Abstract
Introduction
Tirzepatide is a novel hypoglycemic agent for type 2 diabetes mellitus (T2DM). However, the pathophysiology of T2DM in Asians is different from that in non-Asians, and there is no evidence to explain the differences in the efficacy and safety of tirzepatide between different races.
Methods
A literature search was conducted in China National Knowledge Infrastructure (CNKI), PubMed, Cochrane Library, Clinical Trials.gov, and Embase databases for clinical studies of tirzepatide for T2DM. The data extraction process was done independently by two authors. All analyses were performed using STATA 14.0 software and Review Manager 5.3 software.
Results
A total of 2118 patients with T2DM from 6 studies were involved, with doses of tirzepatide ranging from 5 to 15 mg administered subcutaneously once weekly. The results showed that compared with control/placebo, tirzepatide was more effective in decreasing fasting blood glucose (FBG) in non-Asians than in Asians, and 10 mg rather than 15 mg was the optimal dose to decrease FBG. Similarly, non-Asians were more effective than Asians in improving glycated hemoglobin (HbA1c). Asians were significantly more effective than non-Asians in reducing body weight and ≥ 5% weight loss. In terms of adverse events, the incidence of gastrointestinal adverse events was higher in Asians than in non-Asians at the same dose, while the incidence of metabolic and nutrition disorders was higher in non-Asians than in Asians.
Conclusion
Tirzepatide is a novel agent for the treatment of diabetes and has different efficacy in Asians and non-Asians. Asians were more likely to experience weight loss and gastrointestinal adverse events, whereas non-Asians were more likely to have better glycemic control and more metabolic and nutritional disorders.
Trial Registration
PROSPERO registration no. CRD42023489588.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
The incidence of type 2 diabetes mellitus (T2DM) has increased in recent years and displays variations in pathophysiological mechanisms between Asians and non-Asians, with Asian patients constituting more than half of the global patient population. |
Tirzepatide is a novel treatment that acts as a dual agonist of GIP and GLP-1 receptors. The 2023 guidelines issued by the American Diabetes Association support tirzepatide as a favorable treatment option for patients with obesity and T2DM. |
The efficacy and safety of tirzepatide in Asian or non-Asian patients with T2DM are unknown. |
What was learned from the study? |
The results of this meta-analysis indicated that tirzepatide exhibited greater efficacy in regulating blood glucose levels among non-Asian patients compared to Asian patients, while demonstrating a more pronounced reduction in body weight among Asian patients as opposed to non-Asian patients. |
Regarding adverse events, Asian patients exhibited a higher incidence of gastrointestinal adverse events compared to non-Asian patients receiving the same dosage. Conversely, non-Asian patients demonstrated a higher incidence of metabolic and nutrition disorders in comparison to Asian patients. |
Introduction
The global incidence of diabetes has experienced a significant surge in recent times and is projected to reach 700 million individuals (constituting 10.9% of the global population aged 20–79) by 2045 if effective interventions are not implemented [1]. The prevalence of type 2 diabetes mellitus (T2DM) exhibits variations across different ethnic groups. Recent epidemiological findings from 2023 indicate that all Asian American subgroups exhibit higher rates of type 2 diabetes compared to their white adult counterparts [2]. Furthermore, individuals within these subgroups are prone to developing the disease at an earlier age and with a lower body mass index (BMI) [3].
China has the highest number of individuals with diabetes, totaling 116.4 million, followed by India with 101 million [4]. Collectively, Asian countries contribute to over 50% of the global population affected by diabetes [5]. Given the significant impact of diabetes complications on patients’ health-related quality of life, it is imperative to prioritize the implementation of effective preventive measures against the onset and progression of diabetes in Asia.
Tirzepatide is a novel dual agonist of glucagon-like peptide 1 receptor (GLP-1R) and glucose-dependent insulinotropic polypeptide receptor (GIP-R). GLP-1 is classified as an incretin hormone and exerts its effects on pancreatic beta cells to stimulate insulin secretion [6]. Similarly, GIP is also an incretin hormone and, like GLP-1, acts as a glucose-dependent agonist to enhance insulin secretion in the presence of high blood sugar levels [7]. Notably, the GIP receptor is expressed in white adipose tissue, allowing GIP to improve insulin sensitivity in this tissue and maintain lipid metabolic equilibrium. Furthermore, it has been observed that GLP-1 receptor agonists (GLP-1 RAs) possess glucagonotropic properties in both euglycemic and hypoglycemic states, resulting in a reduction in the occurrence of hypoglycemia [8]. Additionally, studies have demonstrated that the combination of GLP-1 and GIP receptor agonists exhibit synergistic effects on glycemic regulation [9, 10]. Phase III clinical trials have provided evidence that treatment with tirzepatide leads to improved glycemic control and offers additional advantages [11], making it a promising therapeutic approach for the management of T2DM in the future [12, 13]. Moreover, the 2023 guidelines from the American Diabetes Association recommend tirzepatide as a favorable option for patients with obesity and type 2 diabetes [14].
The heterogeneous pathophysiology of diabetes results in Asians without diabetes exhibiting higher fasting glycemic indices compared to white people, as well as greater levels of insulin resistance [15,16,17]. When considering individuals with the same BMI, Asian populations demonstrate higher body fat percentage and rates of visceral adiposity compared to non-Asians [18]. The presence of increased visceral fat is associated with insulin resistance-related abnormalities and contributes to the pathophysiology of type 2 diabetes [19,20,21]. In addition to insulin resistance, East Asian patients with type 2 diabetes exhibit beta cell dysfunction, as evidenced by inadequate insulin responses to meals [22]. The association between BMI and diabetes risk is notably more pronounced in Asian populations compared to other populations [23]. The aging demographic in Asia, particularly East Asia, further amplifies the prevalence of type 2 diabetes [24]. Consequently, finding more effective strategies to manage blood glucose levels, reduce body weight, and improve insulin resistance has become an urgent issue. It is noteworthy that differences have been found between Asians and non-Asians with respect to response to therapy and perhaps even between Asians. Thus, the dipeptidyl peptidase 4 (DPP4) inhibitor sitagliptin had a much better effect in Indians and Koreans and somewhat less than in Chinese compared to that reported in Europeans [17]. Later, a systematic review comparing Asians and non-Asians also found that DPP4 inhibitors work better in Asians as a whole [16]. Surprisingly, sodium-dependent glucose transporter 2 (SGLT2) inhibitors also worked better in Asians while GLP-1 RAs did not work better in Asians [16].
This study seeks to provide evidence on the varying efficacy and safety of tirzepatide with different dose formulations for individuals with type 2 diabetes, looking for possible differences in efficacy and safety in Asians, taking into account the significant heterogeneity observed between Asians and non-Asians in terms of BMI, age of onset, and fat distribution. In the context of this study, Asian populations are represented by patients from Japan, China, South Korea, and India.
Methods
This study strictly followed the systematic review and meta-analytic approach of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [25]. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Search Strategy
A thorough review of the literature was undertaken utilizing the databases of China National Knowledge Infrastructure (CNKI), PubMed, Cochrane Library, Clinical Trials.gov, and Embase to identify clinical studies pertaining to the use of tirzepatide for the treatment of T2DM until March 21, 2023. The English database search employed the medical subject terms tirzepatide, LY3298176, and T2DM, while the Chinese database search utilized the medical subject terms tirzepatide, LY3298176, and erxing tangniaobing (the Chinese term for T2DM). The CNKI database was utilized to expand Chinese free terms, while the MeSH database was employed to expand English free terms. Subsequently, the subject terms and free terms were amalgamated for the purpose of conducting the search. Additionally, a manual search of the database was conducted to identify pertinent references, conference proceedings, and review articles.
Study Selection
Criteria for inclusion were as follows: (1) Study design: randomized controlled trial (RCT). (2) Participants: had type 2 diabetes for at least 8 weeks (HbA1c 6.5–11% inclusive), all subjects were Asian or none were Asian. (3) Intervention: Patients in the experimental and control groups were treated with different doses of tirzepatide or GLP-1 RAs. (4) Results: HbA1c, fasting plasma glucose, and body weight were selected as efficacy endpoints. Exclusion criteria were as follows: (1) Studies, such as reviews, animal experiments, and case reports. (2) Repeated published studies. (3) Studies published in abstract form. (4) Studies with incomplete or unclear data. (Studies where means and standard deviations for continuous variables were not provided and these data could not be obtained by transformation.)
Two authors screened the titles/abstracts of all identified records to identify studies eligible for inclusion. Additional assessments of references from identified eligible studies were also performed to look for more potential articles. Following this process, selected records were then screened in full for inclusion and exclusion criteria. If any discrepancies are identified, they will be resolved by discussion.
Data Extraction
The process of data extraction was conducted by two authors in an independent manner. Several elements were extracted from each article, including the first author, study year, clinical trial registration number, study type, study duration, participant count in each group, age, weight, duration of diabetes, and other relevant outcomes. In cases where multiple search results were associated with the same clinical trial registration number, priority was given to the clinical trial registration number. Additionally, a standardized predefined table of clinical trial registration numbers was utilized for data extraction. In this study, the results were categorized into three different doses of tirzepatide (5 mg, 10 mg, and 15 mg) compared to control groups. The efficacy outcomes assessed were the change in HbA1c levels from baseline, the change in fasting plasma glucose levels from baseline, and the reduction in body weight from baseline. The safety outcomes encompassed various factors, such as all-cause mortality, gastrointestinal adverse events (GIAEs), incidence of hypoglycemia (blood glucose < 54 mg/dL), adverse events leading to treatment discontinuation, and serious adverse events.
Quality Assessment
The risk of bias for eligible RCTs was performed using the Risk of Prejudice Assessment Tool in Review Manager version 5.3 software. This was done independently by three investigators. The following points were taken into consideration: whether the sequence generation was sufficient and whether allocation was sufficiently concealed (selection bias); whether knowledge of the allocated interventions was adequately prevented during the study and whether participants and personnel were appropriately blinded (performance bias); whether the result assessor was blind (detection bias); whether incomplete result data were adequately handled (attrition bias); whether the study report had recommendations for selective result reporting (reporting bias); and whether the study was clearly free of other issues that could put them at risk of bias. Any disagreement was resolved by a fourth author.
Data Analysis
All analyses were performed using STATA 14.0 software and Review Manager 5.3 software. The effect size was represented by mean difference (MD). The fixed-effect or random-effect model was applied for the present meta-analysis in terms of the heterogeneity among all studies [26]. Heterogeneity was tested using the Higgins I2 statistical method (I2 < 25% indicated no heterogeneity, 25% < I2 < 50% indicated moderate heterogeneity, and I2 > 50% indicated strong heterogeneity) [27]. To study publication bias, we used Egger’s funnel plot [28], and a two-tailed P value > 0.05 was considered meaningful.
Results
Search Results and Characteristics of Included Studies
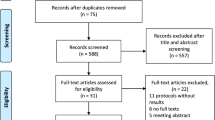
Initially, a comprehensive search yielded a total of 367 relevant studies. Following an initial review of the topics and summaries, 87 articles were selected for further examination and subsequently downloaded. The complete texts of these articles underwent further scrutiny, resulting in the inclusion of six articles for the purpose of this analysis (Fig. 1). The pertinent attributes of these studies are concisely summarized in Table 1. Among the six included articles, two focused on Japanese populations [29, 30], while one study encompassed participants from China, South Korea, and India [31].
Studies in non-Asians populations have been conducted in the USA, Germany, Poland, Puerto Rico, and Slovakia [9, 32, 33]. Overall, 2118 patients with T2DM were involved in these studies. Of these, 1601 patients were Asian and 517 patients were non-Asians. Strikingly, Asians have a lower BMI than non-Asians, and other additional information is detailed in Table 1. In terms of risk of bias assessment, the six studies were rated as medium to low risk, and the quality of evidence for each indicator was high (Fig. 2).
Efficacy of Tirzepatide on Fasting Blood Glucose in Different Ethnic Groups
The fasting plasma glucose data from all six studies were included in the analysis, as depicted in Fig. 3. A random-effects model was employed to combine the effect sizes. The meta-analysis of the available data revealed that a dosage of 5 mg tirzepatide resulted in a decrease of 24.7 mg/dL (MD − 24.7, 95% confidence interval [CI] − 38.1 to − 11.3, P = 0.0003, I2 = 88%) in Asians and a decrease of 37.8 mg/dL (MD − 37.8, 95% CI − 73.8 to − 1.8, P = 0.04, I2 = 88%) in non-Asians. Similarly, at a dosage of 10 mg, FBG decreased by 51.8 mg/dL (MD − 51.8, 95% CI − 91.7 to − 11.9, P = 0.01, I2 = 93%) in Asians and 57.8 mg/dL (MD − 57.8, 95% CI − 93.8 to − 21.8, P = 0.002, I2 = 88%) in non-Asians. At a dosage of 15 mg, FBG exhibited a reduction of 50.3 mg/dL (MD − 50.3, 95% CI − 82.4 to − 18.3, P = 0.002, I2 = 88%) in Asians and 53.6 mg/dL (MD − 53.6, 95% CI − 65.7 to − 41.6, P < 0.0001, I2 = 58%) in non-Asians.
Efficacy of Tirzepatide on Glycated Hemoglobin in Different Ethnic Groups
Glycated hemoglobin data were available in five studies, as depicted in Fig. 4. A random-effects model was employed to amalgamate effect sizes. The findings indicated that the administration of 5 mg tirzepatide resulted in a reduction of HbA1c by 1.18% (MD − 1.18, 95% CI − 1.39 to − 0.98, P < 0.00001, I2 = 57%) compared to placebo/controls among Asians. However, no statistically significant differences were observed among non-Asians. Among Asians, the administration of 10 mg tirzepatide led to a reduction of HbA1c by 1.37% (MD − 1.37, 95% CI − 1.60 to − 1.15, P < 0.00001, I2 = 63%) compared to placebo/controls, whereas among non-Asians, the reduction was 1.5% (MD − 1.50, 95% CI − 2.67 to − 0.32, P = 0.01, I2 = 93%). Similarly, the administration of 15 mg tirzepatide exhibited a significant reduction in HbA1c levels, with a decrease of 1.53% (MD − 1.53, 95% CI − 1.67 to − 1.40, P < 0.00001, I2 = 0%) observed in Asians and a decrease of 2.08% (MD − 2.08, 95% CI − 2.50 to − 1.65, P < 0.00001, I2 = 79%) observed in non-Asians.
Efficacy of Tirzepatide on Body Weight in Different Ethnic Groups
As a result of a significant disparity in BMI between Asians and non-Asians, a separate statistical analysis was conducted. Among Asians (Fig. 5a), meta-analyses revealed a noteworthy decrease in body weight (BW) when compared to placebo/control (MD − 7.87, 95% CI − 9.17 to − 6.56, P < 0.00001, I2 = 90%). Specifically, tirzepatide at 5 mg exhibited a reduction of 5.95 kg (MD − 5.95, 95% CI − 7.12 to − 4.78, P < 0.00001, I2 = 62%), while the 10 mg dosage resulted in a decrease of 8.31 kg (MD − 8.31, 95% CI − 9.02 to − 7.59, P < 0.00001, I2 = 0%), and the 15 mg dosage led to a reduction of 9.41 kg (MD − 9.41, 95% CI − 10.88 to − 7.94, P < 0.00001, I2 = 75%).
In the case of non-Asian individuals, the meta-analyses demonstrated a statistically significant decrease in BW compared to placebo/control. The reductions in BW were 6.29 kg (MD − 6.29, 95% CI − 8.23 to − 4.36, P < 0.00001, I2 = 83%), 3.24 kg (MD − 3.24, 95% CI − 5.49 to − 0.99, P = 0.005, I2 = 54%), 7.14 kg (MD − 7.14, 95% CI − 9.39 to − 4.89, P < 0.00001, I2 = 52%), and 7.42 kg (MD − 7.42, 95% CI − 10.19 to − 4.64, P < 0.00001, I2 = 83%) for tirzepatide doses of 5 mg, 10 mg, and 15 mg, respectively (Fig. 5b).
People Achieving Weight Loss of ≥ 5% in Different Ethnic Groups
The analysis of data from three studies was conducted to evaluate the impact of tirzepatide on achieving a weight loss of at least 5% (Fig. 6). Asian individuals receiving tirzepatide had over 33.9-fold (odds ratio [OR] 33.9, 95% CI 20.5–56.1, P < 0.00001, I2 = 72%) increased odds of achieving weight loss more than 5% overall and 16.4-fold (OR 16.4, 95% CI 10.0–26.7, P < 0.00001, I2 = 21%), 42.2-fold (OR 42.2, 95% CI 26.6–67.0, P < 0.00001, I2 = 0%), and 57.3-fold (OR 57.3, 95% CI 35.4–92.7, P < 0.00001, I2 = 0%) increased odds of losing weight versus placebo/control at doses of 5 mg, 10 mg, and 15 mg, respectively. In non-Asians (Fig. 6b), patients receiving tirzepatide had higher odds (OR 15.2, 95% CI 4.7–49.2, P < 0.00001, I2 = 77%) of achieving weight loss more than 5% versus control. However, there was no statistically significant difference in the subgroup analysis for the three doses.
Adverse Events
Six adverse events of tirzepatide were evaluated: all-cause mortality, serious adverse events (an adverse event that results in death, is life-threatening, requires inpatient hospitalization or extends a current hospital stay, results in an ongoing or significant incapacity or interferes substantially with normal life functions, or causes a congenital anomaly or birth defect; medical events that do not result in death, are not life-threatening, or do not require hospitalization may be considered serious adverse events if they put the participant in danger or require medical or surgical intervention to prevent one of the results listed above), gastrointestinal adverse events (GIAEs), metabolism and nutrition disorders, anti-drug antibodies (ADAs), and hypoglycemia. All doses of tirzepatide did not result in a higher incidence of events compared with control/placebo in terms of all-cause mortality, serious adverse events, and hypoglycemia. However, 5 mg tirzepatide could lead to a higher incidence of GIAEs in Asians (OR 8.02, 95% CI 2.97–21.68) but not in non-Asians (OR 1.35, 95% CI 0.75–2.44). Both 10 mg and 15 mg tirzepatide could lead to a higher incidence of GIAEs in Asians (10 mg: OR 11.12, 95% CI 4.17–29.63; 15 mg: OR 12.28, 95% CI 4.63–32.55) and non-Asians (10 mg: OR 2.90, 95% CI 1.61–5.21; 15 mg: OR 3.53, 95% CI 2.22–5.61). For metabolism and nutrition disorders, all doses of tirzepatide resulted in a higher incidence of adverse events in both Asians (5 mg: OR 2.65, 95% CI 1.39–5.03; 10 mg: OR 3.63, 95% CI 1.95–6.77; 15 mg: OR 3.69, 95% CI 1.99–6.85) and non-Asians (5 mg: OR 27.38, 95% CI 3.63–206.80; 10 mg: OR 8.70, 95% CI 2.90–26.09; 15 mg: OR 5.53, 95% CI 2.66–11.49). For anti-drug antibodies, data were limited in Asians, and either dose can lead to an increased incidence in non-Asians (5 mg: OR 56.61, 95% CI 7.61–421.30; 10 mg: OR 94.40, 95% CI 12.69–702.05; 15 mg: OR 102.11, 95% CI 13.75–758.24) (Table 2).
Sensitivity Analysis and Publication Bias
Because there was heterogeneity in this study, we performed a sensitivity analysis, which showed that NCT04093752 and NCT03951753-1 could be the source of heterogeneity (Fig. 7).
We assessed the risk of bias for the primary outcome (FBG), and visual inspection showed no publication bias (Fig. 8). Begg’ s test (P = 0.917) and Egger’ s test (P = 0.142) showed no evidence of publication bias.
Discussion
We conducted a comparative analysis to assess the efficacy and safety of tirzepatide in relation to ethnicity, specifically comparing Asians and non-Asians, considering that Asian individuals constitute 60% of the global population of patients with T2DM [34, 35]. This becomes particularly important since an earlier systematic review showed that DPP4 and SGLT2 inhibitors worked better in Asians but not GLP-1 RA compared to Europeans [16].
Our findings indicate that tirzepatide exhibited greater effectiveness in reducing FBG levels among non-Asians, regardless of the administered dose (5 mg, 10 mg, 15 mg), when compared to Asians. It is noteworthy that the optimal dose for FBG reduction in both Asian and non-Asian patients was 10 mg, rather than 15 mg. This finding deviates from previous research, which suggested a dose-dependent decrease in FBG with tirzepatide [36]. On the basis of the current limited data, although there is large heterogeneity in the dose of 10 mg in lowering FBG in Asians, the difference in efficacy between Asians and non-Asians is undeniable. Regarding the reduction of HbA1c, the outcomes of administering 5 mg tirzepatide to non-Asians did not exhibit any statistically significant variance due to the restricted availability of data. Moreover, both 10 mg and 15 mg tirzepatide demonstrated greater effectiveness in non-Asians compared to Asians, aligning with the findings concerning FBG. However, the efficacy of tirzepatide in reducing HbA1c displayed a dose-dependent trend in both Asian and non-Asian populations, which diverged from the conclusions drawn regarding FBG. This may be related to the different intervention times for the included studies.
On the basis of the different characteristics of obesity in Asian and non-Asian patients with type 2 diabetes, we assessed the effect of different doses on weight loss. As a result of the disparity in baseline BMI between these two groups, we performed separate analyses for Asians and non-Asians. Remarkably, despite Asians having a lower BMI, they exhibited significantly greater weight loss compared to non-Asians. This discrepancy may be attributed to the higher prevalence of visceral obesity among Asians [18]. In the SURPASS clinical trial [37], tirzepatide treatment was beneficial in all BMI of categories in Asians and can reduced BMI to within the normal range. Notably, a subset of participants even achieved a weight that fell below the BMI threshold for “underweight”. These findings highlight the remarkable efficacy of tirzepatide in facilitating weight loss among Asians, emphasizing the importance of carefully adjusting dosage to avoid the risk of underweight status during clinical implementation. However, it is worth noting that as a result of limitations in available data, statistical significance in terms of 5% body weight loss was only observed with the 10 mg dosage in non-Asians. In relation to the rate of weight achievement, it10 mg tirzepatide yielded significantly superior outcomes among Asians compared to non-Asians. In this regard, more non-Asian data are needed to support this in the future. In addition to data limitations, this further confirms that tirzepatide is more effective in reducing body weight in Asians than in non-Asians.
Regarding safety, our investigation revealed a dose-dependent escalation in gastrointestinal adverse events, with a notably higher incidence among Asians compared to non-Asians. This is consistent with previous studies [38]. Because the definition of gastrointestinal adverse events such as nausea and abdominal pain is somewhat vague, this result needs to be treated with caution. Conversely, non-Asians exhibited a significantly higher incidence of metabolic and nutrition disorders compared to Asians. Previous studies have indicated that the majority of adverse events were of mild or moderate severity [9, 32, 39, 40]. The predominant adverse events associated with tirzepatide were gastrointestinal in nature, accounting for 44% of reported cases, specifically encompassing symptoms such as nausea and diarrhea. These adverse events were similar to those observed with current GLP-1 analogues [41]. Furthermore, the incidence of these adverse events increased in correlation with the dosage administered, indicating a dose-dependent relationship. In addition to gastrointestinal adverse events, existing research highlights the significance of endocrine and metabolic disturbances as noteworthy adverse events. These disturbances manifest in various ways, including decreased appetite, dehydration, hyperglycemia, hyperlipasemia, gout, hyperuricemia, and hypokalemia. It is important to acknowledge that the evidence presented, specifically regarding gastrointestinal adverse events (Table 2), exhibits considerable heterogeneity. Consequently, this evidence can be classified as moderate to low in terms of quality. Thus, there remains a requirement for higher-quality data.
Furthermore, it was unexpected to discover that the frequency of ADAs as an unfavorable occurrence seemed to vary according to the dosage of tirzepatide in non-Asian individuals. The development of ADAs triggered by weekly formulations of GLP-1 RAs is prevalent [42], whereas the immunogenicity of the GLP-1 RA dulaglutide is low [43]. However, our study demonstrated that all three doses of tirzepatide exhibited a significant increase in ADAs when compared to the control/placebo group among non-Asian individuals. This finding suggests that the enhanced molecular composition of tirzepatide may contribute to its heightened immunogenicity. Consequently, it is imperative to exercise appropriate caution when considering the immunogenicity and long-term safety of tirzepatide in subsequent investigations.
To the best of our knowledge, this study represents the first systematic review and meta-analysis examining the effects of tirzepatide across diverse ethnic populations. However, we must acknowledge the inherent limitations of our survey, such as its focus on Asian populations from China, India, Japan, and South Korea. Additionally, it is important to note that as a result of the lack of clinical data on this novel agent, further high-quality clinical studies are needed in the future.
Conclusions
Our systematic review and meta-analysis of six RCTs showed that tirzepatide has different efficacy in controlling glycemic index and body weight among Asians and non-Asians, when compared with placebo and other glucose-lowering agents. Asians were more likely to experience weight loss and gastrointestinal adverse events, whereas non-Asians were more likely to have better glycemic control and more metabolic and nutritional disorders. This provides a basis for dose adjustment in different races. This paper adds to the growing body of knowledge showing different antidiabetic drug responses in Asians and non-Asians. Such studies if confirmed by other groups could pave the way for precision treatment of diabetes and obesity which are rapidly escalating globally in Asia.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Sun H, Saeedi P, Karuranga S, et al. IDF diabetes atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119.
Kwan TW, Wong SS, Hong Y, et al. Epidemiology of diabetes and atherosclerotic cardiovascular disease among Asian American adults: implications, management, and future directions: a scientific statement from the American Heart Association. Circulation. 2023;148(1):74–94.
McKeigue PM, Shah B, Marmot MG. Relation of central obesity and insulin resistance with high diabetes prevalence and cardiovascular risk in South Asians. Lancet. 1991;337(8738):382–6.
Anjana RM, Unnikrishnan R, Deepa M, et al. Metabolic non-communicable disease health report of India: the ICMR-INDIAB national cross-sectional study (ICMR-INDIAB-17). Lancet Diabetes Endocrinol. 2023;11(7):474–89.
Seino Y, Yamazaki Y, Yabe D. The AsianAssociation for the Study of Diabetes: the first 10 years and the next 10 years. J Diabetes Investig. 2020;11(5):1079–84.
Seino Y, Fukushima M, Yabe D. GIP and GLP-1, the two incretin hormones: similarities and differences. J Diabetes Investig. 2010;1(1–2):8–23.
Min T, Bain SC. The role of tirzepatide, dual GIP and GLP-1 receptor agonist, in the management of type 2 diabetes: the SURPASS clinical trials. Diabetes Ther. 2021;12(1):143–57.
Nauck MA, Meier JJ. The incretin effect in healthy individuals and those with type 2 diabetes: physiology, pathophysiology, and response to therapeutic interventions. Lancet Diabetes Endocrinol. 2016;4(6):525–36.
Frias JP, Nauck MA, Van J, et al. Efficacy and safety of LY3298176, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: a randomised, placebo-controlled and active comparator-controlled phase 2 trial. Lancet. 2018;392(10160):2180–93.
Finan B, Yang B, Ottaway N, et al. A rationally designed monomeric peptide triagonist corrects obesity and diabetes in rodents. Nat Med. 2015;21(1):27–36.
Dahl D, Onishi Y, Norwood P, et al. Effect of subcutaneous tirzepatide vs placebo added to titrated insulin glargine on glycemic control in patients with type 2 diabetes: the SURPASS-5 randomized clinical trial. JAMA. 2022;327(6):534–45.
Bhagavathula AS, Vidyasagar K, Tesfaye W. Efficacy and safety of tirzepatide in patients with type 2 diabetes mellitus: a systematic review and meta-analysis of randomized phase II/III trials. Pharmaceuticals (Basel). 2021;14(10):991.
Karagiannis T, Avgerinos I, Liakos A, et al. Management of type 2 diabetes with the dual GIP/GLP-1 receptor agonist tirzepatide: a systematic review and meta-analysis. Diabetologia. 2022;65(8):1251–61.
ElSayed NA, Aleppo G, Aroda VR, et al. 9. Pharmacologic approaches to glycemic treatment: standards of care in diabetes-2023. Diabetes Care. 2023;46(Suppl 1):S140-s57.
Ghouri N, Purves D, McConnachie A, Wilson J, Gill JM, Sattar N. Lower cardiorespiratory fitness contributes to increased insulin resistance and fasting glycaemia in middle-aged South Asian compared with European men living in the UK. Diabetologia. 2013;56(10):2238–49.
Gan S, Dawed AY, Donnelly LA, et al. Efficacy of modern diabetes treatments DPP-4i, SGLT-2i, and GLP-1RA in white and Asian patients with diabetes: a systematic review and meta-analysis of randomized controlled trials. Diabetes Care. 2020;43(8):1948–57.
Mohan V, Yang W, Son HY, et al. Efficacy and safety of sitagliptin in the treatment of patients with type 2 diabetes in China, India, and Korea. Diabetes Res Clin Pract. 2009;83(1):106–16.
Ma RC, Chan JC. Type 2 diabetes in East Asians: similarities and differences with populations in Europe and the United States. Ann N Y Acad Sci. 2013;1281(1):64–91.
Sandeep S, Velmurugan K, Deepa R, Mohan V. Serum visfatin in relation to visceral fat, obesity, and type 2 diabetes mellitus in Asian Indians. Metabolism. 2007;56(4):565–70.
Sandeep S, Gokulakrishnan K, Velmurugan K, Deepa M, Mohan V. Visceral & subcutaneous abdominal fat in relation to insulin resistance & metabolic syndrome in non-diabetic south Indians. Indian J Med Res. 2010;131:629–35.
Anjana M, Sandeep S, Deepa R, Vimaleswaran KS, Farooq S, Mohan V. Visceral and central abdominal fat and anthropometry in relation to diabetes in Asian Indians. Diabetes Care. 2004;27(12):2948–53.
Ke C, Narayan KMV, Chan JCN, Jha P, Shah BR. Pathophysiology, phenotypes and management of type 2 diabetes mellitus in Indian and Chinese populations. Nat Rev Endocrinol. 2022;18(7):413–32.
Chiu M, Austin PC, Manuel DG, Shah BR, Tu JV. Deriving ethnic-specific BMI cutoff points for assessing diabetes risk. Diabetes Care. 2011;34(8):1741–8.
Shibayama T, Noguchi H, Takahashi H, Tamiya N. Relationship between social engagement and diabetes incidence in a middle-aged population: results from a longitudinal nationwide survey in Japan. J Diabetes Investig. 2018;9(5):1060–6.
Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16.
Melsen WG, Bootsma MC, Rovers MM, Bonten MJ. The effects of clinical and statistical heterogeneity on the predictive values of results from meta-analyses. Clin Microbiol Infect. 2014;20(2):123–9.
Cumpston M, Li T, Page MJ, et al. Updated guidance for trusted systematic reviews: a new edition of the cochrane handbook for systematic reviews of interventions. Cochrane Database Syst Rev. 2019;10(10):000142.
Inagaki N, Takeuchi M, Oura T, Imaoka T, Seino Y. Efficacy and safety of tirzepatide monotherapy compared with dulaglutide in Japanese patients with type 2 diabetes (SURPASS J-mono): a double-blind, multicentre, randomised, phase 3 trial. Lancet Diabetes Endocrinol. 2022;10(9):623–33.
Furihata K, Mimura H, Urva S, Oura T, Ohwaki K, Imaoka T. A phase 1 multiple-ascending dose study of tirzepatide in Japanese participants with type 2 diabetes. Diabetes Obes Metab. 2022;24(2):239–46.
Gao L, Lee BW, Chawla M, et al. Tirzepatide versus insulin glargine as second-line or third-line therapy in type 2 diabetes in the Asia-Pacific region: the SURPASS-AP-Combo trial. Nat Med. 2023;29(6):1500–10.
Frias JP, Nauck MA, Van J, et al. Efficacy and tolerability of tirzepatide, a dual glucose-dependent insulinotropic peptide and glucagon-like peptide-1 receptor agonist in patients with type 2 diabetes: a 12-week, randomized, double-blind, placebo-controlled study to evaluate different dose-escalation regimens. Diabetes Obes Metab. 2020;22(6):938–46.
Heise T, Mari A, DeVries JH, et al. Effects of subcutaneous tirzepatide versus placebo or semaglutide on pancreatic islet function and insulin sensitivity in adults with type 2 diabetes: a multicentre, randomised, double-blind, parallel-arm, phase 1 clinical trial. Lancet Diabetes Endocrinol. 2022;10(6):418–29.
Ogurtsova K, da Rocha Fernandes JD, Huang Y, et al. IDF diabetes atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. 2017;128:40–50.
Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94(3):311–21.
Permana H, Yanto TA, Hariyanto TI. Efficacy and safety of tirzepatide as novel treatment for type 2 diabetes: a systematic review and meta-analysis of randomized clinical trials. Diabetes Metab Syndrome. 2022;16(11): 102640.
Kiyosue A, Dunn JP, Cui X, et al. Safety and efficacy analyses across age and body mass index subgroups in East Asian participants with type 2 diabetes in the phase 3 tirzepatide studies (SURPASS programme). Diabetes Obes Metab. 2023;25(4):1056–67.
Kim YG, Hahn S, Oh TJ, Park KS, Cho YM. Differences in the HbA1c-lowering efficacy of glucagon-like peptide-1 analogues between Asians and non-Asians: a systematic review and meta-analysis. Diabetes Obes Metab. 2014;16(10):900–9.
Frías JP, Davies MJ, Rosenstock J, et al. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N Engl J Med. 2021;385(6):503–15.
Rosenstock J, Wysham C, Frías JP, et al. Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): a double-blind, randomised, phase 3 trial. Lancet. 2021;398(10295):143–55.
Sun F, Chai S, Yu K, et al. Gastrointestinal adverse events of glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes: a systematic review and network meta-analysis. Diabetes Technol Ther. 2015;17(1):35–42.
Tovey MG, Lallemand C. Immunogenicity and other problems associated with the use of biopharmaceuticals. Ther Adv Drug Saf. 2011;2(3):113–28.
Milicevic Z, Anglin G, Harper K, et al. Low incidence of anti-drug antibodies in patients with type 2 diabetes treated with once-weekly glucagon-like peptide-1 receptor agonist dulaglutide. Diabetes Obes Metab. 2016;18(5):533–6.
Funding
This work was funded by National Natural Science Foundation of China Grants (No. 82170847). The authors are funding the journal’s Rapid Service Fee.
Author information
Authors and Affiliations
Contributions
YC: conceptualization, methodology, visualization, and writing-original draft; JY and XQ: formal analysis and data curation; CG: writing review and editing; DK: writing review and editing; JD: supervision; LL: project administration and funding acquisition.
Corresponding authors
Ethics declarations
Conflict of Interest
Yuying Cui, Jinming Yao, Xiaodong Qiu, Congcong Guo, Degang Kong, Jianjun Dong, and Lin Liao have nothing to disclose.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Cui, Y., Yao, J., Qiu, X. et al. Comparative Efficacy and Safety of Tirzepatide in Asians and Non-Asians with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Diabetes Ther 15, 781–799 (2024). https://doi.org/10.1007/s13300-024-01540-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-024-01540-7