Abstract
Introduction
Recent studies have shown that the quality of life (QOL) of people living with type 1 diabetes (T1D) is poor and must be improved. However, the living situation and QOL of adults living with T1D in Japan have not been fully clarified. This study will examine their lifestyle, QOL, and clinical situation, as well as the relationships between them.
Methods
This is a prospective, 5-year follow-up observational study. Between December 2019 and September 2021, we enrolled adults in Japan who were living with T1D and receiving insulin therapy, and are acquiring longitudinal clinical data and the responses to seven questionnaires regarding lifestyle and QOL. The primary study outcomes are (1) the relationship between Problem Areas in Diabetes (PAID) scores and various factors including demographic data, clinical characteristics, medical history, lifestyle habits, treatment history, biochemical data, and the scores of questionnaires; and (2) the relationship between Beck Depression Inventory (BDI)-II scores and various factors aforementioned. The secondary outcomes are the relationships between various factors aforementioned and each of the following: (1) blood glucose control, (2) blood lipid control, (3) dietary patterns, (4) fear of hypoglycemia, (5) sleep patterns, and (6) physical activity.
Planned outcome
We registered 352 participants. The median age was 49 (41–63) years, and the median duration of T1D was 13 (8–20) years. All the results will be available in 2026. We expect to clarify the factors associated with decreased QOL, and that this knowledge will contribute to improving QOL in adults in Japan who are living with T1D and receiving insulin therapy.
Trial Registration
Clinical Trials.gov identifier, UMIN000044088.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Recent studies have demonstrated that the quality of life (QOL) of people living with type 1 diabetes (T1D) is poor and must be improved. However, the living situation and QOL of adults living with T1D in Japan have not been fully clarified. |
This is a prospective, 5-year follow-up observational study in which 352 participants completed seven questionnaires at baseline. The study will investigate participant lifestyle, QOL, and clinical situation, as well as the relationships between them. |
We expect to clarify the factors associated with decreased QOL, and that this knowledge will contribute to improved QOL in adults in Japan who are living with T1D and receiving insulin therapy. |
Introduction
Type 1 diabetes (T1D) is a disease caused by the destruction of insulin-producing beta cells in the pancreas [1]. In many cases, insulin secretion is severely impaired, resulting in frequent, unexpected episodes of hyperglycemia and hypoglycemia [2], making strict glycemic control difficult. In addition, quality of life (QOL) in people with T1D is often significantly reduced for a number of reasons, such as the need for injection therapy, and therefore depression [3, 4] and eating disorders [5, 6] are common. Furthermore, people with T1D are more strongly affected by the stigma of diabetes than those with type 2 diabetes [7]. In Japan, as in other countries, new treatment approaches continue to be introduced, including insulin pumps, continuous glucose monitoring (CGM), and intermittently scanned CGM (isCGM). However, many individuals cannot fully benefit from them because of financial burdens and the lack of necessary skills.
In Japan, it is estimated that there are approximately 100,000–140,000 people with T1D (prevalence rate for all ages: approximately 0.09–0.11%) [8]. Individuals with pediatric-onset T1D are registered for public financial support, and many studies have been conducted [9,10,11,12,13,14]. On the other hand, epidemiological studies on adult-onset and adult T1D are in progress [15,16,17,18,19,20], but detailed data, especially on lifestyle and QOL, remain insufficient.
In this study, we will mainly be investigating lifestyle and QOL in adults with T1D using questionnaires such as the Problem Areas in Diabetes (PAID) and Beck Depression Inventory (BDI)-II, and will also analyze the relationships between the scores of these questionnaires and various factors including demographic data, clinical characteristics, medical history, lifestyle habits, treatment history, biochemical data, and the scores of questionnaires.
Methods
Study Design
This is a prospective, observational study. The study has been registered in the University Hospital Medical Information Network Clinical Trials Registry (UMIN-CTR), which is a nonprofit organization in Japan that meets the requirements of the International Committee of Medical Journal Editors (ICMJE) (UMIN000044088).
Study Population
The inclusion criteria of the study subjects were as follows: (1) aged 20 years or older (regardless of gender), (2) clinically diagnosed with T1D according to the diagnostic criteria of the Japan Diabetes Society, and (3) receiving insulin therapy. The exclusion criteria were as follows: (1) diabetes mellitus other than T1D; (2) inability to understand the nature and purpose of the study, inability to read or write, or inability to perform the study protocol, e.g., inability or unwillingness to complete the questionnaires.
People with T1D who regularly attended the outpatient diabetes clinics of Juntendo University (Tokyo) and Aso clinic (Shizuoka) in Japan were consecutively screened, and those who met the aforementioned eligibility criteria were asked to participate. All individuals who agreed to participate were enrolled in the study.
Ethical Approval
The protocol was approved by the institutional review board of each participating institution in compliance with the Declaration of Helsinki and current legal regulations in Japan (Juntendo University Ethics Committee Approval Nos. H19-0169, H19-0231, Aso Clinic Ethics Committee Approval Nos. A2020001, A2019002). Written informed consent to participate and for publication was obtained from all participants after they have received a full explanation of the study.
Observation Parameters and Schedule
The observation parameters and schedule are shown in Table 1. The study period was defined as the 260 weeks after the end of registration (registration period: December 2019 to September 2021; full study duration: December 2019 to September 2026). Patients will visit the study institutions for routine visits during the study period. The following data were collected at baseline using medical records and standardized case record forms after informed consent was obtained: demographic data; clinical characteristics, including T1D subtypes according to the diagnostic criteria of the Japan Diabetes Society (fulminant T1D: FT1D; acute-onset T1D: AT1D; and slowly progressive T1D: SPIDDM) [21,22,23,24]; medical history; lifestyle habits; and treatment history.
This is an observational study, and all treatment decisions will be made by the treating investigators according to usual care protocols. All patients will be followed until the end of the scheduled study period regardless of any changes in treatment for diabetes or other conditions. Clinical and biochemical data were collected at baseline with a ± 8-week buffer period, followed by further collections every 52 weeks with a ± 8-week buffer period. Blood samples were obtained at baseline, with additional samples obtained every 52 weeks thereafter. Standard techniques were used to assess the following blood parameters: white blood cell count, red blood cell count, hemoglobin, hematocrit, platelet count, aminotransferase, alanine aminotransferase, γ-glutamyl transpeptidase, creatinine, uric acid lipids, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides, HbA1c (National Glycohemoglobin Standardization Program) [25], casual glucose, and casual C-peptide. The estimated glomerular filtration rate (eGFR) will be calculated using the following formula: eGFR (ml/min per 1.73 m2) = 194 × age−0.287 × serum creatinine−0.1094 (× 0.739 for females) [26]. Urinary albumin excretion was measured by a latex agglutination assay using a spot urine sample at baseline, followed by additional measurements every 52 weeks with a ± 8-week buffer period. Events such as death, hospitalization, cancer, ischemic heart disease, cerebrovascular disease, and severe hypoglycemia will be recorded during the 260-week follow-up period.
The following self-survey questionnaires were administered at the beginning of the study, and will also be administered at its end: (1) PAID, a survey on diabetes and QOL with questions divided into four main areas, specifically diabetes-related emotional problems, social support-related problems, treatment-related problems, and food-related problems [27]; (2) BDI-II, a questionnaire on depression where the total score indicates depression severity, with 0–13 points being extremely mild, 14–19 points being mild, 20–28 points being moderate, and 29–63 points being severe [28, 29]; (3) Hypoglycemic Fear Survey (HFS-II), a survey on anxiety about hypoglycemia, divided into two scales, specifically 15 items related to behavior (HFS-B) and 18 items related to worry (HFS-W) [30,31,32]; (4) Morningness–eveningness Questionnaire (MEQ), a survey to determine if peak alertness is in the morning or evening [33]; (5) Pittsburgh Sleep Quality Index (PSQI), a survey on sleep quality [34, 35]; (6) International Physical Activity Questionnaire (IPAQ), a survey on physical activity [36]; and (7) Brief, Self-administered Diet History Questionnaire (BDHQ), a survey on dietary energy intake [37]. A standardized case record form was used to record other lifestyle-related data (work details, family structure, educational background, number of meals per day, etc.) and events (adverse event onset date, outcome, and nature of event).
Study outcomes
The primary study outcomes are:
-
1.
The relationship between PAID scores and various factors including demographic data, clinical characteristics, medical history, lifestyle habits, treatment history, biochemical data, and the scores of questionnaires;
-
2.
The relationship between BDI-II scores and various factors aforementioned.
The secondary outcomes are:
-
1.
The relationship between blood glucose control and various factors including demographic data, clinical characteristics, medical history, lifestyle habits, treatment history, biochemical data and the scores of questionnaires;
-
2.
The relationship between blood lipid control and various factors aforementioned;
-
3.
The relationship between dietary patterns, defined using the BDHQ, and various factors aforementioned;
-
4.
The relationship between fear of hypoglycemia, defined using the HFS-II, and various factors aforementioned;
-
5.
The relationship between sleep patterns, defined using the MEQ and PSQI, and various factors aforementioned;
-
6.
The relationship between physical activity, defined using the IPAQ, and various factors aforementioned;
Statistical Analysis
Data are expressed as the mean ± standard deviation for normally distributed data, and as medians (interquartile range [IQR, i.e.,25th–75th percentiles]) for data with skewed distributions. Analyses of study outcomes will be performed mainly on the full analysis set.
For the primary study outcome, the relationships between the scores of the questionnaires (PAID and BDI-II) and various factors will be evaluated using t tests, Wilcoxon signed-rank tests, paired t tests and Mann-Whitney U tests when significance tests are needed for two groups. ANOVA and the Kruskal–Wallis tests will be performed when significance tests are needed for three or more groups. Pearson’s correlation coefficient and Spearman’s rank correlation coefficient will be calculated to examine correlation. Chi-square and Fisher’s exact tests will be performed for categorical data. A multiple regression analysis will be performed to identify significant predictors. For the secondary study outcomes, the relationships between blood glucose control, blood lipid control, dietary patterns, fear of hypoglycemia, sleep patterns, physical activity, and various factors will be analyzed in the same manner as the primary outcomes. The statistical tests will be two-sided with a significance level of 5%, and will be carried out using the JMP Pro statistical software package, version 16.2.0 (SAS Institute, Cary, NC, USA).
Data Collection and Analysis
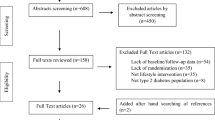
This is a prospective, 5-year follow-up observational study. Enrollment began in December 2019 and ended in September 2021. Patients who met the aforementioned eligibility criteria were consecutively asked to participate in the study, and 352 were finally enrolled. Five individuals with less than one year of history of T1D were included in this cohort. The baseline characteristics of the 352 subjects with T1D are summarized in Table 2. The median age was 49 (41–63) years, 41% were male, the median age of onset was 34 (23–49) years, 9.6% of subjects developed T1D before 15 years old, and the median duration of T1D was 13 (8–20) years. The percentages of subjects with each type of T1D as classified by the Japan Diabetes Society in 2020 [21,22,23,24] were as follows: FT1D, 4.3%; AT1D, 75.5%; and SPIDDM, 20.2%. The median BMI was 22.5 kg/m2 (20.7–24.7), and about 21% of subjects were obese (BMI ≥ 25 kg/m2 is considered obese in Japan [38]). The median HbA1c at enrollment was 7.8% (7.1–8.6), and blood pressure and lipids were well controlled. Liver and renal function were well preserved.
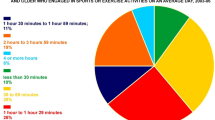
With respect to treatment regimens and technologies to manage T1D, insulin pumps were used by 23% of all patients, and CGM or isCGM was used by 41%. The total daily insulin use was 0.56 (0.41–0.7) units/kg (32 [23–45.6] units/day) (Table 3). With respect to diabetic microvascular complications, the prevalences of diabetic retinopathy, nephropathy, and neuropathy were 21, 20, and 24%, respectively. The prevalences of ischemic heart disease and cerebrovascular disease were 8% and 4%, respectively (Table 4). The questionnaire scores were as follows: PAID (Total), 47 (33–59); BDI-II (Total), 10 (6–16); HSF-II (Total), 23 (11–34); MEQ, 55 (49–61); and PSQI, 5 (4–8). Physical Activity on the IPAQ was 1211 (480–2772) (METs/minutes/week). Dietary data from the BDHQ showed that total energy intake was 1651 (1316–2026) kcal/day, carbohydrate intake was 200 (153–254) g/day, protein intake was 64 (51–80) g/day, and fat intake was 53 (40–68) g/day (Table 5). Regarding the PAID score, Fujii et al. previously reported that the mean score of 591 Japanese individuals with type 2 diabetes was 41.2 ± 16.5 [39], which, as expected, was better than the mean score of the subjects with T1D in this study. Also, the BDI-II score in a Japanese general population (N = 886) was reported to be 8.74 ± 6.44 [29], which was better than that in this study, as anticipated.
Strengths and Limitations of the Study Protocol
Research on adult T1D has certainly progressed in Japan [15,16,17,18,19,20]. However, no reports thus far have been as detailed as our study, which involves the administration of seven different questionnaires on various areas such as diet, exercise, sleep, and QOL, and tracking this information for 5 years. Detailed data from this cohort will provide a more detailed picture of adult T1D. On the other hand, our study protocol has several limitations. The main one is the explanatory nature of the study. The use of a two-center cohort in Japan may lead to limited conclusions. Additionally, the results may not perfectly reflect reality due to withdrawal of subject consent, missing questionnaire data, and the fact that questionnaires were not obtained from all subjects due to the exclusion criteria.
Conclusions
We completed the subject registration in September 2021, and the results will be available in 2026. At the baseline, 352 participants completed seven questionnaires. This study will help determine what factors, if any, contribute to a decline in QOL by yielding information on lifestyle, QOL, and clinical situation, as well as the relationships between them. If, while providing medical care, we are able to eliminate as much as possible the factors that burden people with T1D, it will certainly improve QOL. We assume that this study will help build a foundation for interventions to optimize the quality of care for Japanese adults with T1D.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet. 2014;383(9911):69–82. https://doi.org/10.1016/s0140-6736(13)60591-7. (Epub 2013/07/26).
Bertuzzi F, Verzaro R, Provenzano V, Ricordi C. Brittle type 1 diabetes mellitus. Curr Med Chem. 2007;14(16):1739–44. https://doi.org/10.2174/092986707781058922.
Bassi G, Mancinelli E, Di Riso D, Salcuni S. Parental stress, anxiety and depression symptoms associated with self-efficacy in paediatric type 1 diabetes: a literature review. Int J Environ Res Public Health. 2020. https://doi.org/10.3390/ijerph18010152. (Epub 2020/12/28).
Buchberger B, Huppertz H, Krabbe L, Lux B, Mattivi JT, Siafarikas A. Symptoms of depression and anxiety in youth with type 1 diabetes: a systematic review and meta-analysis. Psychoneuroendocrinology. 2016;70:70–84. https://doi.org/10.1016/j.psyneuen.2016.04.019. (Epub 2016/04/29).
Winston AP. Eating disorders and diabetes. Curr Diab Rep. 2020;20(8):32. https://doi.org/10.1007/s11892-020-01320-0. (Epub 2020/06/15).
Toni G, Berioli MG, Cerquiglini L, Ceccarini G, Grohmann U, Principi N, et al. Eating disorders and disordered eating symptoms in adolescents with type 1 diabetes. Nutrients. 2017;9(8):906. https://doi.org/10.3390/nu9080906. (Epub 2017/08/19).
Liu NF, Brown AS, Folias AE, Younge MF, Guzman SJ, Close KL, et al. Stigma in people with type 1 or type 2 diabetes. Clin Diabetes. 2017;35(1):27–34. https://doi.org/10.2337/cd16-0020.
Report of a research grant from the Ministry of Health, Labor and Welfare (H26-Junkanki-Ippan-003). https://mhlw-grants.niph.go.jp/project/25298.
Kitagawa T, Owada M, Urakami T, Tajima N. Epidemiology of type 1 (insulin-dependent) and type 2 (non-insulin-dependent) diabetes mellitus in Japanese children. Diabetes Res Clin Pract. 1994;24(Suppl):S7-13. https://doi.org/10.1016/0168-8227(94)90221-6.
Matsuura N, Yokota Y, Kazahari K, Sasaki N, Amemiya S, Ito Y, et al. The Japanese Study Group of Insulin Therapy for Childhood and Adolescent Diabetes (JSGIT): initial aims and impact of the family history of type 1 diabetes mellitus in Japanese children. Pediatr Diabetes. 2001;2(4):160–9. https://doi.org/10.1034/j.1399-5448.2001.20404.x.
Nakamura N, Sasaki N, Kida K, Matsuura N. Health-related and diabetes-related quality of life (QOL) in Japanese children and adolescents with type 1 and type 2 diabetes. Pediatr Int. 2009;1:1–52. https://doi.org/10.1111/j.1442-200X.2009.02918.x. (Epub 2009/07/06).
Onda Y, Nishimura R, Morimoto A, Sano H, Utsunomiya K, Tajima N. Causes of death in patients with childhood-onset type 1 diabetes receiving dialysis in Japan: Diabetes Epidemiology Research International (DERI) Mortality Study. J Diabetes Complic. 2015;29(7):903–7. https://doi.org/10.1016/j.jdiacomp.2015.05.014. (Epub 2015/05/29).
Onda Y, Sugihara S, Ogata T, Yokoya S, Yokoyama T, Tajima N. Incidence and prevalence of childhood-onset type 1 diabetes in Japan: the T1D study. Diabet Med. 2017;34(7):909–15. https://doi.org/10.1111/dme.13295. (Epub 2017/02/02).
Takaike H, Miura J, Ishizawa K, Babazono T. High prevalence of depressive symptoms among people with pediatric-onset and adolescent-onset type 1 diabetes: a cross-sectional analysis of the Diabetes Study from the Center of Tokyo Women’s Medical University. J Diabetes Investig. 2022;13(9):1626–32. https://doi.org/10.1111/jdi.13835. (Epub 2022/06/16).
Abiru N, Shimada A, Nishimura R, Matsuhisa M, Ozaki A, Ikegami H. Glycemic control status, diabetes management patterns, and clinical characteristics of adults with type 1 diabetes in Japan: study of Adults’ Glycemia in T1DM subanalysis. Diabetol Int. 2021;12(4):460–73. https://doi.org/10.1007/s13340-021-00504-7. (Epub 2021/04/25).
Chujo D, Imagawa A, Yasuda K, Abiru N, Awata T, Fukui T, et al. Japanese type 1 diabetes database study (TIDE-J): rationale and study design. Diabetol Int. 2022;13(1):288–94. https://doi.org/10.1007/s13340-021-00541-2. (Epub 2021/09/06).
Oikawa Y, Hashimoto K, Hara K, Morimoto J, Namai K, Tanaka A, et al. Current clinical state of type 1 diabetes in Saitama prefecture. Diabetol Int. 2022;13(2):436–46. https://doi.org/10.1007/s13340-021-00557-8. (Epub 2021/11/01).
Takagi S, Miura J, Takita M, Mochizuki S, Asanuma T, Hoshina S, et al. Factors associated with hypoglycemia unawareness and severe hypoglycemia in type 1 diabetes mellitus patients. J Diabetes Investig. 2022;13(12):2018–26. https://doi.org/10.1111/jdi.13886. (Epub 2022/08/16).
Kawasaki E, Shimada A, Imagawa A, Abiru N, Awata T, Oikawa Y, et al. Bivalent GAD autoantibody ELISA improves clinical utility and risk prediction for adult autoimmune diabetes. J Diabetes Investig. 2023;14(4):570–81. https://doi.org/10.1111/jdi.13980. (Epub 2023/01/23).
Nishimura R, Shimada A, Abiru N, Matsuhisa M, Takahashi Y, Ikegami H. Association between glycemic control and patient-reported outcomes in adults with type 1 diabetes in Japan: the SAGE study subanalysis. Diabetol Int. 2023. https://doi.org/10.1007/s13340-023-00668-4.
Araki E, Goto A, Kondo T, Noda M, Noto H, Origasa H, et al. Japanese clinical practice guideline for diabetes 2019. J Diabetes Investig. 2020;11(4):1020–76. https://doi.org/10.1111/jdi.13306.
Imagawa A, Hanafusa T, Awata T, Ikegami H, Uchigata Y, Osawa H, et al. Report of the Committee of the Japan Diabetes Society on the Research of Fulminant and Acute-onset Type 1 Diabetes Mellitus: New diagnostic criteria of fulminant type 1 diabetes mellitus (2012). J Diabetes Investig. 2012;3(6):536–9. https://doi.org/10.1111/jdi.12024. (Epub 2012/11/30).
Kawasaki E, Maruyama T, Imagawa A, Awata T, Ikegami H, Uchigata Y, et al. Diagnostic criteria for acute-onset type 1 diabetes mellitus (2012): Report of the Committee of Japan Diabetes Society on the Research of Fulminant and Acute-onset Type 1 Diabetes Mellitus. J Diabetes Investig. 2014;5(1):115–8. https://doi.org/10.1111/jdi.12119. (Epub 2013/08/13).
JDS. Diagnostic Criteria for SPIDDM (2023). 2023. http://www.jds.or.jp/modules/study/index.php?content_id=50].
Kashiwagi A, Kasuga M, Araki E, Oka Y, Hanafusa T, Ito H, et al. International clinical harmonization of glycated hemoglobin in Japan: from Japan Diabetes Society to National Glycohemoglobin Standardization Program values. J Diabetes Investig. 2012;3(1):39–40. https://doi.org/10.1111/j.2040-1124.2012.00207.x.
Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53(6):982–92. https://doi.org/10.1053/j.ajkd.2008.12.034. (Epub 2009/04/01).
Polonsky WH, Anderson BJ, Lohrer PA, Welch G, Jacobson AM, Aponte JE, et al. Assessment of diabetes-related distress. Diabetes Care. 1995;18(6):754–60. https://doi.org/10.2337/diacare.18.6.754.
Beck ATSR, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio: Psychological Corporation; 1996.
Beck AT, Steer RA, Brown GK, Kojima M, Furukawa T. Manual for the Beck Depression Inventory. 2nd ed. Tokyo: Nihon Bunka Kagakusha; 2003.
Cox DJ, Irvine A, Gonder-Frederick L, Nowacek G, Butterfield J. Fear of hypoglycemia: quantification, validation, and utilization. Diabetes Care. 1987;10(5):617–21. https://doi.org/10.2337/diacare.10.5.617.
Gonder-Frederick LA, Schmidt KM, Vajda KA, Greear ML, Singh H, Shepard JA, et al. Psychometric properties of the hypoglycemia fear survey-ii for adults with type 1 diabetes. Diabetes Care. 2011;34(4):801–6. https://doi.org/10.2337/dc10-1343. (Epub 2011/02/23).
Maclean RH, Jacob P, Choudhary P, Heller SR, Toschi E, Kariyawasam D, et al. Hypoglycemia subtypes in type 1 diabetes: an exploration of the hypoglycemia fear survey-II. Diabetes Care. 2022;45(3):538–46. https://doi.org/10.2337/dc21-1120.
Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness–eveningness in human circadian rhythms. Int J Chronobiol. 1976;4(2):97–110.
Tsai YW, Kann NH, Tung TH, Chao YJ, Lin CJ, Chang KC, et al. Impact of subjective sleep quality on glycemic control in type 2 diabetes mellitus. Fam Pract. 2012;29(1):30–5. https://doi.org/10.1093/fampra/cmr041. (Epub 2011/07/27).
Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. https://doi.org/10.1016/0165-1781(89)90047-4.
Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–95. https://doi.org/10.1249/01.Mss.0000078924.61453.Fb.
Kobayashi S, Murakami K, Sasaki S, Okubo H, Hirota N, Notsu A, et al. Comparison of relative validity of food group intakes estimated by comprehensive and brief-type self-administered diet history questionnaires against 16 d dietary records in Japanese adults. Public Health Nutr. 2011;14(7):1200–11. https://doi.org/10.1017/s1368980011000504. (Epub 2011/04/11).
The Examination Committee of Criter. New criteria for ‘obesity disease’ in Japan. Circ J. 2002;66(11):987–92. https://doi.org/10.1253/circj.66.987.
Fujii H, Karube N, Tokunaga R, Hakogi M, Nakama K, Hayashi M, BTS, Tamura K, Tsuchiya N, Takamura H, Miyakawa AT. Clinical usefulness of “Problem Areas in Diabetes survey (PAID). J Jpn Diabetes Soc. 2008;51(6):497–505.
Acknowledgements
The authors thank all the study participants, all the clinical staff members for their assistance with the execution of the clinical trial, and Ms. Sayaka Wada, clinical research assistant.
Funding
This research including the journal’s Rapid Service Fee is supported by a 2021 grant from the Japan Diabetes Association (Tokyo, Japan), and by MEXT KAKENHI Grant Number JP23K10809.
Author information
Authors and Affiliations
Contributions
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript. Every author had full access to all of the data in this study, take complete responsibility for the data integrity and the accuracy of data analysis, and have given final approval of the version to be published. Conceptualization: [Junko Sato], Methodology: [Junko Sato, Tomoya Mita, Hirotaka Watada], Formal analysis and investigation: [Junko Sato, Kenichi Nakajima, Mami Koshibu, Ayako Sato], Writing—original draft preparation: [Junko Sato]; Writing—review and editing: [Junko Sato, Hirotaka Watada], Funding acquisition: [Junko Sato, Hirotaka Watada], Resources: [Junko Sato, Kenichi Nakajima, Tomoya Mita, Hiromasa Goto, Fuki Ikeda, Yuya Nishida, Katsumi Aso, Hirotaka Watada], Supervision: [Hirotaka Watada].
Corresponding author
Ethics declarations
Conflict of Interest
Junko Sato has received honoraria for lectures for Abbott and for research activities for Dexcom. Hirotaka Watada has received honoraria for lectures for Bayer Pharma Japan, Teijin Pharma Ltd., MSD, Sanofi-Aventis K.K., Novo Nordisk, Nippon Boehringer Ingelheim, Eli Lilly, Sumitomo Pharma, Mitsubishi Tanabe Pharma, Daiichi Sankyo Company, Ltd, Abbott, Kowa Co., Ltd., Taisho Pharmaceutical, Astellas Pharma, Kissei Pharmaceutical Co., Ltd., AstraZeneca K.K., Ono Pharmaceutical Co. Ltd. Sanwa Kagaku, and Takeda Pharmaceuticals, and for research activities for Takeda Pharmaceuticals, Nippon Boehringer Ingelheim, Kissei Pharmaceutical, Novo Nordisk, Mitsubishi Tanabe Pharma, Lifescan Japan, Kyowa Kirin, Sumitomo Pharma, Eli Lilly, Teijin Pharma, Taisho Pharmaceutical, Abbott, Ono Pharmaceutical Co. Ltd., Soiken Inc., Sanwa Kagaku, and Kowa. Other authors declare no conflicts of interest.
Ethical Approval
All procedures followed are in accordance with the ethical standards of the responsible committees on human experimentation (institutional and national) and with the 1964 Declaration of Helsinki, as revised in 2013. The protocol was approved by the institutional review boards of Juntendo University and Aso Clinic in compliance with the 1964 Declaration of Helsinki and its later amendments, and current legal regulations in Japan (Juntendo University Ethics Committee Approval Nos. H19-0169, H19-0231; Aso Clinic Ethics Committee Approval Nos. A2020001, A2019002). Written informed consent to participate and for publication had been obtained from all study participants.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Sato, J., Nakajima, K., Mita, T. et al. Protocol of a Prospective Observational Study on Lifestyle and Quality of Life in Adults with Type 1 Diabetes in Japan. Diabetes Ther 15, 883–892 (2024). https://doi.org/10.1007/s13300-024-01539-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-024-01539-0