Abstract
Adequate glycemic control is key to prevent morbi-mortality from type 2 diabetes (T2D). Despite the increasing availability of novel, effective, and safe medications for the treatment of T2D, and periodically updated guidelines on its management, the overall rate of glycemic goal attainment remains low (around 50%) and has not improved in the past decade. Therapeutic inertia (TI), defined as the failure to advance or de-intensify medical therapy when appropriate to do so, has been identified as a central contributor to the lack of progress in the rates of HbA1c goal attainment. The time to treatment intensification in patients not meeting glycemic goals has been estimated to be between 1 and 7 years from the time HbA1c exceeded 7%, and often, even when an intervention is carried out, it proves insufficient to achieve glycemic goals, which led to the concept of intensification inertia. Therefore, finding strategies to overcome all forms of TI in the management of T2D is a fundamental initiative, likely to have an enormous impact in health outcomes for people with T2D. There are several factors that have been described in the literature leading to TI, including clinician-related, patient-related, and healthcare system-related factors, which are discussed in this review. Likewise, several interventions addressing TI had been tested, most of them proving limited efficacy. Within the most effective interventions, there appear to be two common factors. First, they involve a team-based effort, including nurses, pharmacists, and diabetes educators. Second, they were built upon a framework based on results of qualitative studies conducted in the same context where they were later implemented, as will be discussed in this article. Given the complex nature of TI, it is crucial to use a research method that allows for an in-depth understanding of the phenomenon. Most of the literature on TI is focused on quantitatively describing its consequences; unfortunately, however, not many study groups have undertaken qualitative studies to deeply investigate the drivers of TI in their diverse contexts. This is particularly true in the United States, where there is an abundance of publications exploring the effects of different strategies to overcome TI in type 2 diabetes, but a severe shortage of qualitative studies aiming to truly understand the phenomenon.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
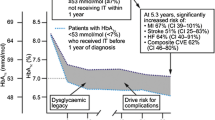
Achieving good glycemic control early in the course of T2D is key to maximizing the protection against micro and macrovascular complications of the disease, leveraging the full benefits of the well-described legacy effect. |
Now that therapeutic options have expanded and proven to be highly effective, therapeutic inertia has become one of the main obstacles to achieving adequate glycemic control in people living with T2D. |
Many approaches and efforts have been tested to address this issue, but after years of such efforts, the results have not been sufficient to overcome it. |
It is reasonable to assume that the factors contributing to TI differ across various contexts. Consequently, we advocate for the use of research methods that facilitate a comprehensive exploration and assessment of the drivers of TI in each setting. We propose the need for locally conducted qualitative studies to gain deeper understanding of the phenomenon and enable the development of context-specific strategies, leveraging team efforts. |
The aim of this narrative review article, compiled by identifying relevant studies via the utilization of PubMed, is to provide a broad overview on the topic of therapeutic inertia as it pertains to type 2 diabetes. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Diabetes: A Global Pandemic
Diabetes is a global pandemic, impacting hundreds of millions worldwide. According to the International Diabetes Federation, as of 2021, more than 537 million adults had diabetes. Projections indicate that its prevalence could escalate to a staggering 643 million cases by 2030 and 783 million cases by 2045. The economic burden of diabetes is immense, exacting a toll of around US $966 billion on global public health systems and accounting for approximately 6.7 million deaths annually [1].
Type 2 diabetes (T2D) accounts for over 90% of the cases of diabetes [1]. It is characterized by a complex interplay of pathophysiologic defects including decreased insulin secretion by pancreatic beta cells, increased glucagon secretion by alpha cells, increased hepatic glucose production, decreased incretin effect, increased lipolysis, increased glucose reabsorption by the kidneys, decreased glucose uptake by muscle cells (insulin resistance), and neurotransmitter dysfunction [2, 3]. These result in the pathogenesis of hyperglycemia, a hallmark of T2D, which in turn increases the risk of microvascular and macrovascular complications that can severely impact patients' health and quality of life [4, 5].
The recognition of the multifaceted nature of T2D has led to the emergence of new therapies targeting different pathophysiologic defects. As a result, there has been an unprecedented expansion of the availability of novel medications to treat T2D, adding significant complexity to its management, but at the same time providing additional therapeutic options to help improve glycemia.
The Relevance of a Proper Therapeutic Approach
The landmark U.K. Prospective Diabetes Study (UKPDS) demonstrated, after a median 10-year follow-up, that in newly diagnosed patients with T2D, tighter glycemic control (measured by HbA1c) significantly reduced the incidence of microvascular complications [5]. During a subsequent observational 10-year follow-up, even though the difference in HbA1c was lost 1 year after the interventional study ended, individuals that had been allocated to the intensive treatment arm still had a 24% lower rate of microvascular complications over the following 10 years [6]. These findings led to the concept of “legacy effect”, in which early (in the course of the disease) intensive glycemic control confers long-term reduction in the risk of chronic complications.
In a recent cohort study including 34,737 individuals with T2D, not achieving an HbA1c of 6.5% in the first year of treatment was associated with a 20% greater risk of microvascular and macrovascular complications over 13 years. Additionally, when a goal of 7.0% was not achieved during the first year, there was a 29% greater likelihood of all-cause death over this period [7].
These findings emphasize not only the need to obtain adequate glycemic control but also the duration of time to achieve it. Despite the overwhelming evidence that early glycemic control offers significant long-term benefits, the reality is that a substantial number of patients still struggle to meet glycemic targets [8, 9].
Current recommendations advocate for a target HbA1c of < 7% or ≤ 6.5% for most individuals with T2D, with a strong emphasis on goal personalization. Aiming for a lower HbA1c level may have value if it can be safely achieved without significant hypoglycemia or other adverse treatment effects. Conversely, less stringent goals are appropriate for patients with limited life expectancy or in whom the potential risks of treatment are greater than the benefits. Moreover, glycemic targets may, and probably will, vary throughout a patient’s life [10, 11].
Besides the glycemic goals, there is strong evidence supporting that in high-risk patients with T2D, newer antidiabetic medications have benefits beyond their glucose-lowering properties. Both SGLT2 inhibitors and GLP-1 receptor agonists have demonstrated to notably impact cardiovascular, renal, and mortality outcomes [12]. The joint American Diabetes Association/European Association for the Study of Diabetes guidelines now recommend the use of SGLT2 inhibitors and/or GLP-1 agonists in individuals with heart failure, chronic kidney disease, multiple cardiovascular risk factors or established cardiovascular disease, independent of HbA1c levels and personalized target goals [11]. Therefore, the use of these medications is now considered a goal in itself, and not just a tool to achieve glycemic targets.
T2D is a progressive disease in terms of gradual decrease of insulin secreting capacity and progression or addition of other pathophysiological defects that also contribute to the development of hyperglycemia [3, 13]. As a result, not only glycemic goals can change but also pharmacological therapy will most likely need periodic modification in order to attain these goals. Even if individuals are diligently following treatment plans, the requirement for further therapeutic interventions is often needed. Therefore, clinicians need to recognize that treatment of T2D is a dynamic process, and they should assist patients in establishing realistic expectations regarding the course of their disease. Patients should be made aware that the necessity for additional therapies over time is not a reflection of a failure in managing their condition; rather, it is an intrinsic part of the natural progression of T2D. The awareness of the anticipated course of the disease may help address some important barriers to achieving optimal glycemic control.
The Concept of Therapeutic Inertia
Inertia, a term originating from the Latin word “iners”, meaning “inactive”, describes a property of matter by which objects tend to remain in their existing state of rest or uniform motion unless that state is changed by an external force [14]. This principle, fundamental in the world of physics, has extended its reach into the realm of healthcare.
The concept of “clinical inertia” (CI) in managing chronic conditions was first introduced in 2001 by Phillips et al. and was defined as “failure of health care providers (HCP) to initiate or intensify therapy when indicated”, despite well-defined therapeutic goals, known evidence-based benefits of achieving them and available effective therapies and practice guidelines to achieve goals (i.e., recognition of the problem, but failure to act) [15].
Okonofua et al. introduced the term “therapeutic inertia” (TI) in 2006, defining it as “providers failure to increase therapy when treatment goals are not met” [16]. In 2017, Khunti and Davies refined the terminology to better differentiate the two concepts. CI, a wider concept, was defined as “lack of adherence to guideline recommendations when appropriate to do so”, encompassing a failure to improve care at many levels of health care beyond medication adjustments, including screenings, referral for prevention programs, cardiovascular risk assessment, prescribing for preventative therapies, nutrition and/or diabetes education referrals, surveillance and management of risk factors, and complications once disease has been diagnosed. Conversely, TI is more specific and was defined as “failure to advance or de-intensify medical therapy when appropriate to do so” [17], and therefore it is mostly related to prescribing decisions made by the healthcare team.
Therapeutic Inertia in Type 2 Diabetes
Despite the availability of multiple evidence-based clinical guidelines that are updated on a yearly basis, and the approval of many new medications and technologies, the translation of the current T2D management guidelines into clinical practice remains suboptimal [9].
An analysis from the National Health and Nutrition Examination Survey (NHANES) reported that almost half of the adults with diabetes in the U.S did not meet the recommended glycemic goals [8], and unfortunately, this does not seem to be improving. A comparative analysis of the NHANES data reports that between the 2007–2010 period and the 2015–2018 period, the percentage of adult participants with diabetes achieving HbA1c < 7% declined from 57.4 to 50.5% [9], despite the increasing availability of newer pharmacological agents that have proven to be highly effective and safe.
Even though population statistics regarding glycemic control provide a general idea of the magnitude of the problem as a whole, it does not provide direct information about TI per se, especially because TI is one key cause for poor metabolic outcomes, but certainly not the only one. To adequately assess TI, it is fundamental to assess what is happening with patient care over time. In that matter, several studies have highlighted the worrisome rates of treatment delays or lack of treatment adjustments in patients with T2D. For instance, in a retrospective analysis using a large US electronic health record (EHR) database, the median time to receive an additional antidiabetic medication was more than 1 year for patients who failed metformin monotherapy [18]. Similarly, a retrospective cohort study with more than 80,000 individuals with T2D in the U.K. demonstrated that treatment intensification in those taking one oral antidiabetic (OAD) agent took about 2 years from the time HbA1c exceeded a threshold of 7%; the delay was about 7 years for adding a third oral agent [19].
Utilizing longitudinal EHR data, Cleveland Clinic conducted an analysis of over 5000 patients with T2D who had not achieved HbA1c goals after 3 months of metformin monotherapy. The study assessed the time taken for intervention, which included addition of an antidiabetic medication, prescription for a weight loss medication, change in metformin dose or regimen, or referral to a dietitian or nutritionist. CI was defined as the absence of intervention within 6 months from the elevated index HbA1c. The average duration until intervention for the entire patient cohort was approximately 14 months. CI was present in 28% of patients with HbA1c ≥ 8% and in 31% and 38% of those with HbA1c ≥ 7.5% and ≥ 7%, respectively [20].
In another retrospective cohort study, conducted at Cleveland Clinic involving 7389 individuals with T2D who maintained HbA1c levels above 7% despite being on a stable regimen of two OAD agents, it was observed that 63% of them did not receive any treatment escalation within 6 months. Most alarming was that even among individuals with HbA1c exceeding 9%, therapy was not intensified in 44% of them [21]. These findings prompted the authors to further study the specific patterns of intensification within the same cohort, finding that among the 37% of patients that did receive a therapeutic intensification intervention, the probability of HbA1c goal attainment was anywhere between 21.6 and 57.3%, depending on the chosen intervention and the baseline HbA1c. Among subjects with HbA1c > 9%, the crude probability of goal attainment was highest with the addition of a GLP1-RA (glucagon-like peptide-1 receptor agonist) or insulin, while OAD dose increase was not significantly different from no intensification at all with regard to probability of goal attainment [22]. This led to the description of another type of inertia, ‘intensification inertia’, which is the implementation of intensification measures that have a very small (or no) chance of getting the HbA1c to target.
In a retrospective analysis of EHR across 22 American Medical Group Association (AMGA) healthcare organizations, including more than 28,000 bolus-naive patients with T2D and two consecutive HbA1c values ≥ 8%, TI (defined as proportion of patients with no observable action and failure to meet the target of HbA1c < 8%) was identified in 46% (range across organizations 24–54%) of patients at 6 months and 27% (21–37%) at 12 months. At 24 months, 12% (9–14%) of patients have still not experienced any observable action and their HbA1c remained above 8%. In this same cohort, the median time taken to achieve control was 272 days from the index date, and it was slightly shorter for patients receiving only oral antidiabetic medications compared to those on baseline regimens that included an injectable medication [23].
A systematic review of 53 studies published between 2004 and 2016, evaluated the global extent of TI in the management of T2D. Most studies reported a median time to treatment intensification of more than 1 year after an HbA1c measurement above target (range, 0.3 to > 7.2 years). It was also demonstrated that TI increased as the number of OAD agents rose and decreased with increasing HbA1c levels [24]. In a retrospective cohort study including more than 105,000 individuals with T2D in the U.K., a delay in treatment intensification by 1 year in conjunction with poor glycemic control significantly increased the risk of myocardial infarction, heart failure, stroke, and composite cardiovascular events [25]. Additionally, TI leading to suboptimal glycemic control has also been associated with a significantly shorter median time to progression of microvascular complications [26].
The Other Side of Therapeutic Inertia
Most literature regarding TI in T2D is based on the lack of treatment intensification. However, as its definition states, TI not only refers to the failure to intensify therapy when goals are not met but also to failure to de-intensify therapy when it is appropriate to do so. In this regard, guidelines are less clear and more subject to individual interpretations in terms of when is it “appropriate to do so”. Moreover, population-based data classically used to assess adequacy of treatment and consequences of TI with measurements such as HbA1c, are less likely to reflect inertia due to failure to de-escalate therapy, since this can occur at any level of HbA1c. Hence, the assessment of the magnitude of TI due to failure to de-intensify is much more complex.
Overtreatment can be defined as the use of a treatment even when the potential harm exceeds the possible benefit [27], and therefore it constitutes a form of TI due to failure to de-intensify or de-prescribe. The concept of overtreatment in T2D is relatively recent. Studies assessing diabetes overtreatment have been mostly published after 2010 and are almost exclusively based on the population aged over 65 years, which is the one for whom diabetes overtreatment is a major issue. Even though overtreatment is considered a bigger issue than undertreatment in older adults [28], given the great heterogeneity in this population, there is no standardized definition of diabetes overtreatment [29]. Nevertheless, it has been reasonably well established that for the vast majority of older patients with diabetes, the harms associated with an HbA1c ≤ 7.5% outweigh the benefits [30]. Additionally, the American Geriatrics Society recommends avoiding the use of short-acting insulin and all sulfonylureas in the treatment of older adults with T2D [31].
A retrospective analysis of 7597 adults with T2D in Europe reported that 44.7% of subjects aged ≥ 65 years treated with insulin or sulfonylureas had an HbA1c ≤ 7%, and 27.1% of these subjects had ischemic heart disease or congestive heart failure. Likewise, 52.9% of people aged ≥ 80 years had HbA1c ≤ 7%, and 50.7% of them were treated with insulin or sulfonylurea [32].
An analysis of 1288 adults aged ≥ 65 years participating in the NHANES between 2001 and 2010, reported that 61.5% of them had HbA1c < 7%, and 41.9% < 6.5%, with no significant differences in glycemic control among the patients’ different health status categories. Among those older adults with HbA1c < 7%, more than half were being treated with insulin or a sulfonylurea, and 4% were being treated with both [33].
On the other hand, it is important to note that the adoption of less strict, personalized goals considering age, life expectancy, frailty, comorbidities, etc., does not explain nor justify poor metabolic control in this population. Deintensification of diabetes treatment does not necessarily entail loosening glycemic control beyond the individualized goals. It is perfectly possible to de-intensify treatment by making it safer and simpler, and yet aim to maintain glycemic control at goals.
It is fundamental to emphasize that overtreatment and TI due to failure to de-intensify can be associated to a wide spectrum of HbA1c levels, and it is not restricted to patients with lower values of HbA1c, nor to older patients. It is not infrequent in clinical practice to encounter patients with above-goal HbA1c who are being treated with medications that can potentially induce hypoglycemia (namely, insulin and/or sulfonylureas) and who are indeed overtreated or overprescribed, and whose poor glycemic control can be explained either by the compensatory physiological and behavioral response to hypoglycemia, or by intentional non-compliance to their prescribed regimen.
Furthermore, with the advent of newer drugs, such as GLP1-RA, dual GLP1/GIP-RA (glucagon-like peptide-1/glucose-dependent insulinotropic polypeptide receptor agonists), SGLT2i (sodium/glucose cotransporter-2 inhibitors) and DPP4i (dipeptidyl peptidase 4 inhibitors), all with a very favorable safety profile, and some of them with very potent glucose lowering properties, de-intensifying (and also simplifying) treatment seems frequently appropriate, not only in older adults, but also in younger patients treated with complex pharmacological regimens, which is a known barrier to achieving adequate glycemic control [34]. There are several reports in the literature supporting that the addition of GLP1-RA or SGLT2i agents to patients already on complex insulin regimens, is able to significantly decrease insulin requirements, number of injections, and even replace mealtime insulin in a significant percentage of patients [35,36,37,38]. This is especially relevant now that it has been demonstrated that many patients with T2D are candidates to these medications (GLP-1RA and/or SGLT2i) not only for glycemic control, but also from a cardiovascular and/or renal perspective.
Factors Contributing to Therapeutic Inertia in Type 2 Diabetes Management
The causes of TI in the management of T2D are complex and multifactorial (hence, not easy to understand in depth), stemming from various factors at the clinician, patient, and healthcare system levels [39, 40]. Recognizing the interplay among these factors is essential to finding effective solutions to overcome TI.
Clinician-Related Factors
Clinician-related factors are central and play a dominant role in the occurrence of TI in the management of T2D. One significant factor is time constraints during patient visits [15], especially affecting clinicians who must manage T2D alongside addressing various other medical conditions. In many cases, intensification of T2D therapy may not occur as frequently as needed due to the limited time available for comprehensive discussions and the decision-making process. This constraint can lead to situations where clinicians may opt for the simpler and quicker approach of increasing doses of existing therapies, even though these interventions might have limited chances of achieving significant improvements in glycemic control. While some clinicians may not be aware of the low likelihood of success, many understand that these incremental changes won't bring patients to their glycemic targets. Nevertheless, they feel that they have somehow addressed the problem by making an adjustment, even if it does not get patients to their goals.
Another important factor is the tendency among some clinicians to overestimate the quality of care they provide [15]. This overestimation inadvertently fosters a sense of complacency in their management, leading them to not proactively initiate necessary adjustments in the treatment plans of their patients, even when clinical targets are not being met.
Insufficient resources and a lack of proper training in care escalation are other significant clinician-related barriers [15]. The rapidly evolving landscape of treatment guidelines frequently creates a sense of uncertainty among clinicians, making it challenging to determine the most suitable treatment strategies for individual patients [39]. Poor communication practices can also often limit the effectiveness of care [41]. Inadequate patient education and communication about their medical conditions can lead to misunderstandings and reluctance of patients to initiate or intensify treatment as recommended by their HCP [42].
Furthermore, clinicians themselves may grapple with certain fears and concerns. For instance, the perceived risk of hypoglycemia and/or weight gain associated with certain diabetes drugs can lead clinicians to exercise extreme caution in prescribing specific medications, fearing potential adverse consequences for their patients [40]. Also, clinicians may overestimate patient resistance to initiate injectable therapies because of fear of injection-induced pain, and/or may not be able to address [42, 43]. These concerns can influence the clinicians’ choice of treatment and their willingness to initiate or intensify it when necessary.
Patient-Related Factors
While TI is considered mostly a clinician-related behavior, its occurrence is heavily influenced by factors beyond the clinician itself. In this context, it is essential to acknowledge the nuanced role that patient-related factors play in the occurrence of TI.
Clinicians frequently recognize that patients themselves encounter barriers that impede the seamless execution of treatment plans, and therefore might become less likely to advance therapy in that context.
Health literacy, defined as the extent to which individuals can acquire, process, understand, and communicate health-related information for informed health decisions [44], is crucial for self-management of T2D. Among patients with T2D, low health literacy is prevalent and closely linked with deficits in their understanding of the disease nature, progression and timely intervention, self-efficacy, and self-care behaviors, and therefore it is a strong predictor of medication non-adherence [45]. When clinicians detect low health literacy, they are less likely to intensify therapy due to safety concerns, especially when this implies adding complexity to the treatment, ultimately compromising goals attainment.
Access to medications and their affordability can also pose substantial barriers to clinicians’ ability to initiate or maintain therapies in the long-term [46]. In many cases, patients may struggle to obtain the necessary medications due to financial constraints, exacerbating the challenges of managing their disease effectively.
Furthermore, psychological barriers can be prevalent among individuals with T2D. This includes a phenomenon known as “psychological insulin resistance”, where patients may resist or delay insulin therapy due to concerns about injections, dependence on medication, or misunderstanding the implications of insulin use [47]. In this same line, the occurrence of diabetes burnout has been described. Burnout typically refers to a state resulting from prolonged exposure to unrelieved stress, which can lead to listlessness, indifference, carelessness, and finally poor self-care, all of which, when experienced by patients with diabetes, are frequently misdiagnosed as non-compliance. People with diabetes need to live tuned-up with their condition 24 h a day, 365 days a year, for their whole lives, with constant need for decision-making, no possibility of delegating responsibilities, and the continuous subliminal fear of complications [48]. When (or if) clinicians become widely sensitive and capable of detecting patients’ burnout, some degree of transient therapeutic inertia could even be the most appropriate approach.
Additionally, some individuals, particularly older adults, may face difficulties due to issues with dexterity and impaired vision, which can restrict their capacity to monitor glucose levels and administer injectable medications [49].
All of these factors, coupled with the common challenge of managing T2D alongside other coexisting health conditions, result in a multifaceted problem for patients that consequently hinders clinicians from prescribing and intensifying treatment, ultimately leading to TI and suboptimal health outcomes.
It is important to make the distinction between the concepts of medication non-adherence and TI. Medication non-adherence occurs when a patient does not initiate or continue care that has been recommended by a HCP, and therefore it is related to patients’ behavior. Therapeutic inertia is a clinician-related behavior that involves the failure to intensify or de-intensify treatment when it is appropriate to do so. It is influenced by several factors that can be intrinsic or extrinsic to the provider, but ultimately manifests as the failure to adjust therapy as needed. Both TI and patient non-adherence are the main contributors to the failure to achieve glycemic targets in the population suffering from T2D [50].
Healthcare System-Related Factors
Factors related to the healthcare systems encompass poor planning and/or coordination in care delivery, hindering effective collaboration among HCP [51]. This can lead to patients experiencing fragmented care, delayed responses, and a lack of continuity in their treatment, ultimately resulting in suboptimal health outcomes. The absence of individualized treatment guidelines, tailored to the unique needs and circumstances of individual patients, further complicates the problem and can also hinder the delivery of optimal care [51].
Additionally, disparities in healthcare settings and available services, influenced by factors like geographic location, socioeconomic status, and healthcare infrastructure, can result in variations in the quality of care received by patients. Furthermore, limitations in insurance coverage can have a profound impact on patients' ability to access the treatments they require [52].
Strategies for Overcoming Therapeutic Inertia
What has been Tried
Several studies have tested interventions to overcome or mitigate TI in the management of T2D.
In a systematic review and meta-analysis, Powell et al. included 36 studies (26 of them performed in USA) and grouped them according to the intervention type in: pharmacist-based interventions (four studies), care management and patient education interventions (20 studies), physician-based interventions (seven studies) or nurse/certified diabetes educator (CDE)-based interventions (five studies). See Fig. 1 for an overview of the different interventions.
Summary and overview of research interventions [53]. 1Certified Diabetes Care and Education Specialist
Among the different categories, all the nurse/CDE and pharmacist-based interventions proved effective at reducing HbA1c, with mean HbA1c reductions ranging from − 1.62% to − 0.40% and − 0.90% to − 0.60%, respectively. The patient education- and physician-based interventions did not achieve statistically significant reductions in HbA1c. As the authors point out, it is important to note that physician-based interventions were directed at the physicians; thus, their impact on patients was indirect, whereas the other intervention types were mediated through direct engagement with the patients, potentially enhancing their efficacy. A shared feature of the nurse/CDE- and pharmacist-led interventions was that providers had the autonomy to initiate and intensify treatment, supported by guidelines, protocols, and collaborative practice agreements. It is worth noting, however, that, with a median intervention duration of 1 year among the studies, a reduction of HbA1c was only observed during the first year, and only in individuals with a baseline HbA1c > 9% [53].
In a systematic review and meta-analysis of clinical trials assessing the impact of information technology on changes in the levels of HbA1c, Alharbi et al. included 32 studies (40,454 patients) using different combinations of technologies: diabetes registry (four studies), electronic medical records (three studies), electronic patient self-management technology (18 studies), electronic decision support systems (seven studies). The use of technologies achieved a significant, but modest reduction in HbA1c in patients with T2D (mean difference − 0.33%, 95% CI − 0.40 to − 0.26, P < 0.001). The subgroup analysis demonstrated that electronic self-management technology had the greatest impact on HbA1c (− 0.5%), while the diabetes registry had the least effect (− 0.05%) [54].
Recently, Pantalone et al. published a retrospective analysis of the effect of an EHR-based diabetes intensification tool on the rate of HbA1c goal achievement, when implemented in a large integrated health system. The tool featured a best practice alert (BPA) notifying physicians of patients with HbA1C ≥ 8% which encouraged therapy intensification. Alternatively, providers could acknowledge the alert and provide a reason for deferring intensification (“other”, “need to assess” or “nonadherence”). Additionally, it facilitated the intensification process through an interactive Smartform offering guidance in the best approach for a particular patient, taking into consideration five key factors: cardiovascular risk reduction, A1C lowering, hypoglycemia risk, treatment cost, and weight loss. After completing the Smartform, providers were guided to a Smartset of pre-populated orders for prescriptions, laboratory tests, consultations, and/or follow-up visits based on the selections and choices made in the Smartform. A total of 5071 HCP were exposed to the tool, but its utilization was only 9.7%, with “other” being the most frequent reason for deferring action. Primary care providers (PCP) had a significantly higher tendency to defer action compared to endocrinology providers. The proportions of patients achieving the HbA1C goal (< 8%) were not significantly improved at sites in which the tool was implemented compared to those in which it was not. The authors conclude that considering the time constraints during office encounters, the use this kind of tool to enhance HbA1C goal achievement might be more effective if implemented asynchronously to office visits and further optimized with provider workflows to facilitate its adoption. They also propose that the scheduling of diabetes-focused visits may also result in greater tool utilization [55].
Filling the Gaps in Knowledge
In-depth knowledge of the context is essential for designing effective interventions. Most of the literature regarding TI in T2D is focused mainly in describing the consequences of therapeutic inertia (i.e., low rate of HbA1c goal attainment, prolonged duration of time to obtain HbA1C goal attainment or to intensify treatment, and failure to improve rates of goal attainment over time), and, when attempting to identify causes of TI, most evidence comes from quantitative studies using structured questionnaire-based surveys.
Qualitative research methodology, originally developed within social sciences, allows researchers to investigate phenomena in a holistic fashion, providing in-depth insights and understanding of real-world problems. To our knowledge, there are only six published studies that have analyzed the causes of TI using a qualitative methodology.
Using semi-structured in-depth interviews to HCP working in primary care in the UK, Zafar et al. observed that they were generally open to accept some responsibility for CI, yet they aimed to temper their accountability by citing barriers associated to patients and the healthcare system. They also discovered that interviewees had inaccurate perceptions about the performance of their clinical practice centers in terms of glycemic control of their patients, with a tendency to overestimation. They tend to utilize their non-specialist role to justify the lack of expertise, citing challenges in staying current with evolving recommendations, new treatments and with interpreting and implementing evidence from trials. Responses regarding the value of expert feedback varied, as some view it positively, while others indicated that it could be perceived as a threat at the individual or practice level [56].
Aiming to elucidate general practitioner’s (GP) beliefs regarding CI and identify modifiable provider-related factors, Aujoulat et al. conducted group interviews including 114 GP in Belgium. They reported that bringing up the topic of CI elicited mixed feelings, initially causing unease for most participants. While they found the discussion interesting, stimulating, and revealing, it also evoked feelings of guilt and the term was often perceived as insulting. There was strong consensus that “failure to initiate or intensify a treatment according to guidelines” is common in general practice. However, participants agreed that most decisions are taken after careful examination of the patients’ context and preferences. They advocated for a redefinition of CI, urging acknowledgement of their health-promoting role, and emphasizing that most decisions arise from complex clinical reasoning, much broader than treating to target. They voiced concern about the numerous, constantly evolving and sometimes conflicting guidelines, making clinical decisions more challenging. Moreover, the validity of guidelines was questioned by some who thought that new guidelines might be issued with marketing purposes of new pharmaceutical products. Ultimately, a feeling of being overwhelmed and experiencing disempowerment emerged as the primary factors associated with the risk of CI. Interestingly, the authors conclude that labeling non-adherence to clinical guidelines as CI without investigating the underlying motives behind decisions not to act or to postpone therapeutic actions could be misleading. They also suggest that the term CI could potentially increase the already existing gap between general practice and specialized care [57].
De Lusignan et al. investigated the patients’ and clinicians’ perceptions on the initiation of injectable therapies in T2D in primary care in the UK, and the context in which those decisions are made. Using a mixed methods design, phase 1 consisted of focus groups with patients and HCP; phase 2 consisted of recorded GP consultations featuring actor-portrayed patient scenarios requiring treatment escalation, and phase 3 were surveys directed to HCP to explore external validity of their findings. Focus groups identified certain barriers to initiation reported by patients, such as lack of knowledge and misconceptions about diabetes and treatment goals; fear (of restriction of lifestyle, of self-administration, of pain, of stigma), and feelings of failure. Facilitators included education, clinician competence and knowledge of diabetes, and good communication. HCP described barriers such as concerns about weight gain, hypoglycemia, and time constraints during consultation. In simulated consultations, GPs acknowledged the need for injectable medication initiation in most consultations where this was the expert recommended option but refrained from offering initiation support themselves. Surveys showed that clinicians lack the required practical skills to initiate injectable therapies and find it difficult to maintain competence [58].
Another mixed-method study by Wrzal et al. performed in Canada aimed to understand patients and providers determinants of behavior related to treatment intensification using focus groups. Some remarkable findings on the providers side were: providers initiate patients on medications following guidelines even when they harbor doubts about long-term efficacy; they reported that they do individualize goals considering age, motivation, comorbidities, etc.; that they tend to not intensify treatment in older patients or those with complex comorbidities, and that referral to specialists is typically reserved as a last resort. They also believe educating patient on the long-term consequences of diabetes does not effectively engage them, and they do not consider hypoglycemia as the primary concern. Their approach involves treatment on a case-by-case basis, emphasizing multiyear strategy for overall health [59].
Berenguera et al. conducted a qualitative study in Spain [60], including patients with uncontrolled T2D (HbA1c ≥ 9%), using semi-structured interviews. The objective was to identify patients’ perceptions, barriers, and facilitators of self-management and then use this information in developing and implementing an intervention strategy they named INTEGRA. Several important findings were reported in terms of patients-related potential drivers of TI:
-
The way in which the diagnosis was communicated impacted how the individual self-managed the disease.
-
The primary challenge they reported was achieving control of the disease (based on laboratory tests), which they attributed to struggles with finding the appropriate glucose-lowering drug.
-
They perceived recommended diets lack individualization and were challenging to implement. Adherence to dietary guidelines was particularly low among immigrant patients due to cultural and socio-economic barriers.
-
Patients felt more or less “diabetic” depending on the treatment they were prescribed, associating injectable treatments with a more serious form of diabetes.
-
To them, “sometimes the visits became an interrogation to identify what they have done incorrectly”, and insufficient time was allocated to address their concerns, fears, and potential disease complications. Few patients recalled the HCP offering reassurance, and some noted a lack of empathy and understanding of their problems, which they attributed to the limited time allowed for the consultation.
Utilizing this information, Molló et al. designed the INTEGRA study [61], a cluster, non-randomized, sham-controlled, pragmatic trial including 406 subjects, testing a multicomponent approach including professional-oriented interventions and patient-centered interventions to improve self-management. The intervention group was given all five components of the tested approach, while the Sham comparator was given four of the five components. After 12 months, the mean HbA1c value decreased from 10.2 to 8.3% in the intervention group (P < 0.001) and from 10.4 to 8.95% (P < 0.001) in the control group. The difference between groups was also statistically significantly (mean difference = − 0.65%, 95% CI − 0.9%, − 0.4%; P < 0.001).
Finally, Furler et al. developed a model of care for supporting GPs and practice nurses (PN) to undertake insulin initiation for patients with T2D. They first used qualitative research involving providers and patients to identify potential facilitators for insulin initiation within general practice in Australia. Using this information, they designed the “Stepping Up” Program, which included simple, understandable tools and algorithms for insulin initiation and titration. The program, led by a diabetes nurse educator (DNE), had three components:
-
1.
Practice briefing visit: the program was detailed to GPs and PNs. Roles and responsibilities of HCP in initiating insulin were discussed.
-
2.
3-h evening training session: Facilitated by a GP with extensive skills in diabetes management and a study DNE. This session focused on the rationale for use of insulin, the protocol for starting and titrating doses and addressing common patient-level barriers (including motivational interviewing and goal-setting strategies).
-
3.
Teamwork: patients came in for a GP consultation. If appropriate, the GP would recommend starting insulin and then the patient saw the PN for further discussion and insulin initiation assessment. If they agreed, the PN gave the first dose of insulin. Follow-up visits with PN were performed on the following day and every 3 days after until the patient was confident to start self-titration protocol.
In 2014, the feasibility study results were published [62], including a qualitative assessment by participating patients and clinicians. The positive quantitative (mean HbA1c decreased from 8.4 to 7.5% at 3 months) and qualitative results led to the evaluation of the Stepping-Up program in a 24-month duration randomized controlled trial [63] including 266 participants from 74 general practices in Australia. The mean HbA1c was 8.9% (95% CI 8.8–9.1%) at baseline for both groups. There was a significant between-group difference at 6 months, which was sustained at 24 months, with a mean HbA1c at 24 months of 7.6% (95% CI 7.5–7.8) in the intervention group and 8.0% (95% CI 7.7–8.4) in the control group.
Discussion
It has been more than 20 years since the concept of TI was introduced. Since then, and especially in the past 15 years, dozens of studies have been published seeking to assess the magnitude of the problem and test strategies to overcome it. We have reviewed here much of the available evidence, and even though there is consensus on some specific issues, it seems clear that most interventions have not resulted in a significant improvement in overcoming TI in the management of T2D, and that to date, the rates of glycemic goals achievement remain alarmingly low despite the availability of drugs with highly proven effectiveness and guidelines to assist their use. In other words, we have the certainty that adequate glycemic control can be achieved in a great majority of the population with T2D, but somehow, we are not being able to achieve it. There seems to be a missing link in the process, that most likely has to do with the failure to properly address TI, but also other issues that are beyond the scope of this review, such as medication coverage and affordability, and patient’s adherence.
TI is a very complex phenomenon, mainly because it relates to human behavior, and therefore it encompasses feelings, beliefs, and cultural backgrounds, as well as different local realities and settings. Given the above, the drivers of TI most likely differ from one setting to another (as can be observed when analyzing the existing qualitative studies) and so does the relative contribution of each of them to the global phenomenon. It is reasonable to assume that the causes and drivers of TI cannot be the same in developing or poor countries versus developed, rich ones; or in publicly funded health systems with universal access versus private or dual (public and private) funded health systems with incomplete or inexistent medication coverage; or in countries where there is no widespread use of electronic medical records; or communities with different levels of health literacy, or even different healthcare providers’ social status. Likewise, the type of intervention that a health system can opt to implement in terms of economic and human resources is highly variable; not all healthcare systems have the possibility of working in a multidisciplinary team with diabetes educators or dedicated diabetes nurses and clinical pharmacists, and therefore strategies that are designed to address TI in a particular healthcare setting could not be feasible to implement in another.
Figure 2 describes the author’s proposed approach to finding strategies to overcome TI in T2D management, based on the Plan-Do-Study-Act cycles for Quality Improvement [64].
Qualitative analysis can offer profound and rich insights about aspects of health care and services that sometimes prove elusive to quantitative research. In-depth understanding of the phenomenon and the setting in which it happens is essential to enable the design of evidence-based and context-targeted interventions. When looking at the different approaches that have been tested to overcome TI, most of the studies were performed in USA, yet none of the published qualitative studies were performed in USA. On the contrary, both interventions that were designed based on the findings of previous qualitative analysis of the phenomenon reported statistically significant favorable results. Qualitative studies help understand why promising clinical interventions do not always work in the real world, how patients experience care, and how practitioners think, and hence we believe that they should be the starting point for the design of any intervention seeking to address TI.
Conclusions
Addressing TI in the management of T2D is a multifaceted challenge that demands a nuanced and context-specific approach. The recognition of the diverse causes and drivers of TI across various healthcare settings and populations is essential. Most of the published research regarding TI in T2D focuses on describing the consequences of therapeutic inertia (i.e., low rate of HbA1c goal attainment, prolonged duration of time to obtain HbA1C goal attainment or to intensify treatment, and the failure to improve rates of goal attainment over time), rather than focusing on identifying the underlying causes of the inertia being observed. Qualitative research plays a pivotal role in providing in-depth insights necessary for the development of evidence-based solutions. By crafting interventions firmly grounded in these qualitative insights, we can navigate the intricacies of T2D management with a comprehensive perspective, ultimately making a meaningful impact on overcoming TI and enhancing the quality of care for individuals living with T2D.
References
IDF Diabetes Atlas, 10th edition. 2021.
Roden M, Shulman GI. The integrative biology of type 2 diabetes. Nature. 2019;576(7785):51–60.
Defronzo RA. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58(4):773–95.
Stratton IM, Adler AI, Neil HAW, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. Br Med J. 2000;321(7258):405–12.
UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352(9131):837–53.
Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HAW. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577–89.
Laiteerapong N, Ham SA, Gao Y, et al. The legacy effect in type 2 diabetes: impact of early glycemic control on future complications (the Diabetes & Aging study). Diabetes Care. 2019;42(3):416–26.
Ali MK, Bullard KM, Saaddine JB, Cowie CC, Imperatore G, Gregg EW. Achievement of goals in US diabetes care, 1999–2010. N Engl J Med. 2013;368(17):1613–24.
Fang M, Wang D, Coresh J, Selvin E. Trends in diabetes treatment and control in US adults, 1999–2018. N Engl J Med. 2021;384(23):2219–28.
Elsayed NA, Aleppo G, Aroda VR, et al. 6. Glycemic targets: standards of care in diabetes—2023. Diabetes Care. 2023;46(Suppl 1):S97–110.
Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycaemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022;45(11):2753–86.
Rodriguez-Valadez JM, Tahsin M, Fleischmann KE, et al. Cardiovascular and renal benefits of novel diabetes drugs by baseline cardiovascular risk: a systematic review, meta-analysis, and meta-regression. Diabetes Care. 2023;46(6):1300–10.
Ahmad E, Lim S, Lamptey R, Webb DR, Davies MJ. Type 2 diabetes. Lancet. 2022;400(10365):1803–20.
Newton I. Axioms, or Laws of Motion. In: Motte A, ed. Newton’s Principia: The Mathematical Principles of Natural Philosophy. New York: Daniel Adee; 1846:83–94.
Phillips LS, Branch WT, Cook CB, et al. Clinical inertia. Ann Intern Med. 2001;135(9):825–34.
Okonofua EC, Simpson KN, Jesri A, Rehman SU, Durkalski VL, Egan BM. Therapeutic inertia is an impediment to achieving the healthy people 2010 blood pressure control goals. Hypertension. 2006;47(3):345–51.
Khunti K, Davies MJ. Clinical inertia—time to reappraise the terminology? Prim Care Diabetes. 2017;11(2):105–6.
Fu AZ, Qiu Y, Davies MJ, Radican L, Engel SS. Treatment intensification in patients with type 2 diabetes who failed metformin monotherapy. Diabetes Obes Metab. 2011;13(8):765–9.
Khunti K, Wolden ML, Thorsted BL, Andersen M, Davies MJ. Clinical inertia in people with type 2 diabetes: a retrospective cohort study of more than 80,000 people. Diabetes Care. 2013;36(11):3411–7.
Pantalone KM, Wells BJ, Chagin KM, et al. Intensification of diabetes therapy and time until A1C goal attainment among patients with newly diagnosed type 2 diabetes who fail metformin monotherapy within a large integrated health system. Diabetes Care. 2016;39(9):1527–34.
Pantalone KM, Misra-Hebert AD, Hobbs TM, et al. Clinical inertia in type 2 diabetes management: evidence from a large, real-world data set. Diabetes Care. 2018;41(7):e113–4.
Pantalone KM, Misra-Hebert AD, Hobbs TM, et al. Intensification patterns and the probability of HbA1c goal attainment in type 2 diabetes mellitus: real-world evidence for the concept of ‘intensification inertia.’ Diabet Med. 2020;37(7):1114–24.
Rattelman CR, Ciemins EL, Stempniewicz N, Mocarski M, Ganguly R, Cuddeback JK. A retrospective analysis of therapeutic inertia in type 2 diabetes management across a diverse population of Health Care Organizations in the USA. Diabetes Therapy. 2021;12(2):581–94.
Khunti K, Gomes MB, Pocock S, et al. Therapeutic inertia in the treatment of hyperglycaemia in patients with type 2 diabetes: a systematic review. Diabetes Obes Metab. 2018;20(2):427–37.
Paul SK, Klein K, Thorsted BL, Wolden ML, Khunti K. Delay in treatment intensification increases the risks of cardiovascular events in patients with type 2 diabetes. Cardiovasc Diabetol. 2015;14:100.
Osataphan S, Chalermchai T, Ngaosuwan K. Clinical inertia causing new or progression of diabetic retinopathy in type 2 diabetes: a retrospective cohort study. J Diabetes. 2017;9(3):267–74.
Makam AN, Nguyen OK. An evidence-based medicine approach to antihyperglycemic therapy in diabetes mellitus to overcome overtreatment. Circulation. 2017;135(2):180–95.
Maciejewski ML, Mi X, Sussman J, et al. Overtreatment and deintensification of diabetic therapy among Medicare beneficiaries. J Gen Intern Med. 2018;33(1):34–41.
Christiaens A, Henrard S, Boland B, Sinclair AJ. Overtreatment of older people with type 2 diabetes—a high impact frequent occurrence in need of a new definition. Diabet Med. 2023;40(2): e14994.
Vijan S, Sussman JB, Yudkin JS, Hayward RA. Effect of patients’ risks and preferences on health gains with plasma glucose level lowering in type 2 diabetes mellitus. JAMA Intern Med. 2014;174(8):1227–34.
American Geriatrics Society 2023 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2023;71(7):2052–2081.
Müller N, Khunti K, Kuss O, et al. Is there evidence of potential overtreatment of glycaemia in elderly people with type 2 diabetes? Data from the GUIDANCE study. Acta Diabetol. 2017;54(2):209–14.
Lipska KJ, Ross JS, Miao Y, Shah ND, Lee SJ, Steinman MA. Potential overtreatment of diabetes mellitus in older adults with tight glycemic control. JAMA Intern Med. 2015;175(3):356–62.
Peyrot M, Barnett AH, Meneghini LF, Schumm-Draeger PM. Insulin adherence behaviours and barriers in the multinational global attitudes of patients and physicians in Insulin Therapy study. Diabet Med. 2012;29(5):682–9.
Rosenstock J, Nino A, Soffer J, et al. Impact of a weekly glucagon-like peptide 1 receptor agonist, albiglutide, on glycemic control and on reducing prandial insulin use in type 2 diabetes inadequately controlled on multiple insulin therapy: a randomized trial. Diabetes Care. 2020;43(10):2509–18.
Giugliano D, Longo M, Caruso P, et al. Feasibility of simplification from a basal-bolus insulin regimen to a fixed-ratio formulation of basal insulin plus a GLP-1RA or to basal insulin plus an SGLT2 inhibitor: BEYOND, a randomized, pragmatic trial. Diabetes Care. 2021;44(6):1353–60.
Taybani Z, Bótyik B, Katkó M, Gyimesi A, Várkonyi T. Simplifying complex insulin regimens while preserving good glycemic control in type 2 diabetes. Diabetes Ther. 2019;10(5):1869–78.
Bonora BM, Rigato M, Frison V, et al. Deintensification of basal-bolus insulin after initiation of GLP-1RA in patients with type 2 diabetes under routine care. Diabetes Res Clin Pract. 2021;173: 108686.
Allen JD, Curtiss FR, Fairman KA. Nonadherence, clinical inertia, or therapeutic inertia? J Manag Care Spec Pharm. 2009;15(8):690–5.
Karam SL, Dendy J, Polu S, Blonde L. Overview of therapeutic inertia in diabetes: prevalence, causes, and consequences. Diabetes Spectr. 2020;33(1):8–15.
Tarn DM, Heritage J, Paterniti DA, Hays RD, Kravitz RL, Wenger NS. Physician communication when prescribing new medications. Arch Intern Med. 2006;166(17):1855–62.
Nakar S, Yitzhaki G, Rosenberg R, Vinker S. Transition to insulin in type 2 diabetes: family physicians’ misconception of patients’ fears contributes to existing barriers. J Diabetes Complicat. 2007;21(4):220–6.
Yoshioka N, Ishii H, Tajima N, Iwamoto Y. Differences in physician and patient perceptions about insulin therapy for management of type 2 diabetes: the DAWN Japan study. Curr Med Res Opin. 2014;30(2):177–83.
Berkman ND, Davis TC, McCormack L. Health literacy: what is it? J Health Commun. 2010;15(Suppl 2):9–19.
Cavanaugh KL. Health literacy in diabetes care: explanation, evidence and equipment. Diabetes Manag (Lond). 2011;1(2):191–9.
Hua X, Carvalho N, Tew M, Huang ES, Herman WH, Clarke P. Expenditures and prices of antihyperglycemic medications in the United States: 2002–2013. JAMA. 2016;315(13):1400–2.
Polonsky WH, Fisher L, Guzman S, Villa-Caballero L, Edelman SV. Psychological insulin resistance in patients with type 2 diabetes. The scope of the problem. Diabetes Care. 2005;28(10):2543–5.
Hoover JW. Patient burnout, and other reasons for noncompliance. Diabetes Educ. 1983;9(3):41–3.
Elsayed NA, Aleppo G, Aroda VR, et al. 13. Older adults: standards of care in diabetes—2023. Diabetes Care. 2023;46(Suppl 1):S216–29.
Giugliano D, Maiorino MI, Bellastella G, Esposito K. Clinical inertia, reverse clinical inertia, and medication non-adherence in type 2 diabetes. J Endocrinol Invest. 2019;42(5):495–503.
Reach G, Pechtner V, Gentilella R, Corcos A, Ceriello A. Clinical inertia and its impact on treatment intensification in people with type 2 diabetes mellitus. Diabetes Metab. 2017;43(6):501–11.
Okemah J, Peng J, Quiñones M. addressing clinical inertia in type 2 diabetes mellitus: a review. Adv Ther. 2018;35(11):1735–45.
Powell RE, Zaccardi F, Beebe C, et al. Strategies for overcoming therapeutic inertia in type 2 diabetes: a systematic review and meta-analysis. Diabetes Obes Metab. 2021;23(9):2137–54.
Alharbi NS, Alsubki N, Jones S, Khunti K, Munro N, de Lusignan S. Impact of information technology-based interventions for type 2 diabetes mellitus on glycemic control: a systematic review and meta-analysis. J Med Internet Res. 2016;18(11): e310.
Pantalone KM, Rajpathak S, Ji X, et al. Addressing therapeutic inertia: development and implementation of an electronic health record-based diabetes intensification tool. Diabetes Spectr. 2023;36(2):161–70.
Zafar A, Stone MA, Davies MJ, Khunti K. Acknowledging and allocating responsibility for clinical inertia in the management of type 2 diabetes in primary care: a qualitative study. Diabet Med. 2015;32(3):407–13.
Aujoulat I, Jacquemin P, Hermans MP, et al. Clinical inertia in general practice, a matter of debate: a qualitative study with 114 general practitioners in Belgium. BMC Fam Pract. 2015;16:13.
De Lusignan S, McGovern A, Hinton W, et al. Barriers and facilitators to the initiation of injectable therapies for type 2 diabetes mellitus: a mixed methods study. Diabetes Ther. 2022;13(10):1789–809.
Wrzal PK, Mohseni AA, Fournier C, et al. Persons with diabetes and general/family practitioner perspectives related to therapeutic inertia in type 2 diabetes mellitus using qualitative focus groups and the theoretical domains framework: results from the MOTION study. Can J Diabetes. 2022;46(2):171–80.
Berenguera A, Molló-Inesta À, Mata-Cases M, et al. Understanding the physical, social, and emotional experiences of people with uncontrolled type 2 diabetes: a qualitative study. Patient Prefer Adher. 2016;10:2323–32.
Molló À, Vlacho B, Gratacòs M, et al. Impact of a multicomponent healthcare intervention on glycaemic control in subjects with poorly controlled type 2 diabetes: the INTEGRA study. Diabetes Obes Metab. 2023;25(4):1045–55.
Furler JS, Blackberry ID, Walker C, et al. Stepping up: a nurse-led model of care for insulin initiation for people with type 2 diabetes. Fam Pract. 2014;31(3):349–56.
Manski-Nankervis JA, Furler J, O’Neal D, Ginnivan L, Thuraisingam S, Blackberry I. Overcoming clinical inertia in insulin initiation in primary care for patients with type 2 diabetes: 24-month follow-up of the Stepping Up cluster randomised controlled trial. Prim Care Diabetes. 2017;11(5):474–81.
Taylor MJ, McNicholas C, Nicolay C, Darzi A, Bell D, Reed JE. Systematic review of the application of the plan-do-study-act method to improve quality in healthcare. BMJ Qual Saf. 2014;23(4):290–8.
Funding
No funding or sponsorship was received for this study or publication of this article.
Author information
Authors and Affiliations
Contributions
All authors participated in the study design and/or conduct and manuscript preparation. Dr. Paloma Rodriguez and Dr. Vicente T. San Martin researched the literature and wrote the manuscript. Dr. Kevin M. Pantalone reviewed the literature and reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest Disclosures
Dr. Kevin M. Pantalone reported receiving consulting honoraria from AstraZeneca, Bayer, Corcept Therapeutics, Diasome, Eli Lilly, Merck, Novo Nordisk, and Sanofi; speaker honoraria from AstraZeneca, Corcept Therapeutics, Merck and Novo Nordisk; and research support from Bayer, Merck, Novo Nordisk, and Twin Health. Dr. Paloma Rodriguez reports no conflicts of interest to disclose. Dr. Vicente T. San Martin reported receiving speaker honoraria from Novo Nordisk.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Rodriguez, P., San Martin, V.T. & Pantalone, K.M. Therapeutic Inertia in the Management of Type 2 Diabetes: A Narrative Review. Diabetes Ther 15, 567–583 (2024). https://doi.org/10.1007/s13300-024-01530-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-024-01530-9