2020;36(Suppl 1): e3267. https://doi.org/10.1002/dmrr.3267.
Gao L, Wang J, Yin Y. Interpretation of 2019 International Working Group on Diabetic Foot guidelines on the prevention and management of diabetic foot disease. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2020;34(1):16–20. https://doi.org/10.7507/1002-1892.201906014.
Sauerbrei W, Royston P, Binder H. Selection of important variables and determination of functional form for continuous predictors in multivariable model building. Stat Med. 2007;26(30):5512–28. https://doi.org/10.1002/sim.3148.
Nakazawa D, Marschner JA, Platen L, Anders HJ. Extracellular traps in kidney disease. Kidney Int. 2018;94(6):1087–98. https://doi.org/10.1016/j.kint.2018.08.035.
Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532–5. https://doi.org/10.1126/science.1092385.
Yang H, Biermann MH, Brauner JM, Liu Y, Zhao Y, Herrmann M. New insights into neutrophil extracellular traps: mechanisms of formation and role in inflammation. Front Immunol. 2016;7:302. https://doi.org/10.3389/fimmu.2016.00302.
Carminita E, Crescence L, Brouilly N, Altie A, Panicot-Dubois L, Dubois C. DNAse-dependent, NET-independent pathway of thrombus formation in vivo. Proc Natl Acad Sci USA. 2021. https://doi.org/10.1073/pnas.2100561118.
Papayannopoulos V. Neutrophil extracellular traps in immunity and disease. Nat Rev Immunol. 2018;18(2):134–47. https://doi.org/10.1038/nri.2017.105.
Denning NL, Aziz M, Gurien SD, Wang P. DAMPs and NETs in Sepsis. Front Immunol. 2019;10:2536. https://doi.org/10.3389/fimmu.2019.02536.
Shafqat A, Abdul Rab S, Ammar O, et al. Emerging role of neutrophil extracellular traps in the complications of diabetes mellitus. Front Med (Lausanne). 2022;9: 995993. https://doi.org/10.3389/fmed.2022.995993.
van Montfoort ML, Stephan F, Lauw MN, et al. Circulating nucleosomes and neutrophil activation as risk factors for deep vein thrombosis. Arterioscler Thromb Vasc Biol. 2013;33(1):147–51. https://doi.org/10.1161/ATVBAHA.112.300498.
Tan C, Aziz M, Wang P. The vitals of NETs. J Leukoc Biol. 2021;110(4):797–808. https://doi.org/10.1002/JLB.3RU0620-375R.
Dharmadas S, Kumar H, Pillay M, et al. Biomarkers for early detection of charcot arthropathy: a prospective study on type 2 diabetes patients with severe neuropathy. Foot Ankle Int. 2023. https://doi.org/10.1177/10711007231213645.
Hinchliffe RJ, Brownrigg JR, Apelqvist J, et al. IWGDF guidance on the diagnosis, prognosis and management of peripheral artery disease in patients with foot ulcers in diabetes. Diabetes Metab Res Rev. 2016;32(Suppl 1):37–44. https://doi.org/10.1002/dmrr.2698.
Wang A, Lv G, Cheng X, et al. Guidelines on multidisciplinary approaches for the prevention and management of diabetic foot disease (2020 edition). Burns Trauma. 2020;8:tkaa017. https://doi.org/10.1093/burnst/tkaa017.
Armstrong DG, Kanda VA, Lavery LA, Marston W, Mills JL Sr, Boulton AJ. Mind the gap: disparity between research funding and costs of care for diabetic foot ulcers. Diabetes Care. 2013;36(7):1815–7. https://doi.org/10.2337/dc12-2285.
Eggert JV, Worth ER, Van Gils CC. Cost and mortality data of a regional limb salvage and hyperbaric medicine program for Wagner Grade 3 or 4 diabetic foot ulcers. Undersea Hyperb Med. 2016;43(1):1–8.
Petersen BJ, Linde-Zwirble WT, Tan TW, et al. Higher rates of all-cause mortality and resource utilization during episodes-of-care for diabetic foot ulceration. Diabetes Res Clin Pract. 2022;184: 109182. https://doi.org/10.1016/j.diabres.2021.109182.
Wang J, Li J, Wen C, Liu Y, Ma H. Predictors of poor glycemic control among type 2 diabetes mellitus patients treated with antidiabetic medications: a cross-sectional study in China. Medicine (Baltimore). 2021;100(43): e27677. https://doi.org/10.1097/MD.0000000000027677.
Gong H, Ren Y, Li Z, et al. Clinical characteristics and risk factors of lower extremity amputation in the diabetic inpatients with foot ulcers. Front Endocrinol (Lausanne). 2023;14:1144806. https://doi.org/10.3389/fendo.2023.1144806.
Mansoor Z, Modaweb A. Predicting amputation in patients with diabetic foot ulcers: a systematic review. Cureus. 2022;14(7): e27245. https://doi.org/10.7759/cureus.27245.
Ugwu E, Adeleye O, Gezawa I, Okpe I, Enamino M, Ezeani I. Predictors of lower extremity amputation in patients with diabetic foot ulcer: findings from MEDFUN, a multi-center observational study. J Foot Ankle Res. 2019;12:34. https://doi.org/10.1186/s13047-019-0345-y.
Armstrong DG, Lipsky BA. Advances in the treatment of diabetic foot infections. Diabetes Technol Ther. 2004;6(2):167–77. https://doi.org/10.1089/152091504773731357.
Jeffcoate WJ, Lipsky BA. Controversies in diagnosing and managing osteomyelitis of the foot in diabetes. Clin Infect Dis. 2004;39(Suppl 2):S115-122. https://doi.org/10.1086/383272.
Potier L, Abi Khalil C, Mohammedi K, Roussel R. Use and utility of ankle brachial index in patients with diabetes. Eur J Vasc Endovasc Surg. 2011;41(1):110–6. https://doi.org/10.1016/j.ejvs.2010.09.020.
Acknowledgements
We thank the participants of the study.
Funding
This research was funded by Xinjiang Autonomous Region Health and Youth Medical Science and Talents Special Research Project (WJWY-202102) and Kashi Regional Science and Technology Project (KS2020012). The journal’s Rapid Service Fee was funded by the authors.
Author information
Authors and Affiliations
Contributions
Conceptualization, Irshat Ibrahim and Yuanquan Wu; Data curation, Gulijianaiti Maimaituxun, Xinling Luo and Azimat Akbar; Funding acquisition, Yuanquan Wu; Investigation, Gulijianaiti Maimaituxun and Xinling Luo; Methodology, Yilimire Nuermaimaiti and Yuanquan Wu; Super-vision, Kahaer Tuerxun; Validation, Irshat Ibrahim; Writing—original draft, Irshat Ibrahim, Yilimire Nuermaimaiti and Gulijianaiti Maimaituxun; Writing—review and editing, Irshat Ibra-him, Mailudemu Maimaituxun, Kahaer Tuerxun and Yuanquan Wu. All authors provided edits to the manuscript and reviewed and approved the final version.
Corresponding authors
Ethics declarations
Conflict of Interest
Irshat Ibrahim, Yilimire Nuermaimaiti, Gulijianaiti Maimaituxun, Xinling Luo, Mailudemu Maimaituxun, Azimat Akbar, Kahaer Tuerxun, Yuanquan Wu declare that they have no competing interests.
Ethical Approval
The presented study received approval from the Ethics Committee of The First People’s Hospital of Kashi Prefecture (KDYY-202023) and conformed with the Helsinki Declaration of 1964 (as re-vised in 2013) concerning human and animal rights. Written consent was required for all participants.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Ibrahim, I., Nuermaimaiti, Y., Maimaituxun, G. et al. Neutrophil Extracellular Traps (NETs) Are Associated with Type 2 Diabetes and Diabetic Foot Ulcer Related Amputation: A Prospective Cohort Study. Diabetes Ther (2024). https://doi.org/10.1007/s13300-024-01579-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13300-024-01579-6